CHAPTER 3 Blood Gas and Acid-Base Analysis
1 What are the normal arterial blood gas values in a healthy patient breathing room air at sea level?
| pH | 7.36–7.44 |
| PaCO2 | 33–44 mm Hg |
| PaO2 | 75–105 mm Hg |
| HCO3 | 20–26 mmol/L |
| Base deficit | +3 to −3 mmol/L |
| SaO2 | 95%–97% |
2 What information does arterial blood gas provide about the patient?
Arterial blood gas (ABG) provides an assessment of the following:
 Oxygenation (PaO2). The PaO2 is the amount of oxygen dissolved in the blood and therefore provides initial information on the efficiency of oxygenation.
Oxygenation (PaO2). The PaO2 is the amount of oxygen dissolved in the blood and therefore provides initial information on the efficiency of oxygenation. Ventilation (PaCO2). The adequacy of ventilation is inversely proportional to the PaCO2 so that, when ventilation increases, PaCO2 decreases, and when ventilation decreases, PaCO2 increases.
Ventilation (PaCO2). The adequacy of ventilation is inversely proportional to the PaCO2 so that, when ventilation increases, PaCO2 decreases, and when ventilation decreases, PaCO2 increases. Acid-base status (pH,
Acid-base status (pH, 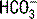 , and base deficit [BD]). A plasma pH of >7.4 indicates alkalemia, and a pH of <7.35 indicates acidemia. Despite a normal pH, an underlying acidosis or alkalosis may still be present.
, and base deficit [BD]). A plasma pH of >7.4 indicates alkalemia, and a pH of <7.35 indicates acidemia. Despite a normal pH, an underlying acidosis or alkalosis may still be present.Oxygenation and ventilation were discussed in Chapter 2 and acid-base status will be the area of focus for this chapter.
5 What are the common acid-base disorders and their compensation?
TABLE 3-2 Major Acid-Base Disorders and Compensatory Mechanisms*
| Primary Disorder | Primary Disturbance | Primary Compensation |
|---|---|---|
| Respiratory acidosis | ↑ PaCO2 | ↑ HCO3 |
| Respiratory alkalosis | ↓ PaCO2 | ↓ HCO3 |
| Metabolic acidosis | ↓ HCO3 | ↓ PaCO2 |
| Metabolic alkalosis | ↑ HCO3 | ↑ PaCO2 |
* Primary compensation for metabolic disorders is achieved rapidly through respiratory control of CO2, whereas primary compensation for respiratory disorders is achieved more slowly as the kidneys excrete or absorb acid and bicarbonate. Mixed acid-base disorders are common.
6 How do you calculate the degree of compensation?
| Primary Disorder | Rule |
|---|---|
| Respiratory acidosis (acute) | 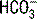 increases 0.1 × (PaCO2 − 40) increases 0.1 × (PaCO2 − 40)pH decreases 0.008 × (PaCO2 − 40) |
| Respiratory acidosis (chronic) | 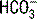 increases 0.4 × (PaCO2 − 40) increases 0.4 × (PaCO2 − 40) |
| Respiratory alkalosis (acute) | 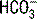 decreases 0.2 × (40 − PaCO2) decreases 0.2 × (40 − PaCO2)pH increases 0.008 × (40 − PaCO2) |
| Respiratory alkalosis (chronic) | 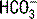 decreases 0.4 × (40 − PaCO2) decreases 0.4 × (40 − PaCO2) |
| Metabolic acidosis | PaCO2 decreases 1 to 1.5 × (24 − 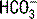 ) ) |
| Metabolic alkalosis | PaCO2 increases 0.25 to 1 × (HCO3− − 24) |
* Compensatory mechanisms never overcorrect for an acid-base disturbance; when ABG analysis reveals apparent overcorrection, the presence of a mixed disorder should be suspected.
Data from Schrier RW: Renal and electrolyte disorders, ed 3, Boston, 1986, Little, Brown.
9 What organs play a major role in acid-base balance?
 The lungs are the primary organ involved in rapid acid-base regulation. Carbon dioxide produced in the periphery is transported to the lung, where the low carbon dioxide tension promotes conversion of bicarbonate to carbon dioxide, which is then eliminated. The respiratory regulatory system can increase and decrease minute ventilation to compensate for metabolic acid-base disturbances.
The lungs are the primary organ involved in rapid acid-base regulation. Carbon dioxide produced in the periphery is transported to the lung, where the low carbon dioxide tension promotes conversion of bicarbonate to carbon dioxide, which is then eliminated. The respiratory regulatory system can increase and decrease minute ventilation to compensate for metabolic acid-base disturbances.12 List the major consequences of acidemia
Severe acidemia is defined as blood pH <7.20 and is associated with the following major effects:
13 List the major consequences of alkalemia
Severe alkalemia is defined as blood pH >7.60 and is associated with the following major effects:
18 List the common causes of elevated and nonelevated anion gap metabolic acidosis
 Nonelevated AG metabolic acidosis is caused by iatrogenic administration of hyperchloremic solutions (hyperchloremic metabolic acidosis), alkaline gastrointestinal losses, renal tubular acidosis (RTA), or ureteric diversion through ileal conduit. Excess administration of normal saline is a cause of hyperchloremic metabolic acidosis.
Nonelevated AG metabolic acidosis is caused by iatrogenic administration of hyperchloremic solutions (hyperchloremic metabolic acidosis), alkaline gastrointestinal losses, renal tubular acidosis (RTA), or ureteric diversion through ileal conduit. Excess administration of normal saline is a cause of hyperchloremic metabolic acidosis.19 Describe a stepwise approach to acid-base interpretation
 If the patient is breathing spontaneously, use the following rules:
If the patient is breathing spontaneously, use the following rules:
 If the PCO2 is increased and the pH is <7.35, the primary disorder is most likely a respiratory acidosis.
If the PCO2 is increased and the pH is <7.35, the primary disorder is most likely a respiratory acidosis. If the PCO2 is decreased and the pH >7.40, the primary disorder is most likely a respiratory alkalosis.
If the PCO2 is decreased and the pH >7.40, the primary disorder is most likely a respiratory alkalosis. Metabolic disorders can also be observed by analyzing the base excess or BD.
Metabolic disorders can also be observed by analyzing the base excess or BD.
 If there is a metabolic acidosis, calculate the AG and determine if the acidosis is a non-AG or AG acidosis, remembering to correct for hypoalbuminemia.
If there is a metabolic acidosis, calculate the AG and determine if the acidosis is a non-AG or AG acidosis, remembering to correct for hypoalbuminemia. If the patient is mechanically ventilated or if the acid-base disorder doesn’t seem to make sense, check electrolytes, albumin, and consider calculating the SID. Also consider the clinical context of the acid-base disorder (e.g., iatrogenic fluid administration, massive blood resuscitation, renal failure, liver failure, diarrhea, vomiting, gastric suctioning, toxin ingestion). This may require further testing, including measuring urine electrolytes, serum, and urine osmolality, and identifying ingested toxins.
If the patient is mechanically ventilated or if the acid-base disorder doesn’t seem to make sense, check electrolytes, albumin, and consider calculating the SID. Also consider the clinical context of the acid-base disorder (e.g., iatrogenic fluid administration, massive blood resuscitation, renal failure, liver failure, diarrhea, vomiting, gastric suctioning, toxin ingestion). This may require further testing, including measuring urine electrolytes, serum, and urine osmolality, and identifying ingested toxins.1. Casaletto J.J. Differential diagnosis of metabolic acidosis. Emerg Med Clin North Am. 2005;23:771-787.
2. Corey H.E. Stewart and beyond: new models of acid-base balance. Kidney Int. 2003;64:777-787.
3. Kraut J.A., Madias N.E. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2:162-174.
4. Morris C.G., Low J. Metabolic acidosis in the critically ill. Part 1. Classification and pathophysiology. Anaesthesia. 2008;63:294-301.
5. Morris C.G., Low J. Metabolic acidosis in the critically ill. Part 2. Cause and treatment. Anaesthesia. 2008;63:396-411.

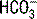 and PaCO2 determine pH as follows:
and PaCO2 determine pH as follows: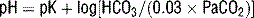
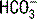 . The importance of other physiologic nonbicarbonate buffers was later recognized and partly integrated into the BD and the corrected anion gap, both of which aid in interpreting complex acid-base disorders.
. The importance of other physiologic nonbicarbonate buffers was later recognized and partly integrated into the BD and the corrected anion gap, both of which aid in interpreting complex acid-base disorders.




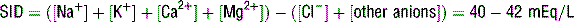
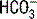 and pH were dependent on the three independent variables, contrary to the Henderson-Hasselbalch and standard base excess approaches. This model has been most useful in interpreting complex acid-base disorders in patients with mixed acid-base disorders and disorders that were not observable with conventional acid-base analysis such as hypoalbuminemia and hyperchloremic metabolic acidosis.
and pH were dependent on the three independent variables, contrary to the Henderson-Hasselbalch and standard base excess approaches. This model has been most useful in interpreting complex acid-base disorders in patients with mixed acid-base disorders and disorders that were not observable with conventional acid-base analysis such as hypoalbuminemia and hyperchloremic metabolic acidosis.






















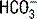 is a calculated value, whereas the CO2 is a measured value. Because the CO2 is measured, it is thought to be a more accurate determination of
is a calculated value, whereas the CO2 is a measured value. Because the CO2 is measured, it is thought to be a more accurate determination of 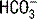 . The ABG
. The ABG 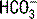 is calculated using the Henderson-Hasselbalch equation and the measured values of pH and PaCO2. In contrast, a chemistry panel reports a measured serum carbon dioxide content (CO2), which is the sum of the measured bicarbonate (
is calculated using the Henderson-Hasselbalch equation and the measured values of pH and PaCO2. In contrast, a chemistry panel reports a measured serum carbon dioxide content (CO2), which is the sum of the measured bicarbonate (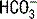 ) and carbonic acid (H2CO3). The CO2 is viewed as an accurate determination of
) and carbonic acid (H2CO3). The CO2 is viewed as an accurate determination of 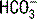 because the
because the 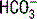 concentration in blood is about 20 times greater than the H2CO3 concentration; thus H2CO3 is only a minor contributor to the total measured CO2.
concentration in blood is about 20 times greater than the H2CO3 concentration; thus H2CO3 is only a minor contributor to the total measured CO2.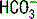 , and the nonbicarbonate buffer hemoglobin. Although the BD is determined in part by the nonbicarbonate buffer hemoglobin, it is criticized because it is derived from a nomogram and assumes normal values for other important nonbicarbonate buffers such as albumin. Thus in a hypoalbuminemic patient the BD should be used with caution since it may conceal an underlying metabolic acidosis.
, and the nonbicarbonate buffer hemoglobin. Although the BD is determined in part by the nonbicarbonate buffer hemoglobin, it is criticized because it is derived from a nomogram and assumes normal values for other important nonbicarbonate buffers such as albumin. Thus in a hypoalbuminemic patient the BD should be used with caution since it may conceal an underlying metabolic acidosis.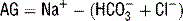
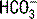 , thereby decreasing the
, thereby decreasing the 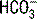 concentration. According to the previous equation, this decrease in
concentration. According to the previous equation, this decrease in 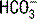 will increase the AG. Keep in mind that hypoalbuminemia has an alkalinizing effect that lowers the AG, which may mask an underlying metabolic acidosis caused by unmeasured anions. This pitfall can be avoided by correcting the AG when evaluating a metabolic acidosis in a hypoalbuminemic patient:
will increase the AG. Keep in mind that hypoalbuminemia has an alkalinizing effect that lowers the AG, which may mask an underlying metabolic acidosis caused by unmeasured anions. This pitfall can be avoided by correcting the AG when evaluating a metabolic acidosis in a hypoalbuminemic patient: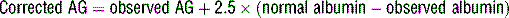

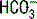 :
:









