Case 3 Asthma
Description of asthma
Definition
Asthma is a chronic inflammatory disease of the airways, characterised by acute exacerbations of reversible airway obstruction. The condition was formerly divided into two main types – extrinsic and intrinsic asthma. These classifications have since changed. Asthma is now separated into more specific aetiological subtypes, including, for example, allergic, exercise-induced, nocturnal, aspirin-sensitive and occupational asthma.
Epidemiology
This distressing, often disabling, and sometimes fatal disorder affects around ten per cent of the Australian population.1 A slightly higher prevalence rate is evident among adult women (eleven per cent) and among the 15–24 and 75 years and over age groups (eleven per cent). Higher rates of asthma are also observed among socially disadvantaged, unemployed and Indigenous populations.1
Aetiology and pathophysiology
The aetiology of asthma is multifactorial, with genetic and familial factors playing a major part in the pathogenesis of the disease. There is some suggestion that the growing prevalence of asthma is also due to a number of environmental contacts, such as vaccination, early introduction of foods, early exposure to antibiotics and food additives,2,3 but there are insufficient data to support these theories. Emerging evidence does indicate that exposure to infectious agents may be a risk factor; one recent longitudinal study reported significantly higher odds of asthma and respiratory wheeze among 5-year-old children who reported severe respiratory infections during infancy, particularly those with atopy.4 Other factors linked to the development of asthma are obesity, exposure to household allergens (e.g. dust mite, cockroaches, pets), omega 3 fatty acid intake and perinatal issues (e.g. lack of breastfeeding, poor maternal nutrition, young maternal age, prematurity, low birthweight).3,5 Evidence linking nutrient deficiency (e.g. vitamin C, vitamin E) with asthma is not convincing.6,7
The acute onset of asthma in susceptible individuals can be initiated by a range of allergic and non-allergic triggers, including household allergens (e.g. dust mite, cockroaches, animal dander), respiratory irritants (e.g. air pollution, cigarette smoke, perfumes, cleaning agents, sulfur dioxide), grass and tree pollens, occupational irritants (e.g. latex, solder, flour), hormonal changes, exercise, emotions (e.g. anger, anxiety, excitement), respiratory infections, cold air, aspirin and gastro-oesophageal reflux disease.3,8 It is not yet established how these factors trigger respiratory distress, although the prevailing theory suggests that heightened inflammatory activity could be a precipitating factor. The predominance of T-helper cell type 2 (Th2) activity observed in asthmatics and the subsequent increase in pro-inflammatory cytokine levels, airway eosinophilia and immunoglobulin (IgE) production, all appear to promote the development of smooth muscle hypertrophy and airway remodelling.9 These changes lead to airway hyper-responsiveness, which, upon exposure to any one of the aforementioned triggers, causes airway inflammation, submucosal oedema, increased mucus production, bronchoconstriction, mucus plugging and respiratory distress.3,9
Clinical manifestations
People with mild asthma are normally asymptomatic between exacerbations. In more severe cases of asthma, and during acute exacerbations of asthma, people typically present with dyspnoea, tachypnoea, chest tightness, cough, audible wheezing and anxiety. As airway obstruction progresses, and oxygen exchange diminishes, more serious manifestations begin to emerge, including hypoxia, cyanosis and altered consciousness, and at worst, respiratory failure and death.3,8
Clinical case
23-year-old woman with exercise-induced asthma
Rapport
Adopt the practitioner strategies and behaviours highlighted in Table 2.1 (chapter 2) to improve client trust, communication and rapport, and the accuracy and comprehensiveness of the clinical assessment.
Medical history
Family history
Medications
Salbutamol inhaler 2 puffs as needed, eformoterol 1 inhalation daily, 1 multivitamin tablet daily.
Lifestyle history
Alcohol consumption
Light social drinker. Consumes 1–2 × 375 mL premixed Vodka drinks per fortnight.
Illicit drug use
| Diet and fluid intake | |
|---|---|
| Breakfast | Wholemeal toast with low-fat cream cheese, coffee. |
| Morning tea | Protein bar, banana, walnuts. |
| Lunch | Tossed salad or wholemeal sandwich with turkey or chicken, tomato, low-fat cheese and lettuce. |
| Afternoon tea | 150 g vanilla yoghurt. |
| Dinner | Egg white omelette with tomato and cheese, grilled salmon or whiting with carrots and beans, stirfry with chicken breast, carrots, capsicum and onion. |
| Fluid intake | 1–2 cups of instant coffee a day, 7–8 cups of water a day. |
| Food frequency | |
| Fruit | 1–2 serves daily |
| Vegetables | 2–3 serves daily |
| Dairy | 2–3 serves daily |
| Cereals | 4–5 serves daily |
| Red meat | 1 serve a week |
| Chicken | 6 serves a week |
| Fish | 1 serve a week |
| Takeaway/fast food | 0–1 times a week |
Physical examination
Clinical assessment tools
The asthma control scoring system (ACSS) revealed a clinical subscore of twenty per cent (a measure of symptom frequency, physical limitation and rescue medication use), a physiological subscore of eighty per cent (a measure of peak expiratory flow rate), and an inflammatory subscore of eighty per cent (a measure of airway eosinophil count).10 This provided a mean global asthma control score of 60 per cent, signifying modest asthma control and moderate asthma severity (a higher percentage score indicates better control and reduced severity).
Diagnostics
Pathology tests
Eosinophil count
This count is a useful marker of asthma activity because activated eosinophils release histamine, which triggers bronchial smooth muscle contraction and mucus production, all of which are implicated in the pathogenesis of asthma.9
Immunoglobulin E (IgE)
IgE is an antibody primarily involved in allergic reactions. When IgE is cross-linked with an antigen, it stimulates the release of vasoactive substances (e.g. histamine) from basophils and mast cells. This results in smooth muscle contraction, increased vascular permeability and inflammation,11 and the subsequent manifestation of symptoms such as wheeze and dyspnoea. Elevated levels of serum IgE are positively associated with the severity of asthma.12
Plasma or red cell fatty acid analysis
This assesses the concentration of fatty acids within the plasma or erythrocyte, including omega 3, omega 6 and omega 9 polyunsaturated fatty acids, saturated fatty acids and trans fatty acids. Given that high dietary omega 3 fatty acid intake is associated with decreased odds of developing wheeze and asthma when compared with lower omega 3 fatty acid intake,5 this test may help to determine whether poor omega 3 fatty acid consumption is a contributing factor in asthma.
Radiology tests
It is not routine practice to use medical imaging in the diagnosis of asthma. Chest X-ray and CT scans may be indicated, however, in complicated asthma, atypical presentations of asthma, and/or suspicions of more serious pathology.7
Functional tests
Pulmonary function tests (PFTs)
PFTs are often used to evaluate the presence and severity of asthma. The PFT can incorporate any number of different measures of lung volume and capacity, including total lung capacity, tidal volume, maximal mid-expiratory flow (MMEF), maximal volume ventilation (MVV), peak inspiratory flow rate (PIFR), forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and peak expiratory flow rate (PEFR). Reductions in the latter three measures are often evident in obstructive airway diseases such as asthma.11
Miscellaneous tests
Bronchial provocation tests use either direct (e.g. inhaled histamine or methacholine) or indirect (e.g. exercise, cold air hyperventilation) stimuli to trigger bronchial smooth muscle contraction in hyperresponsive airways. There is some debate as to whether the results of these tests are specific to a diagnosis of asthma.13,14
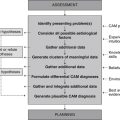
Diagnosis
Planning
Goals
Expected outcomes
Based on the degree of improvement reported in clinical studies that have used CAM interventions for the management of asthma,15,16 the following are anticipated.
Application
The range of interventions reported in the CAM literature that may be used in the treatment of asthma are appraised below.
Diet
Low-calorie diet (Level I, Strength C, Direction +)
As previously stated, there are a number of factors that elevate a person’s risk of developing asthma. A risk factor that is generally responsive to dietary change is obesity. There is, for instance, convincing evidence that high body weight at birth and/or during middle childhood increases the risk of developing asthma.17 Whether weight-reduction strategies are able to reverse this risk or improve asthma outcomes requires evidence from intervention studies. According to a Cochrane review, only one RCT has explored this hypothesis. The trial found the consumption of a low-energy diet plus education for 14 weeks to be statistically significantly superior to normal diet plus education at improving FEV1, FVC and rescue medication use in obese people with asthma.18 Given that low-calorie diets also reduce serum levels of inflammatory markers19 suggests that the low-energy diet could have improved respiratory function via an anti-inflammatory effect.
Miscellaneous diets (Level I, Strength C, Direction o)
Dietary modification is central to the overall management of asthma in many fields of CAM. While there is adequate theoretical justification to recommend many of these dietary interventions to people with asthma, such as a low-reactive or anti-inflammatory diet, there is a paucity of evidence to support these practices. A number of systematic reviews have also found insufficient or inconclusive evidence to link fish oil supplementation,20,21 dietary salt reduction22 and tartrazine avoidance23 to improvements in asthma outcomes. A recent meta-analysis of 10 observational studies also failed to find a significant correlation between dietary intake of antioxidants (including vitamin C, vitamin E and beta-carotene) and risk of asthma.6 By contrast, the consumption of whole foods (e.g. apples, pears, whole milk, butter) appears to offer some protection against asthma, according to a community-based, cross-sectional study of 1601 young adults.24 Several controlled trials have also found the dietary consumption of sulfur dioxide to exacerbate asthma in adults25 and to significantly reduce lung function in children with asthma.26 Nonetheless, these studies were small and, despite being published more than 15 years ago, have yet to be replicated using larger samples and more rigorous methodology.
Lifestyle
Buteyko breathing (Level II, Strength B, Direction + (for bronchodilatator use) and o (for respiratory function))
The Buteyko breathing technique (BBT) is a set of breathing exercises that serve to control the rate and depth of respirations. In doing so, Buteyko is believed to benefit people suffering from conditions characterised by hyperventilation, such as asthma. Five small RCTs have tested this claim,27–31 none of which found BBT to be effective at improving the physiological outcomes of asthma (e.g. FEV1, PEFR). Changes in quality of life and asthma control were also inconsistent across studies. On the other hand, all studies consistently showed BBT to be statistically significantly superior to controls at reducing inhaled beta-2 agonist and corticosteroid medication use. This suggests that the inclusion of BBT within a client’s asthma management plan may help to reduce the overall cost of asthma treatment.
Relaxation therapy (Level I, Strength C, Direction + (for bronchodilatator use) and o (for respiratory function))
The relaxation response can be induced by a number of behavioural therapies, such as progressive muscle relaxation, guided imagery and autogenic training. Many RCTs and systematic reviews have examined the effect of these therapies on asthma outcomes, although the results of these studies have been inconsistent. There are conflicting findings regarding, for instance, the effect of relaxation therapy (RT) on asthma symptoms and respiratory function (e.g. FEV1 and PEFR) in asthmatic adults.32–35 In asthmatic children, evidence suggests that RT may be effective at improving PEFR.36 A meta-analysis of two small RCTs indicates that RT may also be effective at reducing bronchodilator use,35 but the small size and methodological limitations of these studies, as well as the short duration of the interventions, limits any conclusions that can be made.
Yoga (Level II, Strength B, Direction o)
Yoga is an ancient Indian practice that integrates stretching, posture, exercise and breathing with meditation. Given that these techniques are likely to bring on a relaxation response, yoga has been thought to be helpful in improving asthma symptoms, but with the exception of one controlled trial,37 many RCTs have found yoga to be no more effective than controls at improving asthma symptoms, physiological parameters or quality of life.38–42 Still, studies using bronchial provocation tests have demonstrated significantly greater tolerance to airway provocation among people practising yoga than people receiving controls.39,41 This suggests that yoga might be more effective in certain subtypes of asthma, although given the uncertain specificity of this test, further investigation is needed.
Nutritional supplementation
Ascorbic acid (Level 1, Strength C, Direction o)
When compared with normal subjects lower levels of vitamin C have been reported in asthmatic adults and children.43,44 Low vitamin C levels are also associated with poor respiratory function.45 A meta-analysis of seven studies (n = 13,653) found no significant association between dietary intake of vitamin C and risk of asthma.6 Similarly, a Cochrane review of nine RCTs that involved 330 adults and children found no significant difference for the effect of orally administered vitamin C on lung function or symptoms.46
Beta-carotene (Level III-1, Strength C, Direction +)
Beta-carotene demonstrates immunomodulatory and antioxidant activity in experimental and clinical studies.47 While a meta-analysis of seven studies (n = 13,653) found no significant association between dietary intake of beta-carotene and risk of asthma,6 this does not seem to apply to supplemental beta-carotene. In a small RCT, the acute effect of beta-carotene supplementation (64 mg daily for 1 week) was compared with placebo in 37 patients with exercise-induced asthma. Exercise-induced asthma was prevented in fifty-two per cent of people receiving the beta-carotene. All people in the placebo group demonstrated a decline in lung function.16 Whether these effects can be replicated using lower doses of beta-carotene over a longer period of time is not yet certain.
Magnesium (Level II, Strength B, Direction o)
Magnesium is involved in many enzymatic reactions and physiological processes throughout the body. Hence, a deficiency of this mineral can result in a number of adverse manifestations. Low serum magnesium levels, for example, have been shown to intensify neuromuscular cell excitability, resulting in increased smooth muscle contractility. High serum levels of magnesium cause smooth muscle relaxation.48 In addition to this bronchodilating effect, magnesium also plays a role in modulating inflammation.49 Although these properties have influenced the use of intravenous magnesium in the emergency management of asthma, for which there is sound evidence,50 this approach to asthma is not congruent with CAM philosophy or compatible with the scope of CAM practice. Using orally administered magnesium to prevent asthma exacerbations would be a more suitable approach. Even though several RCTs have examined the effectiveness of orally administered magnesium in children and adults with asthma (with doses ranging from 200–450 mg/day of Mg chelate, to 18.3 g/day of Mg), results have been conflicting with regards to lung function, bronchial reactivity, asthma symptoms and bronchodilator use.51–53
Omega 3 fatty acids (Level I, Strength B, Direction o)
High dietary omega 3 fatty acid intake is associated with decreased odds of developing wheeze and asthma when compared with lower omega 3 fatty acid intake.5 It is likely that this protective effect may be attributed, among other reasons, to the anti-inflammatory action of the fatty acids.54 While findings from epidemiological studies are promising, a systematic review of 26 clinical studies (including 11 RCTs) found inadequate or inconclusive evidence to support the use of fish oil supplementation in the primary and secondary prevention of asthma.20 Studies postdating this review55–57 have also demonstrated conflicting results. There is some evidence to suggest that prenatal administration of fish oil may be effective in the primary prevention of asthma, allergic asthma58 and asthma symptoms.59
Pyridoxine (Level III-1, Strength C, Direction + (for medication use only))
Vitamin B6 is a water-soluble vitamin and an important coenzyme in carbohydrate, lipid and protein metabolism. Emerging data suggest pyridoxine also may be useful in the treatment of inflammatory disorders. Clinical data indicate that, for instance, pyridoxine supplementation (300 mg twice a day for 7 days) significantly reduces thromboxane B2 and leukotriene E4 excretion (p<0.05) in male subjects, although the effect in females is not certain.60 Despite the results of this small mechanistic study, evidence from two double-blind controlled clinical trials is somewhat difficult to interpret because of the small size of the studies, the lack of randomisation, different control groups, the variable dosage and duration of B6 treatment, and the different outcomes measured. In the first study, oral pyridoxine (200 mg daily for 20 weeks) significantly reduced bronchodilator and cortisone use when compared with lower dose pyridoxine (100 mg daily).61 In the second study, oral pyridoxine (300 mg daily for 9 weeks) was found to be no more effective than placebo at improving asthma symptoms or lung function,62 which highlights the need for further research in this area.
Selenium (Level II, Strength B, Direction o)
Selenium is a mineral involved in the regulation of inflammation and immunity, specifically, the inhibition of nuclear factor-kappaB activation,63 the enhancement of T-cell function, and B-cell activation and proliferation.64 Selenium has also been shown to lower oxidative stress in asthmatics with selenium deficiency.65 Despite these favourable results, findings from controlled trials have yet to support the use of this mineral in people with asthma. Two controlled clinical trials have, for instance, failed to demonstrate a statistically significant difference between patients receiving selenium (100 μg daily for 14–24 weeks) and patients receiving placebo with regards to lung function, medication use, asthma symptoms and quality of life.66,67 While selenium supplementation was significantly (p = 0.04) more effective than placebo at improving a composite score of these measures (excluding quality of life), the validity and reliability of this score is questionable. The effectiveness of higher doses of selenium in people with asthma is a matter for further research.
Vitamin E (Level II, Strength C, Direction o)
Tocopherols exhibit a number of actions that may target the mechanisms of asthma; for instance, the inhibition of proinflammatory cytokine release, and the reduction in monocyte adhesion to endothelial tissue.68 Even so, observational studies have failed to find a significant correlation between dietary vitamin E intake and risk of asthma.6 Likewise, a 6-week double-blind RCT involving 72 adults with asthma found vitamin E supplementation (500 mg daily) to be no more effective than placebo at improving bronchial hyperresponsiveness, lung function, asthma symptom scores and bronchodilator use.69
Herbal medicine
Boswellia serrata (Level II, Strength B, Direction +)
Frankincense has long been used as an anti-inflammatory herb in Ayurvedic medicine. Data from experimental studies support this anti-inflammatory effect; the boswellic acids in frankincense gum resin have been shown to inhibit 5-lipo-oxygenase and cyclo-oxygenase activity, and the subsequent release of proinflammatory mediators.70 While frankincense was traditionally used as a treatment for arthritis, the action of the herb suggests it may also be useful in the management of asthma. Findings from one well-designed RCT (n = 80 adults with asthma) lend support to this claim. This trial found B. serrata extract (350 mg three times a day for 6 weeks) to be significantly more effective than placebo at improving lung function, particularly FEV1 (p<0.0001). B. serrata was also effective at reducing asthma symptoms, number of asthma attacks, eosinophilic count and erythrocyte sedimentation rate.15 Further research is now required to corroborate these findings.
Ginkgo biloba (Level III-2, Strength C, Direction +)
Maidenhair tree has demonstrable anti-inflammatory and antioxidant effects.71,72 Despite these useful properties, and the long history of its use in traditional Chinese medicine, only one published study has explored the clinical efficacy of ginkgo in people with asthma. This study, which compared the effectiveness of a concentrated ginkgo leaf liquor (15 g three times a day) to placebo in 61 adults and children with asthma, found ginkgo to be significantly more effective than placebo (p<0.05) at improving FEV1 at 8 weeks.72 While this outcome is promising, further research is needed to determine if standardised extracts of G. biloba exhibit similar activity to ginkgo liquor.
Picrorhiza kurroa (Level II, Strength B, Direction o)
Katuka is traditionally used in Ayurvedic medicine for the treatment of respiratory disease, particularly asthma and bronchitis. The anti-inflammatory, antioxidant, smooth muscle relaxant and immunomodulatory effects of the herb, which have been demonstrated experimentally,73,74 offer a pathophysiological rationale for the use of katuka in asthma. That said, the best available evidence fails to support this theory. In the only known RCT of katuka and asthma, P. kurroa root (300 mg three times a day for 14 weeks) was found to be no more effective than placebo at improving lung function (i.e. FEV1) or asthma symptoms in 72 children and adults with asthma.75
Tylophora indica (Level I, Strength B, Direction o)
Indian ipecac is an Ayurvedic herb traditionally prescribed for the treatment of asthma, bronchitis, allergy and respiratory complaints. The anti-inflammatory, antihistaminic and smooth muscle relaxant properties of T. indica, which are supported by a number of experimental studies,76 are desirable for the effective management of asthma. At least five controlled clinical trials have explored the effectiveness of monopreparations of T. indica in adults and children with asthma.77 Firm conclusions cannot be made about the effectiveness of this herb, as improvements in lung function and asthma symptoms have not been consistent across studies. This may be partly explained by differences in treatment duration (i.e. 1–12 weeks), as well as differences in the active and control preparations used. One study did find the alkaloid extract of Indian ipecac to be significantly more effective than control at reducing medication use at 12 weeks, though the relevance of this preparation to conventional CAM practice is not certain. Given that all five studies are more than 30 years old, and that the clinical efficacy of T. indica in people with asthma is still inconclusive, further research in this area is well justified.
Other herbs
Adhatoda zeylanica (adhatoda), Albizia lebbeck (albizzia), Aloe barbadensis (aloe vera), Althaea officinalis (marshmallow), Chamomilla recutita (German chamomile), Coleus forskohlii (coleus), Euphorbia hirta (euphorbia), Inula helenium (elecampane), Grindelia camporum (grindelia), Rehmannia glutinosa (rehmannia), Schisandra chinensis (schisandra), Scutellaria baicalensis (baical skullcap), Viburnum prunifolium (black haw) and Withania somnifera (ashwaganda) have all been used traditionally and/or tested under experimental conditions for their antiallergic, antimicrobial, anti-inflammatory and/or immunostimulant activity,78,79 but there is insufficient clinical data to support the use of these herbs in asthma.
Other
Acupuncture (Level I, Strength C, Direction o)
Acupuncture originated in China more than 4000 years ago.80 Since then, the therapy has established a large traditional evidence base. In the case of asthma, the best available evidence indicates that acupuncture is not an effective treatment for this condition. According to a Cochrane review of 12 RCTs (n = 350), for instance, neither needle nor laser acupuncture were found to be effective at improving lung function or wellbeing in people with asthma, while changes in asthma symptoms were shown to be inconsistent across studies. Needle acupuncture did demonstrate statistically significant superiority to sham acupuncture in reducing medication usage.81 Given the range of interventions or techniques used, the lack of consideration given to contextual effects, and the differences in study design and outcomes measures, no firm conclusions can be drawn.
Chiropractic (Level II, Strength C, Direction o)
Chiropractic manipulation is generally indicated in the treatment of nervous and musculoskeletal disorders. While chiropractic is also used to treat a range of non-musculoskeletal complaints, such as asthma,82,83 there is insufficient evidence to support the use of chiropractic manipulation in asthma. Findings from three small RCTs indicate that chiropractic spinal manipulation is no more effective than sham manipulation at reducing bronchodilator use or improving PEFR, FEV1 or FVC in children and adults with asthma.84–86 As for asthma severity and quality of life, results have not been consistent across studies.
Homeopathy (Level I, Strength C, Direction o)
Homeopathy is a system of medicine that uses highly diluted and potentised remedies to influence the body’s vital force and restore balance. The therapy can be used to treat a wide range of acute and chronic complaints, including asthma. A number of trials have investigated the effectiveness of homeopathy in asthma, the results of which have been synthesised in a Cochrane review.87 The six randomised double-blind placebo-controlled trials included in the review examined the effect of individualised and formula homeopathy in adults and children with mild to severe asthma, for a period ranging from 1 day to 12 months. Changes in lung function (e.g. FEV1, FVC, PEFR), asthma symptoms, quality of life, frequency, duration or intensity of asthma exacerbations and medication use were not consistent across studies; thus the effectiveness of homeopathy for asthma is still uncertain. There is some concern that the outcomes of these studies might have been influenced by the concurrent administration of allopathic medication, as well as the omission of important contextual effects of homeopathic treatment (i.e. client–practitioner interaction). A more appropriate way of investigating the effectiveness of homeopathic management in future may be through whole systems research.
Massage therapy (Level II, Strength C, Direction + (for some measures of respiratory function))
Massage is the systematic manipulation of soft tissues of the body. This therapy may be helpful in reducing the symptoms of asthma, such as anxiety, wheezing and tachypnoea, by stimulating parasympathetic nervous system activity.88 The best available evidence to date comes from two small RCTs, both of which investigated the effects of parent-administered massage (20 minutes every night for 30 days) in children. In the first study (n = 32), massage was found to be more effective than progressive muscle relaxation in reducing child anxiety,89 although the level of significance was not clear. In the second trial (n = 44), massage was shown to be more effective than standard care in improving FEV1 (p = 0.04) and FVC (p = 0.05), though the difference between groups was only marginally significant.90 Reported changes in MMEF were inconsistent across studies. Whether massage is effective in adults with asthma or at reducing the frequency or severity of asthma attacks is yet to be investigated.
Reflexology (Level II, Strength B, Direction o)
Reflexology is a tactile therapy based on a theory that stimulating specific zones of the feet, hands and/or ears can generate neurophysiological reflexes or responses in distant organs, glands and tissues. Some claim that this therapy may be of benefit to those with asthma. Findings from two RCTs have failed to support this claim. Both studies found foot reflexology (45–60 minutes per week for 10 weeks) to be no more effective than usual care91 or simulated foot reflexology92 at improving objective lung function, quality of life or reducing beta-2 agonist use in patients with asthma.
CAM prescription
Primary treatments
Secondary treatments
Consider one of the following treatments.
Referral
Review
To determine whether pertinent client goals and expected outcomes have been achieved at follow-up, and if any aspects of the client’s care need to be improved, consider the factors listed in Table 8.2 (chapter 8), as well as the questions listed below.
1. Australian Bureau of Statistics (ABS). 2007–08 National health survey: summary of results. Canberra: Australian Bureau of Statistics; 2009. Cat 4364.0
2. Pizzorno J.E., Murray M.T. Textbook of natural medicine, 3rd ed. Philadelphia: Elsevier; 2006.
3. Porter R., et al, editors. The Merck manual. Whitehouse Station: Merck Research Laboratories, 2008.
4. Kusel M.M.H., et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. Journal of Allergy and Clinical Immunology. 2007;119(5):1105-1110.
5. Burns J.S., et al. Low dietary nutrient intakes and respiratory health in adolescents. Chest. 2007;132(1):238-245.
6. Gao J., et al. Observational studies on the effect of dietary antioxidants on asthma: a meta-analysis. Respirology. 2008;13(4):528-536.
7. Woods A.Q., Lynch D.A. Asthma: an imaging update. Radiologic Clinics of North America. 2009;47(2):317-329.
8. Levy M., Weller T., Hilton S. Asthma, 4th ed. London: Class Publishing; 2006.
9. Barrios R.J., et al. Asthma: pathology and pathophysiology. Archives of Pathology and Laboratory Medicine. 2006;130(4):447-451.
10. Boulet L.P., Boulet V., Milot J. How should we quantify asthma control. Chest. 2002;122(6):2217-2223.
11. Pagana K.D., Pagana T.J. Mosby’s diagnostic and laboratory test reference, 9th ed. St Louis: Elsevier Mosby; 2008.
12. Borish L., et al. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Annals of Allergy. Asthma and Immunology. 2005;95:247-253.
13. Brannan J.D., Koskela H., Anderson S.D. Monitoring asthma therapy using indirect bronchial provocation tests. Clinical Respiratory Journal. 2007;1(1):3-15.
14. Freed R., Anderson S.D., Wyndham J. The use of bronchial provocation tests for identifying asthma: a review of the problems for occupational assessment and a proposal for a new direction. ADF Health. 2002;3:77-85.
15. Gupta I., et al. Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. European Journal of Medical Research. 1998;3(11):511-514.
16. Neuman I., Nahum H., Ben-Amotz A. Prevention of exercise-induced asthma by a natural isomer mixture of beta-carotene. Annals of Allergy. Asthma and Immunology. 1999;82(6):549-553.
17. Flaherman V., Rutherford G.W. A meta-analysis of the effect of high weight on asthma. Archives of Disease in Childhood. 2006;91(4):334-339.
18. Cheng J., Pan T. Calorie controlled diet for chronic asthma. Cochrane Database of Systematic Reviews. 2003. (2): CD004674
19. Forsythe C.E., et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43(1):65-77.
20. Agency for Healthcare Research and Quality. Health effects of omega-3 fatty acids on asthma. Rockville: Agency for Healthcare Research and Quality; 2004.
21. Thien F.C.K., et al. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database of Systematic Reviews. 2002. (2): CD001283
22. Ardern K. Dietary salt reduction or exclusion for allergic asthma. Cochrane Database of Systematic Reviews. 2004. (2): CD000436
23. Ardern K. Tartrazine exclusion for allergic asthma. Cochrane Database of Systematic Reviews. 2001. (4): CD000460
24. Woods R.K., et al. Food and nutrient intakes and asthma risk in young adults. American Journal of Clinical Nutrition. 2003;78(3):414-421.
25. Freedman B.J. Asthma induced by sulphur dioxide, benzoate and tartrazine contained in orange drinks. Clinical allergy. 1977;7(5):407-415.
26. Steinman H.A., Le Roux M., Potter P.C. Sulphur dioxide sensitivity in South African asthmatic children. South African Medical Journal. 1993;83(6):387-390.
27. Bowler S.D., Green A., Mitchell C.A. Buteyko breathing techniques in asthma: a blinded randomised controlled trial. Medical Journal of Australia. 1998;169(11–12):575-578.
28. Cooper S., et al. Effect of two breathing exercises (Buteyko and pranayama) in asthma: a randomised controlled trial. Thorax. 2003;58(8):674-679.
29. Cowie R.L., et al. A randomised controlled trial of the Buteyko technique as an adjunct to conventional management of asthma. Respiratory Medicine. 2008;102(5):726-732.
30. McHugh P., et al. Buteyko breathing technique for asthma: an effective intervention. New Zealand Medical Journal. 2003;116(1187):U710.
31. Opat A.J., et al. A clinical trial of the Buteyko breathing technique in asthma as taught by a video. Journal of Asthma. 2002;37(7):557-564.
32. Chiang L.C., et al. Effect of relaxation-breathing training on anxiety and asthma signs/symptoms of children with moderate-to-severe asthma: a randomized controlled trial. International Journal of Nursing Studies. 2009;46(8):1061-1070.
33. Huntley A., White A.R., Ernst E. Relaxation therapies for asthma: a systematic review. Thorax. 2002;57(2):127-131.
34. Lahmann C., et al. Functional relaxation and guided imagery as complementary therapy in asthma: a randomized controlled clinical trial. Psychotherapy and Psychosomatics. 2009;78(4):233-239.
35. Yorke J., Fleming S.L., Shuldham C. Psychological interventions for adults with asthma. Cochrane Database of Systematic Reviews. 2006. (1): CD002982
36. Yorke J., Fleming S.L., Shuldham C. Psychological interventions for children with asthma. Cochrane Database of Systematic Reviews. 2005. (4): CD003272
37. Nagarathna R., Nagendra H.R. Yoga for bronchial asthma: a controlled study. British Medical Journal. 1985;291(6502):1077-1079.
38. Fluge T., et al. Long-term effects of breathing exercises and yoga in patients suffering from bronchial asthma. Pneumologie. 1994;48(7):484-490.
39. Manocha R., et al. Sahaja yoga in the management of moderate to severe asthma: a randomised controlled trial. Thorax. 2002;57(2):110-115.
40. Sabina A.B., et al. Yoga intervention for adults with mild-to-moderate asthma: a pilot study. Annals of Allergy. Asthma and Immunology. 2005;94(5):543-548.
41. Singh V., et al. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet. 1990;335(8702):1381-1383.
42. Vedanthan P.K., et al. Clinical study of yoga techniques in university students with asthma: a controlled study. Allergy and Asthma Proceedings. 1998;19(1):3-9.
43. Aderele W.I., et al. Plasma vitamin C (ascorbic acid) levels in asthmatic children. African Journal of Medical Science. 1985;14(3–4):115-120.
44. Olusi S.O., et al. Plasma and white blood cell ascorbic acid concentrations in patients with bronchial asthma. Clinica Chimica Acta. 1979;92(2):161-166.
45. Schwartz J., Weiss S.T. Relationship between dietary vitamin C intake and pulmonary function in the first national health and nutrition examination survey (NHANES I). American Journal of Clinical Nutrition. 1994;59(1):110-114.
46. Kaur B., Rowe B.H., Arnold E. Vitamin C supplementation for asthma. Cochrane Database of Systematic Reviews. 2009. (1): CD000993
47. Hughes D. Effects of carotenoids on human immune function. Proceedings of the Nutrition Society. 1999;58(3):713-718.
48. Kowal A., et al. The use of magnesium in bronchial asthma: a new approach to an old problem. Archivum Immunologiae et Therapiae Experimentalis. 2007;55(1):35-39.
49. Cairns C.B., Kraft M. Magnesium attenuates the neutrophil respiratory burst in adult asthmatic patients. Academic Emergency Medicine. 1996;3:1093-1097.
50. Cheuk D.K., Chau T.C., Lee S.L. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Archives of Disease in Childhood. 2005;90(1):74-77.
51. Bede O., et al. Urinary magnesium excretion in asthmatic children receiving magnesium supplementation: a randomized, placebo-controlled, double-blind study. Magnesium Research. 2003;16(4):262-270.
52. Fogarty A., et al. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clinical and Experimental Allergy. 2003;33(10):1355-1359.
53. Gontijo-Amaral C., et al. Oral magnesium supplementation in asthmatic children: a double-blind randomized placebo-controlled trial. European Journal of Clinical Nutrition. 2007;61(1):54-60.
54. Jho D.H., et al. Role of omega-3 fatty acid supplementation in inflammation and malignancy. Integrative Cancer Therapies. 2004;3(2):98-111.
55. Marks G.B., et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. Journal of Allergy and Clinical Immunology. 2006;118(1):53-61.
56. Mickleborough T.D., et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129(1):39-49.
57. Moreira A., et al. Pilot study of the effects of n-3 polyunsaturated fatty acids on exhaled nitric oxide in patients with stable asthma. Journal of Investigational Allergology and Clinical Immunology. 2007;17(5):309-313.
58. Olsen S.F., et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. American Journal of Clinical Nutrition. 2008;88(1):167-175.
59. Mihrshahi S., et al. Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatric Allergy and Immunology. 2004;15(6):517-522.
60. Saareks V., et al. Opposite effects of nicotinic acid and pyridoxine on systemic prostacyclin, thromboxane and leukotriene production in man. Pharmacology and Toxicology. 2002;90(6):338-342.
61. Collipp P.J., et al. Pyridoxine treatment of childhood bronchial asthma. Annals of Allergy. 1975;35(2):93-97.
62. Sur S., et al. Double-blind trial of pyridoxine (vitamin B6) in the treatment of steroid-dependent asthma. Annals of Allergy. 1993;70(2):147-152.
63. Vunta H., et al. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. Journal of Biological Chemistry. 2007;282(25):17964-17973.
64. Hawkes W.C., et al. The effects of dietary selenium on the immune system in healthy men. Biological Trace Element Research. 2001;81(3):189-213.
65. Voitsekhovskaia IuG, et al. Assessment of some oxidative stress parameters in bronchial asthma patients beyond add-on selenium supplementation. Biomeditsinskaia Khimiia. 2007;53(3):577-584.
66. Hasselmark L., et al. Selenium supplementation in intrinsic asthma. Allergy. 1993;48(1):30-36.
67. Shaheen S.O., et al. Randomised, double blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax. 2007;62(6):483-490.
68. Singh U., Devaraj S., Jialal I. Vitamin E, oxidative stress, and inflammation. Annual Review of Nutrition. 2005;25:151-174.
69. Pearson P.J., et al. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59(8):652-656.
70. Ammon H.P. Boswellic acids in chronic inflammatory diseases. Planta Medica. 2006;72(12):1100-1116.
71. Li G.H., et al. Studies on the effect of Ginkgo biloba extracts on NF-kappaB pathway. Journal of Chinese Medicinal Materials. 2008;31(9):1357-1360.
72. Li M.H., Zhang H.L., Yang B.Y. Effects of Ginkgo leaf concentrated oral liquor in treating asthma. Chinese Journal of Integrated Traditional and Western Medicine. 1997;17(4):216-218.
73. Govindarajan R., et al. Free radical scavenging potential of Picrorhiza kurrooa Royle ex Benth. Indian Journal of Experimental Biology. 2003;41(8):875-879.
74. Khare C.P. Indian herbal remedies: rational Western therapy, Ayurvedic, and other traditional usage, botany. New York: Springer Verlag, 2004.
75. Doshi V.B., et al. Picrorrhiza kurroa in bronchial asthma. Journal of Postgraduate Medicine. 29(2), 1983. 89–9
76. Patel N.J., et al. Anti-inflammatory and antinociceptive activities of leaf extracts of Tylophora indica. Pharmacognosy Magazine. 2008;4(Suppl 15):S31-S36.
77. Huntley A., Ernst E. Herbal medicines for asthma: a systematic review. Thorax. 2000;55(11):925-929.
78. Bone K. A clinical guide to blending liquid herbs. St Louis: Churchill Livingstone; 2003.
79. Yaniv Z., Bachrach U., editors. Handbook of medicinal plants. New York: Haworth Press, 2005.
80. O’Brien K.A., Xue C.C. Acupuncture. In: Robson T., editor. An introduction to complementary medicine. Sydney: Allen & Unwin, 2003.
81. McCarney R.W., et al. Acupuncture for chronic asthma. Cochrane Database of Systematic Reviews. 2003. (3): CD000008
82. Andrews L., et al. The use of alternative therapies by children with asthma: a brief report. Journal of Paediatrics and Child Health. 1998;34:131-134.
83. Sidora-Arcoleo K., et al. Complementary and alternative medicine use in children with asthma: prevalence and sociodemographic profile of users. Journal of Asthma. 2007;44(3):169-175.
84. Balon J., et al. A comparison of active and simulated chiropractic manipulation as adjunctive treatment for childhood asthma. New England Journal of Medicine. 1998;339(15):1013-1020.
85. Bronfort G., et al. Chronic pediatric asthma and chiropractic spinal manipulation: a prospective clinical series and randomized clinical pilot study. Journal of Manipulative and Physiological Therapeutics. 2001;24(6):369-377.
86. Nielsen N.H., et al. Chronic asthma and chiropractic spinal manipulation: a randomized clinical trial. Clinical and Experimental Allergy. 1995;25(1):80-88.
87. McCarney R.W., Linde K., Lasserson T.J. Homeopathy for chronic asthma. Cochrane Database of Systematic Reviews. 2004. (1): CD000353
88. Moyer C.A., Rounds J., Hannum J.W. A meta-analysis of massage therapy research. Psychological Bulletin. 2004;130(1):3-18.
89. Field T., et al. Children with asthma have improved pulmonary functions after massage therapy. Journal of Pediatrics. 1998;132(5):854-858.
90. Nekooee A., et al. Effect of massage therapy on children with asthma. Iranian Journal of Pediatrics. 2008;18(2):123-129.
91. Petersen L.N., et al. Foot zone therapy and bronchial asthma: a controlled clinical trial. Ugeskrift for Laeger. 1992;154(30):2065-2068.
92. Brygge T., et al. Reflexology and bronchial asthma. Respiratory Medicine. 2001;95(3):173-179.