CHAPTER 29
Dupuytren Contracture
Definition
Dupuytren disease is a nonmalignant fibroproliferative disorder causing progressive and permanent contracture of the palmar fascia; subsequent flexion contracture usually begins with the fourth and fifth digits on the ulnar side of the hand (Fig. 29.1). The eponym Cooper contracture has been suggested for Astley Cooper, who first described and lectured on the entity in 1822. A nodule in the palm is the primary lesion in Dupuytren contracture. It is a firm, soft tissue mass fixed to both the skin and the deeper fascia. It is characterized histologically by dense, noninflammatory, chaotic cellular tissue and appears on the anterior aspect of the palmar aponeurosis.
The key cell response for tissue contraction in Dupuytren disease is thought to be the fibroblast and its differentiation into a myofibroblast [1]. This idiopathic activation happens in response to the fibrogenic cytokines interleukin-1, prostaglandin F2, prostaglandin E2, platelet-derived growth factor, connective tissue–derived growth factor, and, most important, transforming growth factor-β and fibroblast growth factor 2. In addition, microRNAs (miRNAs) identified in Dupuytren contracture samples, including miR-29c, miR-130b, miR-101, miR-30b, and miR-140-3p, were found to regulate important genes related to the β-catenin pathway: WNT5A, ZIC1, and TGFB1 [2]. As the nodule extends slowly, it induces shortening and tension on the longitudinal fascial bands of the palmar aponeurosis, resulting in cords of hypertrophied tissue. It is unique among ailments of the hand, and one could conceive of it as a focal autoimmune collagen vascular phenomenon. Dupuytren disease is thought to begin in the overlying dermis. Unlike the nodule, the cord is strikingly different histologically; it contains few or no myofibroblasts and few fibroblasts in a dense collagen matrix with less vascularity. Skin changes are the earliest signs of Dupuytren disease, including thickening of the palmar skin and underlying subcutaneous tissue. Rippling of the skin can occur before the development of a digital flexion deformity [3].
A controversy exists as to whether there is a relationship between Dupuytren disease and repetitive microtrauma [4], but more recent meta-analysis does seem to suggest some degree of occupational correlation with manual work and vibration exposure [5]. It is now thought that microruptures of the palmar fascia are related to the contracture rather than a primary cause [6]. Cessation of manual labor and immobilization can lead to acceleration of the disease, which has been noted in laborers after retirement [7]. One study seems to suggest that there may be an association with rock climbing in men [8].
A genetic predisposition is thought to be inherited as an autosomal dominant trait with variable penetrance [9]. Family history is often unreliable as many individuals are unaware they have family members with the disease. Dupuytren disease has been termed Viking disease [10] because it has a high prevalence in areas that were populated by the Vikings and where the Vikings migrated. Global prevalence is 3% to 6% of the white population, and it is rare in nonwhite populations. Dupuytren disease occurs more commonly in the elderly but tends to be associated with greater functional compromise in younger patients. Women are affected half as often as men [11]. There is no relationship to handedness; however, affected individuals tend to complain more frequently about the dominant hand. Other associations with the condition include diabetes mellitus [12] (specifically with an increased risk from diet-controlled diabetes to sulfonylureas to metformin to insulin requiring), alcohol consumption [13], cigarette smoking [13,14], human immunodeficiency virus infection [15], and antecedent Colles fracture. Conflicting reports exist of an association with epilepsy, but antiepileptic drugs do not present an increased risk [12,16].
There are potential secondary findings in Dupuytren disease that are rarely seen but when present suggest a strong Dupuytren diathesis (genetic penetrance of the disease). These findings include knuckle pads (Garrod nodes), plantar fascial disease, and Peyronie disease. The contractile tissue in all of these conditions resembles the pathologic findings of Dupuytren disease in the palm [17], and alterations in the expression of certain gene families, fibroblast to myofibroblast differentiation among others, are similar [18]. However, these associated conditions are found in only an estimated 1% or less of patients with Dupuytren disease [19]. All patients with the disease have a diathesis. The association with these conditions as well as onset at an early age and family history suggests that the diathesis is strong. Recognition of a strong diathesis is important for planning an appropriate rehabilitation protocol, including long-term follow-up and awareness of possible poor prognosis and likelihood of recurrence with surgical treatment.
Symptoms
Dupuytren disease typically has a painless onset and progression. Decreased range of motion, loss of dexterity, and getting the hand “caught” when trying to place it in one’s pant-pocket are common presenting symptoms. Pain can be a result of concomitant injuries to the hand and fingers that can precede the development or worsening of Dupuytren disease. Abrasions or ecchymosis to the distal interphalangeal and proximal interphalangeal joints of the affected digits may be seen and may be the reason for the initial consultation. With use of the relatively new Dupuytren disease scale of subjective well-being of patients questionnaire, which covers four areas of the quality of life, there were no differences in quality of life in patients affected in the left or right hand regardless of hand dominance of the patients [20].
The progression of the condition is generally considered to be a result of immobility after an injury in a predisposed individual rather than of the injury itself. Pure sensory symptoms in digits four and five may arise from palmar digital nerves against the relatively inelastic deep transverse metacarpal ligament.
Physical Examination
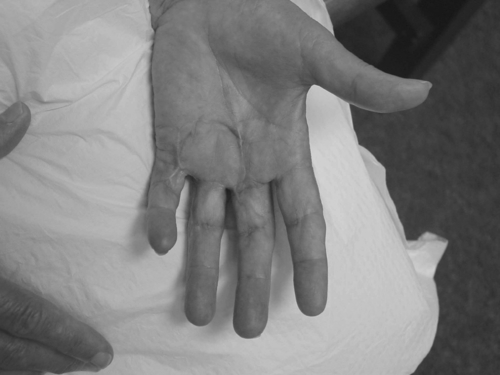
The most common first sign of Dupuytren disease is a lump in the palm close to the distal palmar crease and in the axis of the ray of the fourth digit (ring finger) (Fig. 29.2). It can also be manifested in the digit, generally over the proximal phalanx. The thumb and index finger are the least affected of the five digits. The nodule can be tender to palpation. In most cases, the skin is closely adherent to the nodule, and movement with tendon excursion often suggests other conditions, such as stenosing tenosynovitis. The condition is more readily apparent when it is manifested in a more advanced stage with palmar nodule, cord, and digital flexion contracture. Conditions associated with this disease include fat pads at the knuckles and evidence of the disease in the plantar fascia. “Swan-neck” deformity as a dorsal variant of Dupuytren disease has been suggested [21].
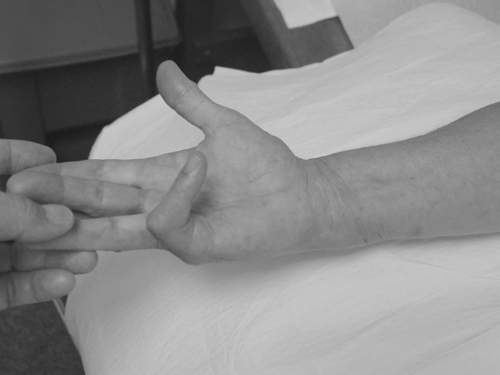
The examination should evaluate the range of motion and kinetic chain of the entire upper limb, including associated adhesive capsulitis, epicondylitis, and other tenosynovitis. Sensory, manual motor, and muscle stretch reflex components of the neurologic examination should be normal.
Functional Limitations
The majority of individuals with this condition have little functional limitation early on. With more advanced contracture, properly opening the palm and grasping can become difficult, making gripping activities such as activities of daily living, opening cans, buttoning shirts, and placing keys in automotive ignitions troublesome. In many cases, the insidious onset allows gradual compensation, and outside observers may notice irregularity during a simple hand shake.
Diagnostic Studies
The diagnosis of Dupuytren disease is generally made on a clinical basis. Biopsy is considered when a palmar soft tissue mass cannot be reliably differentiated from sarcoma [22]. The suspicion for sarcoma is higher in a younger individual with no strong evidence of Dupuytren disease because sarcoma is more likely in younger age groups. Unfortunately, histologic differentiation is not always easy because a Dupuytren nodule can appear cellular with mitotic figures and closely resemble an aggressive sarcoma. Blood work relevant to underlying secondary disease (e.g., hemoglobin A1c level, human immunodeficiency virus testing, uric acid level, erythrocyte sedimentation rate) should be entertained. Electrodiagnostic studies are usually normal. However, concomitant median neuropathy at the wrist or spinal cord may cause sensory loss due to impingement of digital nerves, or Dupuytren tissue may compress the palmar digital nerves against the relatively inelastic deep transverse metacarpal ligament [23]. Sensory nerve action potentials should be recorded to digit four and not just digit five.
Treatment
Initial
Many patients who seek consultation for Dupuytren disease are merely looking for reassurance that they do not have a malignant neoplasm and are satisfied to learn that the contracture is not a sign of a more ominous disease.
Conventional noninvasive treatment has generally been of little or no value in the prevention of contracture or recurrence in Dupuytren disease. This includes the use of steroid injections, splinting, ultrasound, and nonsteroidal anti-inflammatory medications. Radiotherapy, topical dimethyl sulfoxide, colchicine, and interferon have also been proposed but lack data demonstrating long-term efficacy. Topical 5-fluorouracil [24], topical imiquimod (Aldara) [25], and oral simvastatin [26] seem to target the underlying fibroblastic process, but long-term outcome studies do not exist. Traumatic rupture has never gained acceptance as a method of correcting flexion contracture; however, anecdotal reports of individuals correcting their deformity in such a fashion exist [27], but recurrence in true Dupuytren disease would be expected. Continuous passive traction has been proposed by some for severely flexed digit contractures [28]; however, this is used as a preoperative adjunctive procedure and not done in isolation.
Ergonomic assessment and equipment modification can be of use in some instances with laborers who are functionally limited by contracture. Tobacco abuse or excessive alcohol consumption should be addressed if applicable.
Rehabilitation
Rehabilitation efforts are minimal preoperatively and focus on adaptive equipment recommendations for work and home (e.g., large-handled tools for gripping). Splinting may be done by therapists with prefabricated or custom designs, but there is no evidence that this delays contracture or affects the underlying pathohistologic changes. Continuous passive traction has been proposed by some for severely flexed digit contractures [26]; however, this is used as a preoperative adjunctive procedure and not done in isolation.
Postoperative rehabilitation is needed to facilitate a satisfactory outcome. The length of the rehabilitation generally reflects the invasiveness of the surgical procedure; limited fasciotomies often involve a period of 4 to 6 weeks, whereas more extensive surgery may necessitate a formal rehabilitation process of up to 3 to 6 months. Stretching, splinting in palmar extension, and continuous passive traction in some individuals are used early postoperatively. Strengthening and functional activities are added later, after incision healing. Again, splinting may be an option to prevent recurrence, and adaptive equipment recommendations can help with resumption of functions that involve gripping or repetitive hand use.
Procedures
Closed needle fasciotomy has been used [29] but is prone to complications, including infection, nerve injury, and skin breakdown as well as recurrence.
Enzymatic fasciotomy with purified collagenase including injection into the central cord has greatly improved nonsurgical relief of contracture [30–32], with recurrence rates in the range of 14% for metacarpophalangeal joints to 23% for proximal interphalangeal joints at 2-year follow-up where initial collagenase treatment was successful [33]. Xiaflex consists of a 1:1 ratio of collagenases, collagenase AUX-I and AUX-II, isolated from the fermentation of Clostridium histolyticum bacteria. This agent has been used for débridement of burns and skin ulcers as well as for Peyronie disease [34]. Steroid injection into the palmar nodule can be a useful adjunct to flatten the nodule. Concern for potential adverse effect on the underlying flexor tendons should be noted. Thus, care must be taken to avoid injection into the underlying flexor tendons. Identification of unique miRNA expression in Dupuytren contracture may eventually lead to the development of novel molecular therapy.
Surgery
Appropriate selection is critical for patients considering surgical treatment. The potential for recurrence and worsening after surgery is higher in patients with a strong diathesis. Recurrence is defined as the development of nodules and contracture in the area of previous surgery. Extension is the development of lesions outside of the surgical area where there had previously been no disease. All patients should be made aware that surgery is not curative of this disease and that recurrence and extension are likely at some time. Extensive recurrence is more likely if surgery is performed during the proliferative phase of the condition.
Nevertheless, many individuals have improved hand function after surgical treatment, compared with their preoperative status, for years on follow-up [35]. The goals of surgical treatment, when it is indicated, are to improve function, to reduce deformity, and to prevent recurrence. Surgical indication is generally thought to include digital flexion contracture of the proximal interphalangeal and metacarpophalangeal joints and web space contracture. Metacarpophalangeal joint contractures are often fully correctable; however, proximal interphalangeal joint contractures often have residual deformity [36].
Multiple surgical procedures have been described for the treatment of Dupuytren contracture. Malingue’s procedure is a modified Z-plasty, making use of Euclidean geometry and avoiding the use of skin grafts or flaps, and may potentially have a lower incidence of reoperation [37].
In addition, variations of subcutaneous fasciotomy, fasciectomy, and skin grafting have been used. A controlled randomized trial between the two most common approaches found no statistical difference at 2 years [38]. Fewer complications are seen with limited fasciectomy, and this is often the procedure of choice for higher risk patients, for whom temporary relief is a therapeutic goal. A diseased cord arising from the abductor digiti minimi is noted to be present in approximately 25% of cases [39], and it should be released at the time of surgery if it is present. Full-thickness skin grafting has been shown to prevent recurrence [40] and is considered in patients with a strong diathesis who have functionally limiting contracture.
Potential Disease Complications
In some individuals, the condition can become functionally limiting because of severe contracture. The thumb and index finger tend to be less affected than the other digits. Secondary contracture of the proximal interphalangeal joints can also develop in long-standing deformity. Vascular compromise is rare and more often part of complex regional pain syndrome after surgery. It appears that there is an advantage with axillary block or intravenous regional anesthesia with clonidine over lidocaine alone or general anesthesia in preventing complex regional pain syndrome in patients undergoing Dupuytren surgery [41].
Potential Treatment Complications
Overall, recurrence of the disease after surgery is common (approximately 31%) [42], which is unsurprising when one considers that surgery does not alter the underlying histopathologic process. Loss of flexion into the palm is particularly disturbing to patients. The presence of thickly calloused hands can result in increased postoperative swelling, leading to longer postoperative follow-up. “Flare reaction” is a postoperative complication in 5% to 10% of patients that occurs 3 to 4 weeks after surgery and is characterized by redness, swelling, pain, and stiffness [43]. Complication rates are higher with greater disease severity, in particular proximal interphalangeal joint flexion contracture of 60 degrees or more. Amputation and ray resections are reported complications more common in surgery for recurrences [43]. Although women with the disease less frequently meet operative criteria, they are thought to have a higher incidence of complex regional pain syndrome postoperatively [44]. Other potential surgical complications are digital hematoma, granulation, scar contracture, inadvertent division of a digital nerve or artery, infection, and graft failure in full-thickness grafting procedures. A potential complication of injection therapy is injury to nearby structures, including the digital artery, nerve, and flexor tendons. One study [31] reported 96.6% of the patients who received collagenase to have had at least one “treatment-related” adverse event (compared with 21.2% with placebo), but care should be taken in interpreting this number as events such as “ecchymosis” and “injection-site pain” are potentially subjective, to be expected with any needle entry, and should not necessarily be attributed to the collagenase.