CASE 26 Severe Thrombocytopenia After Coronary Intervention
Cardiac catheterization
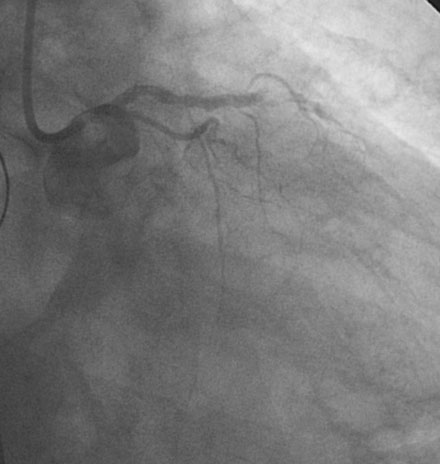
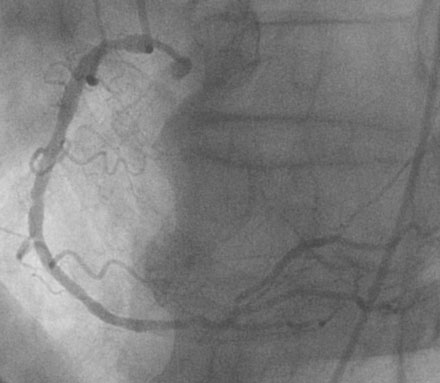
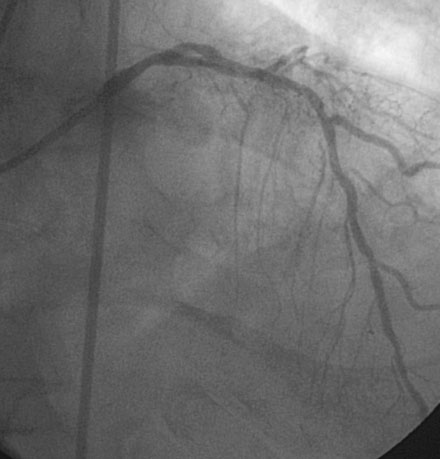
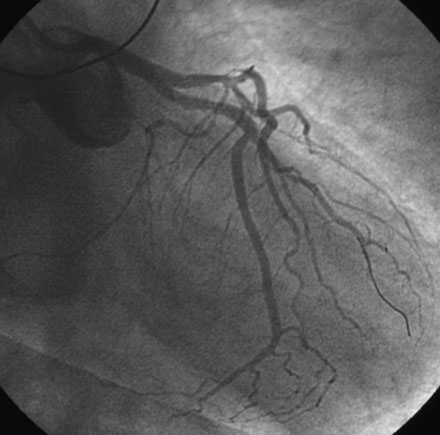
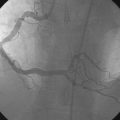
Coronary angiography confirmed complete occlusion of the previously placed stent in the proximal left anterior descending artery and total occlusion of the left circumflex, as previously noted (Figure 26-1 and Video 26-1). There was moderate disease in the right coronary artery (Figure 26-2). After administration of a heparin bolus of 50 units/kg and an eptifibatide bolus (12.6 mg) followed by an infusion (2 mcg/kg/min), balloon angioplasty using a 2.5 mm diameter by 20 mm long balloon inflated to high pressures was performed. This restored TIMI-3 flow (Figure 26-3 and Video 26-2).
Discussion
Originally described with abciximab, thrombocytopenia has been associated with all three of the available glycoprotein IIb/IIIa receptor antagonists (abciximab, eptifibatide, and tirofiban). The mechanism is poorly understood but is believed to be an immune-mediated phenomenon.1 For patients receiving abciximab, thrombocytopenia, defined as a platelet count of less than 100 × 109/L, occurs in 2.7% of patients. Severe thrombocytopenia (platelet count less than 50 × 109/L) occurs in 0.8% of patients receiving abciximab and profound thrombocytopenia (platelet count less than 20 × 109/L) is observed in 0.3% of patients. 2 Severe thrombocytopenia may be less frequent with infusions of eptifibatide and tirofiban than with abciximab, with platelet counts under 50 × 109/L occurring in 0.2% to 0.5% of patients after receiving these agents.1
As observed in this case, most occurrences of severe and profound thrombocytopenia develop within the first few hours after administration of the drug bolus; for this reason, it is recommended that clinicians measure platelet counts 2 to 4 hours after the bolus is administered and again at 24 hours.1 Risk factors for the development of thrombocytopenia have been studied and include advanced age, lower body weight, and lower baseline platelet count.2,3
Patients developing severe thrombocytopenia are at increased risk of bleeding and have increased mortality compared to those without thrombocytopenia.2,3 The management of severe thrombocytopenia includes maintaining platelet counts with platelet transfusions and close monitoring for bleeding complications. Immediate discontinuation of the glycoprotein IIb/IIIa receptor antagonist is obviously necessary. Adjunctive use of heparin or other anticoagulants should be avoided until platelet counts recover. The clinician is often faced with a dilemma regarding continued use of clopidogrel and aspirin. Although the cessation of these agents may result in stent thrombosis, continuation in the case of profound thrombocytopenia may lead to life-threatening bleeding and thus the risks and benefits of discontinuation of antiplatelet therapy should be carefully considered. Transfusion of platelets is recommended for platelet counts less than 20 × 109/L. Platelet counts recover fairly rapidly with cessation of the agent. The case presented here is typical, with full recovery of the platelet count by 5 to 7 days after eptifibatide and 3 to 14 days for abciximab.1
1 Huxtable L.M., Tafreshi M.J., Rakkar A.N.S. Frequency and management of thrombocytopenia with the glycoprotein IIb/IIIa receptor antagonists. Am J Cardiol. 2006;97:426-429.
2 Berkowitz S.D., Sane D.C., Sigmon K.N., Shavender J.H., Harrington R.A., Tcheng J.E., Topol E.J., Califf R.M. Occurrence and clinical significance of thrombocytopenia in a population undergoing high risk percutaneous coronary revascularization. J Am Coll Cardiol. 1998;32:311-319.
3 Kereiakes D.J., Berkowitz S.D., Lincoff A.M., Tcheng J.E., Wolski K., Achenbach R., Melsheimer R., Anderson K., Califf R.M., Topol E.J. Clinical correlates and course of thrombocytopenia during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade. Am Heart J. 2000;140:74-80.