CASE 24 Early Stent Thrombosis
Case presentation
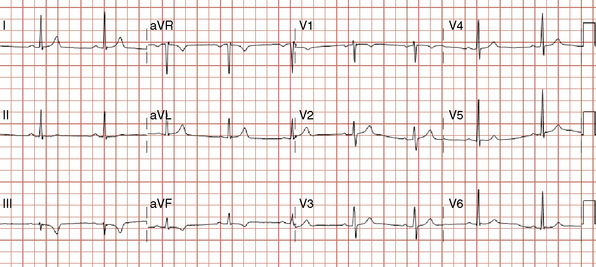
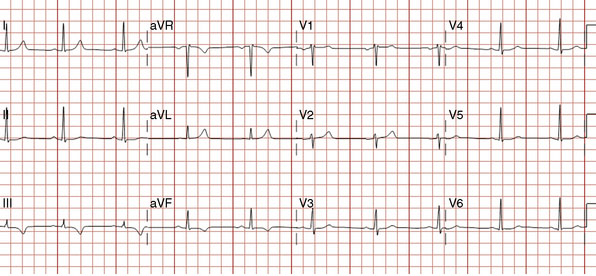
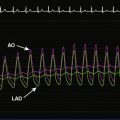
The onset of chest pain occurring during heavy exertion prompted a healthy 44-year-old man to seek medical attention. For 6 weeks, he had noted symptoms occurring only when running or performing strenuous labor. The chest pain always promptly resolved with rest. For many years he had maintained a high level of fitness for his job as a physical trainer, and he had no significant medical history except for a family history of premature coronary disease (both father and paternal grandfather suffered fatal acute myocardial infarctions at age 52 and 55, respectively). A 12-lead electrocardiogram was notable for T-wave inversions inferiorly (Figure 24-1). To further evaluate his symptoms, his family physician ordered an exercise stress test. He exercised to 12.9 METS but experienced chest pain in the third stage and developed 2 mm of downsloping ST-segment depression. His physician prescribed aspirin and metoprolol and referred him for cardiac catheterization.
Cardiac catheterization
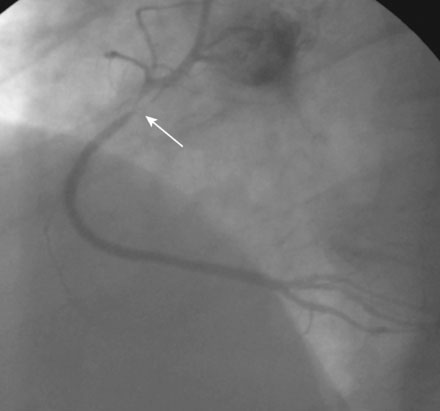
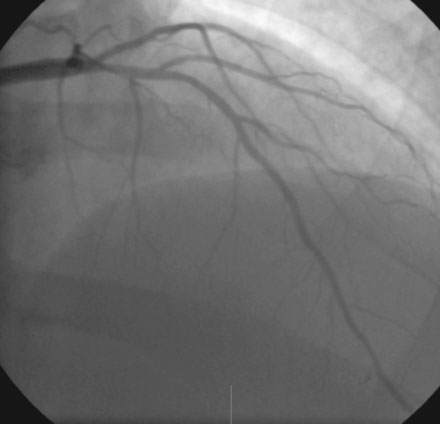
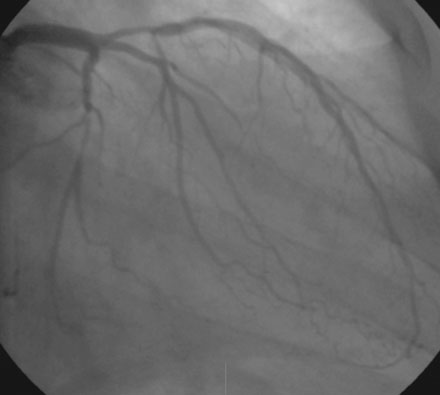
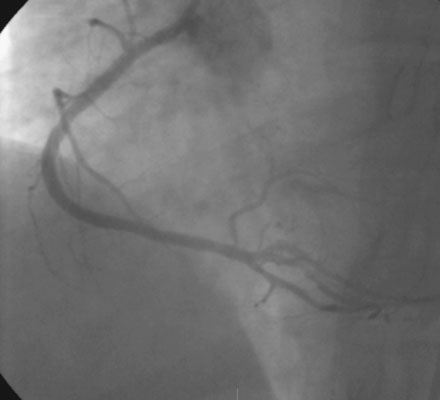
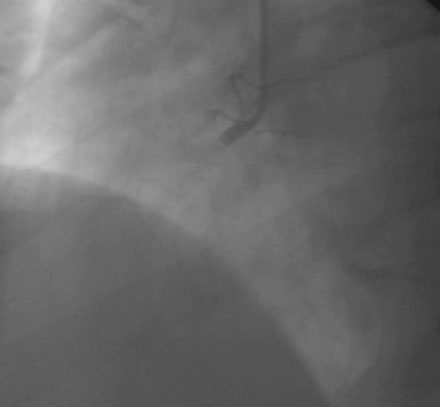
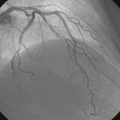
A severe stenosis present in the proximal right coronary artery explained his symptoms (Figure 24-2 and Videos 24-1, 24-2). Atherosclerotic disease evident in the left coronary artery did not appear to cause significant luminal obstruction (Figures 24-3, 24-4). The operator proceeded with percutaneous intervention of the right coronary artery using a bolus and infusion of bivalirudin as the procedural anticoagulant. The patient received 325 mg of aspirin prior to catheterization and was dosed with 600 mg of clopidogrel at the start of the percutaneous coronary intervention. Using first a 2.5 mm diameter by 20 mm long compliant balloon to predilate the lesion, the operator then deployed a 3.0 mm diameter by 23 mm long sirolimus-eluting stent (Figure 24-5). Not satisfied with the angiographic result, the operator postdilated the stent with a 3.0 mm diameter noncompliant balloon to 18 atmospheres (Figure 24-6). This resulted in a satisfactory angiographic result (Figure 24-7 and Videos 24-3, 24-4) and the patient left the cardiac catheterization laboratory without chest pain. An electrocardiogram obtained approximately 1 hour after the procedure showed no changes compared to baseline (Figure 24-8).
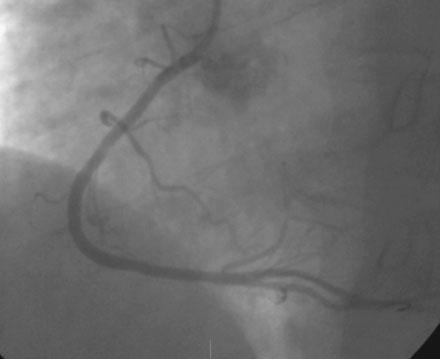
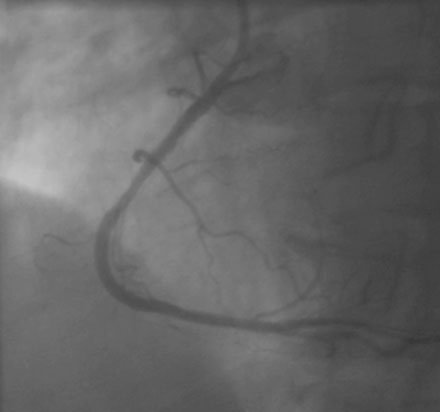
FIGURE 24-5 This angiogram was obtained following stent placement in the proximal portion of the right coronary artery.
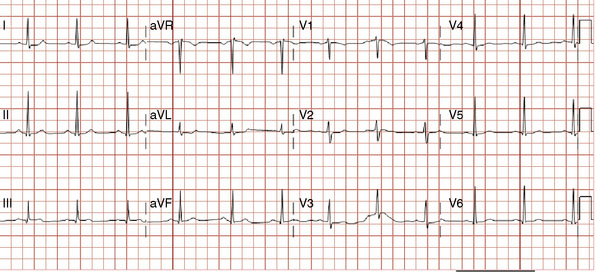
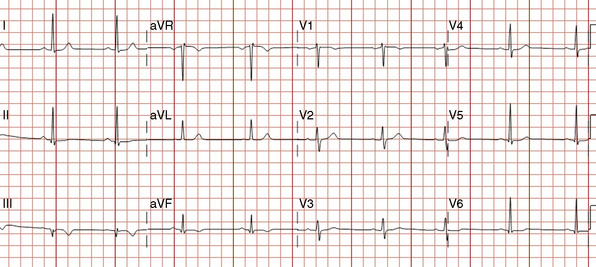
Over the course of the afternoon, the patient reported continual low-grade chest discomfort, but repeat electrocardiograms showed no changes. Although the pain was attributed to noncardiac causes, it continued throughout the afternoon and failed to respond to nitrates, antacids, or positional changes. Approximately 6 hours after the intervention, the chest pain suddenly became acutely worse and was associated with diaphoresis, nausea, and bradycardia. An electrocardiogram obtained at this point revealed normalization of the previously present inferior T-wave changes and new ST-segment elevation in leads III and aVF (Figure 24-9). Emergency coronary angiography confirmed occlusion of the right coronary stent (Figure 24-10 and Video 24-5).
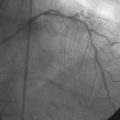
Balloon angioplasty using a 3.0 mm diameter by 15 mm long noncompliant balloon rapidly restored normal flow to the right coronary artery, relieving the patient’s chest pain and resolving the ST-segment elevation. Vasospasm observed distal to the stent (Video 24-6) resolved after administration of intracoronary nitroglycerin (Video 24-7). Because the operator thought there might be a small dissection at the proximal end of the earlier-placed stent, an additional stent (3.0 mm diameter by 8 mm long) was deployed. The final angiographic result is shown in Figure 24-11 and Videos 24-8 and 24-9.
Postprocedural course
Following the second procedure, he left the cardiac catheterization laboratory without chest pain and was observed in the coronary care unit. Serial troponin I assays confirmed a periprocedural infarction, peaking 12 hours after the second intervention at 33.23 ng/mL (normal <0.10 ng/mL); a 12-lead electrocardiogram obtained the next day demonstrated inferior Q waves (Figure 24-12). The remainder of his hospital stay was uneventful, and he was discharged free of symptoms on the third day postprocedure.
Discussion
This case provides an example of early stent thrombosis. In the current era, stent thrombosis is a rare but potentially catastrophic complication of stent implantation. Stent thrombosis is called “acute” when it occurs within 24 hours of stent placement and “subacute” when it happens 24 hours to 30 days after intervention.1 These two groups are often combined and described as “early” stent thrombosis. The term “late” stent thrombosis describes events happening 30 days to 1 year after intervention; stent thrombosis occurring after 1 year is called “very late”.1
Initial clinical experiences with coronary stents witnessed early stent thrombosis rates of around 10%. The incorporation of routine high-pressure balloon inflations during stent deployment along with the adjunctive use of dual antiplatelet therapy reduced the rate of early stent thrombosis to the current level of less than 1% and similar rates for bare-metal and drug-eluting stents.1–4
Patient factors relating to early stent thrombosis include the presence of an acute coronary syndrome, diabetes mellitus, chronic renal failure, and premature discontinuation of antiplatelet drugs.1 Procedural factors include bifurcation stenting, presence of residual dissection or thrombus, stent underexpansion, tissue protrusion, long lesion and stent length, small vessel or stent diameter, and smaller postprocedural luminal diameter.2,3,5 Intravascular ultrasound studies have shown that an underlying cause is present in 78% of events and no cause apparent in 22% of cases.5 The most common abnormality observed on intravascular ultrasound is underexpansion of the stent, resulting in a smaller minimal luminal area compared to patients without stent thrombosis. Additionally, residual dissection, thrombus, or tissue protrusion may be evident. Interestingly, incomplete stent apposition found on intravascular ultrasound is not related to stent thrombosis.6
The underlying cause of the stent thrombosis observed in this case cannot be readily discerned. Angiographically, the stent appeared well-deployed with no evidence of dissection, tissue prolapse, or thrombus. The operator performed high-pressure balloon inflation post-stenting. Intravascular ultrasound was not performed during the initial intervention or when the patient returned with stent thrombosis, which might have provided a clue to the cause. Inadequate adjunctive pharmacology may have played a role. Based on the REPLACE-2 trial, elective coronary intervention in low-risk lesions using bivalirudin and aspirin with a 300 mg clopidogrel load 2 to 12 hours prior to intervention is associated with similar ischemic endpoints as with unfractionated heparin plus glycoprotein IIb/IIIa inhibitors, but with a lower rate of bleeding.7 The optimal dose and time of clopidogrel load relative to the coronary intervention is not clearly defined. Maximum inhibition of platelet aggregation is achieved 2 hours after a load of 600 mg of clopidogrel, and the ISAR-REACT trial enrolling low-risk and intermediate-risk patients undergoing elective intervention who received 600 mg of clopidogrel at least 2 hours prior to stenting found no benefit from the platelet glycoprotein IIb/IIIa inhibitor abciximab suggesting that this dose and timing provides adequate levels of platelet inhibition to safely allow elective stenting in the absence of high-risk features.8 In the present case, clopidogrel was loaded just prior to the intervention, and it is interesting to note that stent thrombosis occurred 6 hours after intervention when the dose of clopidogrel should have already achieved optimal platelet inhibition. Thrombosis in this case may have been caused by aspirin or clopidogrel resistance, estimated to be present in as high as 10% to 15% of patients undergoing coronary intervention.
Stent thrombosis typically manifests as an ST-segment elevation acute myocardial infarction. These are very serious events associated with death in 10% to 15% of cases at 30 days and myocardial infarction in about 60%.2,3 In the present case, although the problem was identified and treated promptly, the patient suffered a sizeable event and developed new Q waves on his electrocardiogram. Obviously, thrombosis of a stent placed in a large proximal artery and stent thrombosis occurring after hospital discharge, resulting in delays in recognition and management, may have devastating and fatal consequences.
1 Lemesle G., Delhaye C., Bonello L., de Labriolle A., Waksman R., Pichard A. Stent thrombosis in 2008: Definition, predictors, prognosis and treatment. Arch Cardiovasc Dis. 2008;101:769-777.
2 Cutlip D.E., Baim D.S., Ho K.K.L., Popma J.J., Lansky A.J., Cohen D.J., Carozza J.P., Chauhan M.S., Rodriguez O., Kuntz R.E. Stent thrombosis in the modern era: A pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103:1967-1971.
3 Ong A.T.L., Hoye A., Aoki J., van Mieghem C.A.G., Granillo G.A.R., Sonnenschein K., Regar E., McFadden E.P., Sianos G., van der Giessen W.J., de Jaegere P.P.T., de Feyter P., van Domburg R.T., Serruys P.W. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005;45:947-953.
4 Daemen J., Wenaweser P., Tsuchida K., Abrecht L., Vaina S., Morger C., Kukreja N., Juni P., Sianos G., Hellige G., van Domburg R.T., Hess O.M., Boersma E., Meier B., Windecker S., Serruys P.W. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: Data from a large two-institutional cohort study. Lancet. 2007;369:667-678.
5 Cheneau E., Leborge L., Mintz G.S., Kotani J., Pichard A.D., Satler L.F., Canos D., Castagna M., Weissman N.J., Waksman R. Predictors of subacute stent thrombosis: Results of a systematic intravascular ultrasound study. Circulation. 2003;108:43-47.
6 Tanabe K., Serruys P.W., Degertekin M., et alTAXUS II Study Group. Incomplete stent apposition after implantation of paclitaxel-eluting stents or bare-metal stents: Insights from the randomized TAXUS II trial. For the Circulation 2005;111:900-905
7 Lincoff A.M., Bittl J.A., Harrington R.A., et alREPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention. REPLACE-2 randomized trial. For the JAMA 2003;289:853-863
8 Kastrati A., Mehilli J., Schuhlen H., et alIntracoronary Stenting and Antithrombotic Regimen-Rapid Early Action for Coronary Treatment (ISAR-REACT) Study Investigators. A clinical trial of abciximab in elective percutaneous coronary intervention after pretreatment with clopidogrel. For the N Engl J Med 2004;350:232-238