CHAPTER 23
Reproductive function in the male
CHAPTER OUTLINE
Production and actions of testosterone
Hypothalamo–pituitary control of testicular function
Endocrine disrupting chemicals
EVALUATION OF TESTICULAR FUNCTION
Endocrine evaluation: hypothalamo–pituitary–gonadal axis
Defective hormone synthesis and hormone receptor defects
INTRODUCTION
Normal male reproductive function – the manufacturing of semen, the ability to create and sustain penile erection and to ejaculate – is dependent on the physiological integration of the hypothalamo–pituitary–testicular axis. Production of androgens, normal responses in the tissues on which these hormones act and intact neurological pathways required for ejaculation are crucial for full operation of this system, to make human reproduction possible.
THE TESTES
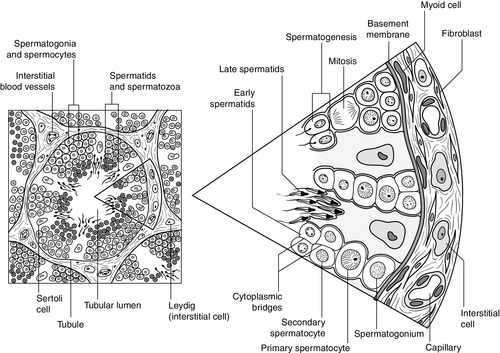
The testes have two distinct roles: androgen production and spermatogenesis. These are achieved by two main units – the interstitial cells and the seminiferous tubules – under the control of the hypothalamo–pituitary axis.
Production and actions of testosterone
Testosterone is the major androgen produced in the interstitial cells of the testes. Also known as Leydig cells, these are found in clumps between the seminiferous tubules, and their mass correlates positively with androgen production. Sertoli cells, along with germ cells, form the seminiferous tubules (Fig. 23.1) and secrete hormones that control embryological sexual differentiation. In addition, they support spermatogenesis by regulating the composition of the seminiferous tubular fluid, thereby providing an environment for meiotic germ cell development.
At week eight of gestation, a human fetus has both Müllerian and Wolffian ducts, with the potential to develop into female and male genital tracts, respectively, and differing only by its sex chromosomes. The Y chromosome is a powerful sex determinant – it confers maleness to the extent that even XXXXY fetuses are phenotypically male at birth. (This topic is covered in more detail in Chapter 21.)
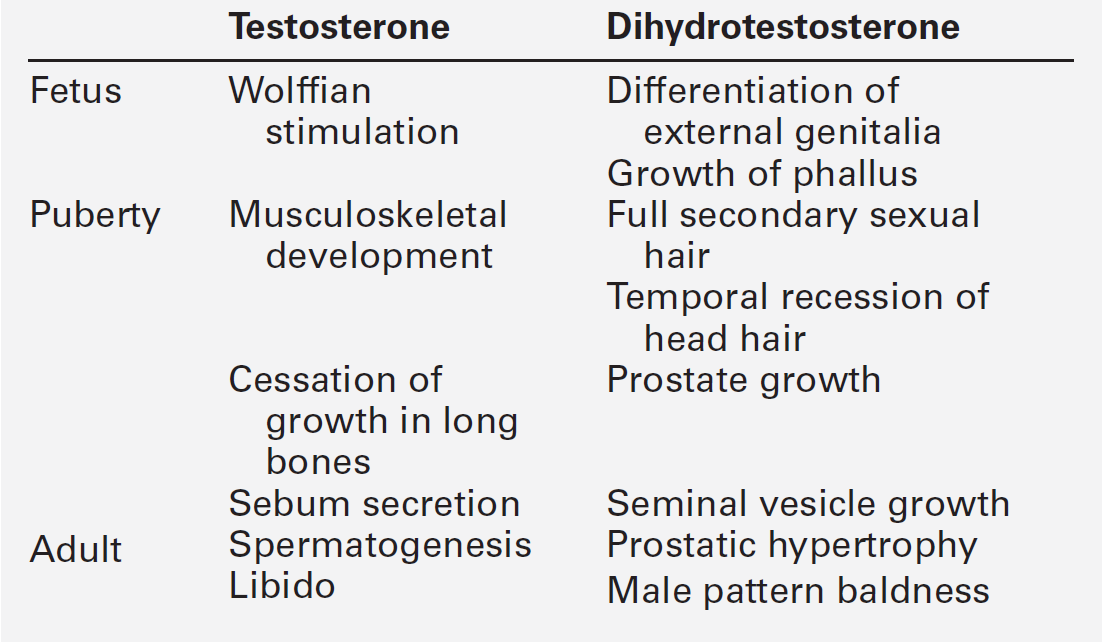
Normal male sexual differentiation in utero depends on the presence of testosterone and other hormones produced by these embryological gonads. Leydig cells are stimulated by placental human chorionic gonadotrophin (hCG) to secrete testosterone from the ninth week of gestation, leading to development of the Wolffian ducts. Sertoli cells secrete anti-Müllerian hormone (AMH), which causes regression of the Müllerian ducts and oogonia (the precursors of primary oocytes). In the absence of AMH and testosterone, the Müllerian ducts differentiate into female internal genitalia. Lower concentrations of testosterone are maintained during late gestation by secretion of luteinizing hormone (LH) from the fetal pituitary. The presence of 5α-reductase, and the subsequent production of dihydrotestosterone (DHT) from testosterone, is required for development of the urogenital sinus. Testosterone secretion may continue for many months post partum before declining until the onset of puberty, when it will again be essential for sexual development.
Testosterone secretion from Leydig cells follows stimulation by LH, which binds to specific membrane receptors. Luteinizing hormone activates adenylyl cyclase, resulting in formation of cAMP, which binds to protein kinase and in turn activates the enzyme 20,22-desmolase, which is responsible for conversion of cholesterol to pregnenolone (see Fig. 22.5, p. 438). The synthesis of testosterone is completed by four other enzymes (3β-hydroxysteroid dehydrogenase, 17α-hydroxylase, 17,20-desmolase and 17β-hydroxysteroid dehydrogenase). At the target organ, testosterone is transported to the nucleus, and gene activation results in the formation of specific messenger RNAs and proteins. Testosterone is usually converted to the active metabolite DHT by the enzyme 5α-reductase. In the adult, there is circadian variation in testosterone secretion with a peak in the early morning and a trough at about 18.00 h, and therefore blood samples for testosterone measurement should ideally be taken at 09.00 h. The biological actions of testosterone and DHT are summarized in Table 23.1.
Hypothalamo–pituitary control of testicular function
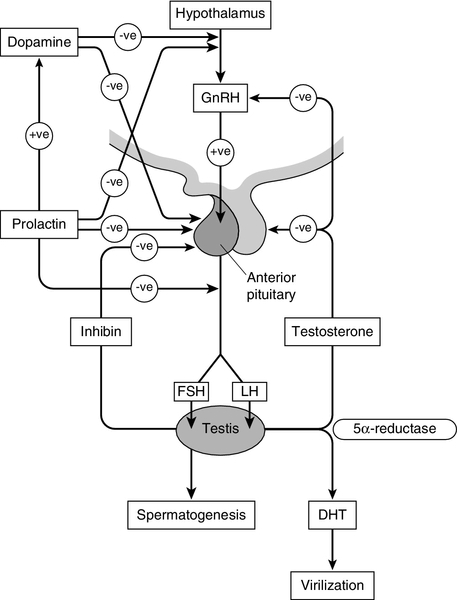
Gonadotrophin releasing hormone (GnRH) is secreted from the hypothalamus in a pulsatile fashion, with peaks occurring approximately every 90 min. It regulates the release of LH, which is crucial for the regulation of Leydig cell number and function, and hence production of testosterone, and follicle stimulating hormone (FSH), which stimulates Sertoli cell division and growth and is therefore responsible for testicular enlargement at puberty. Faster pulse frequencies favour LH release and slower ones, FSH. The pulsatility of GnRH secretion is important in the regulation of gonadotrophin secretion, as continuous exposure results in down-regulation of the GnRH receptor. Luteinizing hormone secretion is also highly pulsatile, which is reflected in measured plasma concentrations. Plasma concentrations of FSH are less variable, presumably as a result of slower metabolic clearance.
The gonadotrophins are, in turn, regulated by a series of feedback mechanisms (Fig. 23.2). Testosterone inhibits LH and, to a lesser extent, FSH secretion from the anterior pituitary. Oestradiol, which is formed in the male through metabolism of androgens by aromatase found in adipose tissue, skin, kidneys and brain, in addition to Leydig cells, also inhibits gonadotrophin secretion by the pituitary. Opioids and prolactin reduce the pulsatile activity of GnRH and thus decrease FSH and LH secretion; prolactin also inhibits the direct action of gonadotrophins on the testes.
Follicle stimulating hormone is also regulated by inhibin B, which is produced by Sertoli cells and inhibits FSH production to regulate spermatogenesis. Conversely, activin works to enhance FSH action in the gonads, increasing spermatogenesis. Both are secreted in the seminal fluid and are thought to work locally in addition to centrally on the pituitary. Inhibin B can be used as a marker for male factor infertility as concentrations directly reflect the function of the Sertoli cells: plasma inhibin B concentrations are significantly higher in fertile men than in infertile men.
Gonadotrophin secretion is affected by a diverse range of regulators, including stress and nutritional status. Recent studies have shown that low concentrations of leptin, an adipose-derived hormone, or decreased sensitivity to leptin, such as occurs in obese individuals, are associated with decreased gonadotrophin concentrations and decreased fertility. This is mediated via hypothalamic kisspeptin neurons rather than directly via GnRH neurons. Kisspeptin is a neurohormone thought to have a key role in puberty, although this is yet to be clearly defined. Patients with mutations in the kisspeptin gene fail to go through puberty owing to hypogonadotropic hypogonadism. In animal models, treatment with kisspeptin can restore GnRH pulsatility and gonadotrophin secretion.
Testicular malignancy
Testicular cancer is the commonest malignancy of men aged 20–39. Histologically, it is commonly a germ cell malignancy – either a seminoma or, more often, a teratoma. Patients are likely to present with a testicular mass, and as this cancer is relatively easily detectable with regular self-inspection/examination, and has a high cure rate if detected prior to metastatic spread, there have been several public campaigns to raise awareness amongst young men.
Approximately 80% of testicular teratomas produce hCG or α-fetoprotein (AFP) (see Chapter 42). Human chorionic gonadotrophin is very similar to LH in structure. It stimulates the LH receptor in the testis eventually resulting in increased secretion of oestradiol, and hence gynaecomastia. Some rarer testicular malignancies, such as Sertoli cell tumours, can also secrete oestradiol directly. Human chorionic gonadotrophin can interact with the thyroid hormone axis as an agonist of the thyroid stimulating hormone (TSH) receptor, to cause hyperthyroidism.
Endocrine disrupting chemicals
Endocrine disruptors are environmental substances that interfere with normal hormonal systems. They are very diverse and can be plastics, chemicals, pesticides and fungicides. They are often able to interact with steroid hormone receptors and can act as androgens and anti-androgens. Exposure may be occupational, but also through water supply, soil and food. There may be a lag between exposure and clinical presentation and there may be trans-generational effects if mutations or modifications of gene expression occur. The developmental stage at which a person is exposed may be crucial. The observations of a declining sperm count, and increasing rates of testicular cancer throughout the last century prompted researchers to ask if these epidemiological trends could be explained by endocrine disruptors. In animal models, exposure to phthalates is significantly related to oligospermia, and epidemiological evidence suggests this may be the case for humans. Endocrine disruptors have also been linked to cryptorchidism, hypospadias, early or delayed puberty and prostatic hyperplasia. More detail on this topic is beyond the scope of this chapter, but the interested reader will find more material in the further reading section, below.
EVALUATION OF TESTICULAR FUNCTION
The laboratory evaluation of testicular function may be conveniently divided into assessment of spermatogenesis and assessment of endocrine gonadal function.
Semen analysis
Semen analysis is used both in the evaluation of male fertility and the follow-up of treatment regimens for male subfertility. It must be noted that there is wide inter-laboratory variation in the results, although this has been reduced in recent years by automated systems using electro-optics or computer-assisted analysis. Furthermore, there is marked variation in sperm output on a day-to-day basis. It is often more useful in clinical practice to assess the actual fertilization capacity of sperm in vitro.
A semen sample is collected by masturbation and should ideally be analysed within 1 h. Basic semen analysis measures the number of spermatozoa (per unit volume and per ejaculate), motility and morphology. These are assessed according to World Health Organization (WHO) criteria, updated in 2010, although it should be noted that individual laboratories will also have developed their own reference ranges. The WHO reference ranges were determined by the analysis of semen from fertile men whose partners became pregnant within one year of trying to conceive. Average sperm count is > 60 million/mL and a count of < 15 million/mL is considered subfertile. There should be > 39 million sperm in a given ejaculate and semen volume should be 1.5 mL or greater.
Motility is defined as the percentage of moving sperm and is subdivided into the following categories:
• non-progressive motility (NP): all other patterns of motility with an absence of progression
• immotility (IM): no movement.
The WHO reference values state that ≥ 40% of the sperm should exhibit either PR or NP motility and ≥ 32% should exhibit PR motility. At least 58% of sperm should be alive – this is especially important for samples with a PR motility of < 40% and must be assessed within 1 h of ejaculation.
Morphology, describing defects in the appearance of spermatozoa, is most commonly assessed according to the WHO morphology standards. The WHO lower reference limit for normal morphology is 4%. Few samples exhibit > 25% normal spermatozoa.
Other parameters measured include volume, pH, white blood cell count, antisperm antibodies, colour, viscosity, agglutination, aggregation and time until liquefaction. Reference ranges for semen analysis are shown in Table 23.2.
TABLE 23.2
Reference ranges for semen variablesa
| Variable | Reference range |
| Volume | > 1.5 mL |
| pH | > 7.2 |
| Sperm concentration | > 15 × 106 sperm/mL |
| Total count | > 39 × 106 sperm/ejaculate |
| Motility | 40% or more with total motility (PR + NP) |
| 32% or more with PR motility | |
| Vitality | 58% or more alive |
| White blood cells | < 1 × 106 cells/ejaculate |
| Fructose | > 13 μmol/ejaculate |
a WHO classification described in text. PR, progressive motility; NP, non-progressive motility.
Endocrine evaluation: hypothalamo–pituitary–gonadal axis
Determination of basal plasma LH and FSH concentrations is a routine part of the assessment of the hypothalamo–pituitary–gonadal axis. As LH and, to a lesser extent, FSH are secreted in a pulsatile fashion, more accurate interpretation may be possible if serial samples are taken over a period of time. However, in routine clinical practice, the measurement of basal plasma concentrations of LH and FSH with testosterone provides most of the information required.
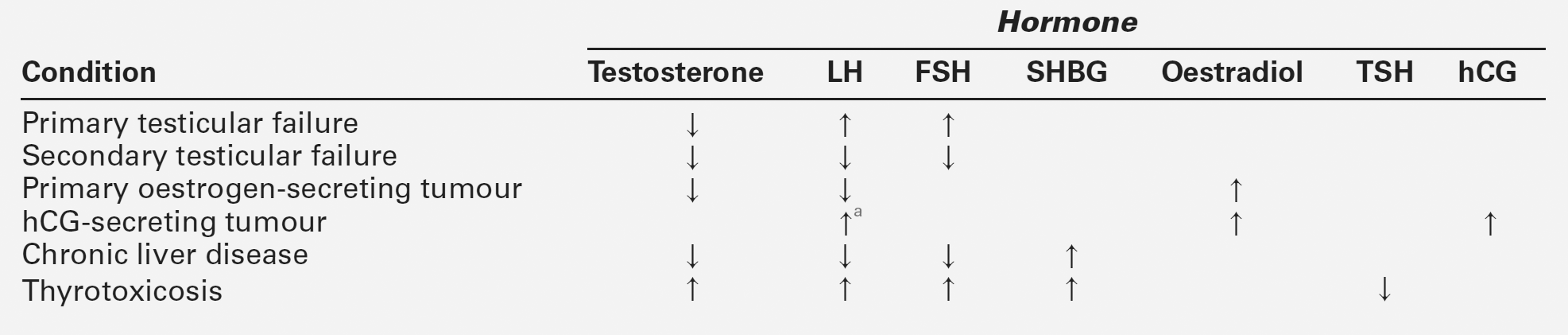
Elevated LH and FSH in the presence of low testosterone concentrations suggest primary testicular failure; low or normal LH and FSH associated with low testosterone concentrations suggest a hypothalamo–pituitary disorder. It is important to recognize that the latter may be caused by any acute illness. Rarely, LH alone may be elevated with a low testosterone (the Sertoli-only syndrome) and, in patients with abnormal spermatogenesis, FSH may be raised with a normal testosterone. It should be remembered that pituitary and gonadal dysfunction may coexist, as in haemochromatosis. In this situation, LH, FSH and testosterone concentrations are low.
The GnRH test (Appendix 23.1i) has been used to assess pituitary reserve of LH and FSH and overall hypothalamo–pituitary function. This test is now used relatively infrequently, as it rarely provides extra information beyond that from basal LH and FSH concentrations.
The clomifene stimulation test (Appendix 23.1ii) can be used to assess the hypothalamo–pituitary axis with respect to gonadotrophin secretion. Clomifene competitively inhibits hypothalamo–pituitary androgen binding. The normal response is a doubling of LH and FSH by day 10 after administration. A low response, however, does not distinguish between abnormalities at the pituitary or hypothalamic level.
Plasma testosterone concentrations exhibit diurnal variation throughout the day – some studies suggesting a variation of as much as 30% between a sample taken at 08.00 h and one at 16.00 h. Consequently, and by convention, testosterone is usually determined on a sample taken before 09.00 h. Analysis of serum testosterone concentrations must take into account sex hormone-binding globulin (SHBG), as 50% of circulating testosterone is bound to SHBG. Low testosterone concentrations are seen both in primary testicular disease, indicating poor Leydig cell function, and hypothalamo–pituitary disorders with depressed gonadotrophin secretion. Elevated concentrations are seen in patients with gonadotrophin- or androgen-secreting tumours (e.g. Leydig cell tumours), in whom gonadotrophin concentrations will be low.
The hCG stimulation test (Appendix 23.1iii) can be helpful in differentiating primary testicular failure from gonadotrophin deficiency. Failure of testosterone to rise after administration of hCG suggests inadequate Leydig cell function. An exaggerated response suggests secondary hypogonadism. The test is also useful in confirming the presence of testicular tissue in cryptorchidism and in conditions in which there may be combined pituitary and testicular dysfunction, for example as a result of iron deposition in haemochromatosis or treated thalassaemia.
MALE HYPOGONADISM
Male hypogonadism may result from either primary testicular failure, in which the testes fail to develop properly or are injured by disease or medical manipulation, or secondary testicular failure owing to hypothalamic or pituitary lesions (hypogonadotrophic hypogonadism). The latter may result from developmental or acquired pituitary disease or a failure of hypothalamic GnRH secretion. Other syndromes resulting in hypogonadism include enzyme defects in which hormonal synthesis is interrupted despite an intact hypothalamo–pituitary–testicular axis.
Clinical features
The physiological effects of testosterone are listed in Table 23.1. Prepubertal testicular failure results in lack of sexual maturation with persistent infantile genitalia and absence of pubic and axillary hair growth. Delayed epiphyseal closure leads to the development of a eunuchoid habitus with increased arm span (> 5 cm greater than height) and increased lower body height (sole-to-pubic symphysis length > 5 cm greater than pubic symphysis-to-head length). The reduction in testosterone is also associated with diminished anabolism and a decrease in normal male muscular development.
Postpubertal onset of androgen deficiency is less obvious and usually presents with decreased libido, impotence and infertility. There may be an associated decrease in growth of facial, pubic and axillary hair and a diminution in skeletal musculature. Gynaecomastia may occur as a result of an increase in the oestradiol:testosterone ratio (see below). Despite some reduction in testicular volume, the penis and prostate do not shrink and erections and orgasms may continue. Nevertheless, spermatogenesis requires testosterone and, even when able to ejaculate, severely hypogonadal males are azoospermic.
Primary hypogonadism
This implies primary testicular failure and is characterized by low plasma testosterone and elevated LH and FSH concentrations. The chief causes can be divided into genetic abnormalities and cryptorchidism. Other causes are orchitis, chronic illness, drugs (including excess alcohol), chemo- and radiotherapy and, rarely, testicular trauma.
Genetic causes
In the male, the most common of these is Klinefelter syndrome, thought to occur in 1 in 500 live births. This was described in 1942 by Klinefelter, Reifenstein and Albright, and comprises eunuchoid stature, gynaecomastia, small testes and infertility. It is caused by non-disjunction of the sex chromosomes during meiosis, the most common resulting abnormality being 46XXY but occasionally other X states or mosaicism (e.g. 48XXXY/46XY) are seen. All these cause Leydig cell abnormalities, resulting in impaired testosterone secretion and seminiferous tubular function, and decreased inhibin secretion. Other genetic causes of primary hypogonadism include XX males, Noonan syndrome, XY/XO gonadal dysgenesis and deletion of the Y chromosome in part or entirety.
Cryptorchidism
In 1–2% of male neonates, the testes fail to descend bilaterally from the abdominal cavity and do not produce adequate concentrations of testosterone. In addition, there is an increased risk of testicular malignancy. Surgery to position the testes in the scrotum, particularly if carried out in early childhood, reduces the risk of later malignancy, and facilitates surveillance. For this reason, it is important to distinguish this condition from anorchia, complete absence of testes in a 46XY male. The presence or absence of gonadal tissue can be assessed using ultrasound or magnetic resonance imaging scanning. If functional testicular tissue is present, there will be an increase in testosterone during an hCG stimulation test.
Secondary hypogonadism
This is hypogonadism caused by hypothalamic or pituitary dysfunction, resulting in deficient gonadotrophin secretion and leading to a low testosterone concentration. The causes may be divided into congenital and acquired disorders.
Congenital causes
The most common of these is Kallmann syndrome. Mutations or deletions of the KAL1 gene, which encodes anosmin-1, result in the X-linked form, leading to an abnormal migration of GnRH-producing neurons to the hypothalamus and failure of episodic GnRH secretion. The syndrome manifests as scant secondary sexual development and eunuchoid habitus in association with anosmia and, occasionally, other craniofacial abnormalities such as cleft lip and palate.
Congenital adrenal hypoplasia presents with primary adrenal failure in association with inadequate GnRH secretion. It is caused by a mutation of the DAX-1 gene on the X chromosome. The fertile eunuch syndrome is a selective LH deficiency leading to partial virilization with normal Sertoli cell function. This can be treated with testosterone replacement or hCG.
Homozygous mutations of the leptin gene and receptor have been found to be associated with idiopathic hypogonadotrophic hypogonadism (IHH) and severe obesity as a result of impaired GnRH release. Idiopathic hypogonadotrophic hypogonadism has also been associated with mutations of the FSH β-subunit, LH β-subunit, PC1 (prohormone convertase 1) and GPR54 (part of the kisspeptin pathway) genes. Other rare congenital causes of hypogonadotrophic hypogonadism include Prader–Willi syndrome and Laurence–Moon–Biedl syndrome. Haemochromatosis is another inherited condition sometimes associated with the development of secondary hypogonadotrophic hypogonadism, although the presentation of this complication is usually rather later in life.
Acquired causes
There are a number of acquired conditions leading to reduced gonadotrophin secretion (Box 23.1). These include excessive exercise or weight loss, physical or psychological stress and systemic illness. Direct damage to the hypothalamus or pituitary, such as from head trauma, radiotherapy, surgery, pituitary tumours and infiltrative disorders (e.g. sarcoidosis) can also be a cause, in addition to prescribed medications causing hyperprolactinaemia and illicit drugs such as cocaine, anabolic steroids and opioids.
Defective hormone synthesis and hormone receptor defects
These conditions lead to ambiguous external genitalia with a 46XY genotype. There are rudimentary or absent Wolffian duct derivatives (seminal vesicles and ductus deferens), but because of the presence of AMH, the uterus and fallopian tubes do not develop either. In addition to this there is usually cryptorchidism.
Five enzymes are responsible for the conversion of cholesterol to testosterone; defects in each of these enzymes have been reported (see Chapter 21). Plasma testosterone concentrations are low and hCG stimulation results in elevated concentrations of precursors proximal to the enzyme block. Defects in androgen receptor proteins can also lead to hypogonadism, but with normal testosterone concentrations.
5α-Reductase deficiency
5α-Reductase converts testosterone to dihydrotestosterone (DHT). Dihydrotestosterone is responsible for masculinization, and normal plasma concentrations are a prerequisite for the full development of the phallus, scrotum and prostate. In 5α-reductase deficiency, external genitalia at birth are ambiguous with a urogenital sinus, clitoromegaly, bifid scrotum, blind vaginal pouch and inguinal or labial testes. Wolffian duct derivatives are present, as they develop under the influence of testosterone rather than DHT. The deficiency is caused by a mutation in the 5α-reductase type 2 gene, which is inherited in an autosomal recessive fashion.
At puberty, patients undergo virilization with some phallic enlargement, development of male habitus and psychosexual orientation and descent of the testes, occasionally with spermatogenesis. This occurs either as a result of a massive increase in testosterone secretion at puberty or by conversion of some testosterone to DHT if the enzyme deficiency is incomplete. Patients may respond to very high doses of testosterone or to DHT (the latter can be applied locally as a 2% cream with some beneficial effects on penile length). If peripubertal virilization is minimal, patients may be raised as infertile females after removal of the cryptorchid testes.
Androgen insensitivity syndromes
These are caused by absence or deficiency of the DHT receptor protein. The complete form results in an apparently normal female phenotype with absent pubic and axillary hair and is transmitted as an X-linked recessive trait. Administration of testosterone has no effect on the generation of secondary sexual hair. As patients usually have normal female psychosexual orientation, treatment involves removal of cryptorchid testes and subsequent oestrogen replacement. Normal female sexual activity is possible, although, of course, the patients remain infertile.
In partial androgen insensitivity syndrome, presentation varies according to the degree of DHT receptor deficiency. It ranges from apparent clitoromegaly in a phenotypically female neonate at birth, to infertility in an apparently normal adult male. Diagnosis is confirmed by identification of the androgen receptor gene mutation. The management of these patients depends on age at diagnosis and the response to treatment with exogenous testosterone.
Treatment of hypogonadism
Testosterone replacement remains the mainstay of treatment. Traditionally, this has been administered by implantation of subcutaneous pellets or by long-acting mixtures of testosterone esters (e.g. Sustanon®). The latter are given by deep intramuscular injection every 2–4 weeks. More recently, patches have been used, although there have been problems with varying degrees of absorption resulting in unpredictable plasma testosterone concentrations, and also problems with adhesion and hypersensitivity at the site of application. Gel and buccal mucoadhesive preparations are now available and are widely used. These are more convenient and result in much more even plasma concentrations, although a disadvantage of gel preparations is inadvertent transfer of gel to women or children. Oral preparations have been used but are poorly absorbed. As the prostate and seminal vesicles are androgen sensitive, there is a risk of stimulating growth of a prostatic carcinoma and therefore plasma prostate specific antigen concentrations should be monitored. (Treatment regimens are discussed in more detail in Chapter 21.)
Testosterone replacement will not achieve fertility in primary testicular failure. Only in secondary hypogonadism can infertility be treated. Treatment in hypogonadotrophic hypogonadism is with intramuscular hCG with the addition of human menopausal gonadotrophin to supply FSH activity as necessary. This will usually induce a good quality ejaculate, which is potentially capable of resulting in conception despite a relatively low sperm count (1–20 million/mL). An alternative treatment is with small pulses of GnRH delivered by a programmed pump, mimicking physiological GnRH pulsatility. These are not long-term treatment options, partly because hCG, whether endogenous or exogenous, causes gynaecomastia. When the need for fertility is over, patients should resume treatment with testosterone.
Fertility can also be achieved using assisted reproduction techniques; in male infertility these include intrauterine insemination and intracytoplasmic sperm injection (ICSI). In ICSI, spermatozoa are injected directly into an oocyte and the fertilized embryo is then inserted into the uterus. ICSI can be appropriate where there is a very low sperm count, poor morphology or motility of sperm. It is also useful where there is an interruption in the pathway from testes to urethra such as occurs post-vasectomy or with an absent vas deferens in patients with cystic fibrosis. Abnormal sperm morphology does not appear to have a negative effect on blastocyst formation and birth defect rates are not significantly different from those conceived using conventional in vitro fertilization. However, as there is some evidence of an increased number of chromosomal abnormalities in children conceived using ICSI, concerns remain regarding health and fertility later in life.
Plasma testosterone concentrations gradually decline with age after the third decade, as a result of a number of factors including decreased GnRH release, increased sensitivity to the negative feedback of testosterone and reduced responsiveness of Leydig cells. Symptoms associated with testosterone deficiency, such as reduced exercise tolerance and increased body fat, overlap with changes linked to the natural process of ageing and hence there is an unavoidable implication that testosterone replacement in the elderly will be beneficial. Positive effects of replacement include increased bone density, increased lean body mass, improvement of lipid profile and stimulation of red blood cell production. There is a concern that such replacement may be associated with an increased risk of prostatic carcinoma, but this has not been proven.
Although testosterone replacement may be indicated in elderly patients with clear testosterone deficiency and no contraindications to treatment, the approach to marginally low concentrations is controversial. Initiation of treatment should always be preceded by evaluation to rule out pre-existing contraindications, for example prostatic carcinoma or polycythaemia, and followed by regular monitoring.
GYNAECOMASTIA
Excessive development of the male mammary glands with increases in both stromal and glandular tissue (gynaecomastia) accounts for ~ 70% of male breast disorders. In the male, breast development may vary from a small subareolar button of tissue to florid breast development with feminization of the nipples and associated breast tenderness.
Gynaecomastia may occur in neonatal life, when it is usually transient and caused by transplacental transfer of maternal oestrogens into the fetal bloodstream. During puberty, gynaecomastia of some degree occurs in up to 60% of adolescent boys: this may reflect normal physiological development and is typically followed by spontaneous regression after 12–18 months.
Causes of gynaecomastia
Physiological gynaecomastia during puberty probably occurs as a result of the relative changes in oestradiol and testosterone concentrations – in normal early puberty, oestradiol concentrations are high in comparison with those of testosterone and this increased oestradiol:testosterone ratio can induce breast development.
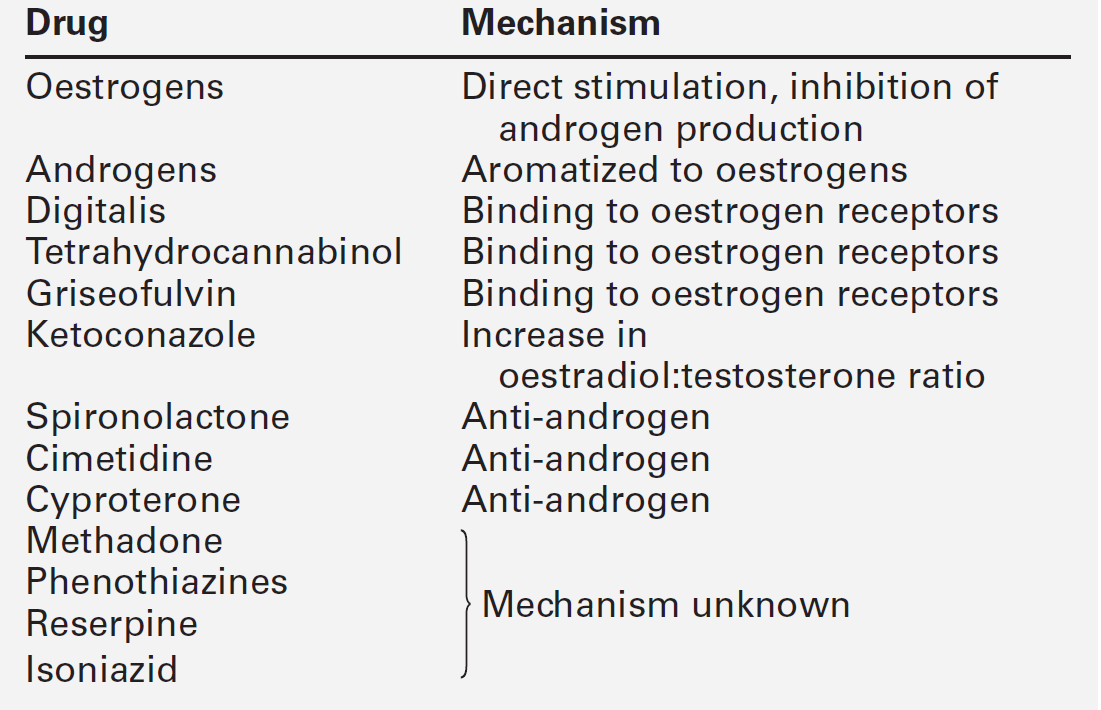
Indeed, pathological causes are invariably related to imbalances between testosterone and oestrogen synthesis and action, and can be broadly divided into hypogonadism, other endocrine disorders, tumour related, systemic disease (Box 23.2) and drugs (Table 23.3).
Investigation
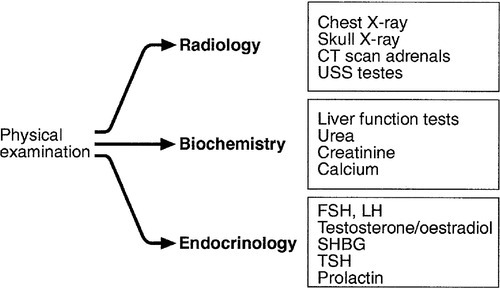
A full drug history and a careful physical examination are essential, with particular reference to the testes, thyroid, liver and lungs.
A suggested schedule for the investigation of symptomatic gynaecomastia is illustrated in Figure 23.3 and the interpretation of endocrine tests in Table 23.4.
IMPOTENCE
Erectile impotence is defined as the inability to achieve and maintain a penile erection of sufficient quality to permit penetrative intercourse. In practice, the commonest physical cause of impotence is diabetes mellitus – some 50% of diabetic males will become impotent after the age of 50. Both autonomic neuropathy and vascular insufficiency may contribute to this. Other endocrine causes include hypogonadism (primary or secondary), hyperprolactinaemia and thyroid disease. Other causes are summarized in Box 23.3.
A psychogenic aetiology is suggested by a history of early morning or sleep erections or full erection on masturbation or with other partners. Treatment with certain drugs can result in impotence – these are summarized in Box 23.4.
Investigation
The initial history and examination is of great importance. Reduced frequency of shaving, reduced libido, small testes and regression or lack of secondary sexual characteristics suggests hypogonadism. Testicular tumours may be palpable and visual field assessment and imaging of the brain and skull should be carried out if there is a possibility of pituitary tumour. A full neurological and cardiovascular examination is essential. The relevant basic biochemical investigations are listed in Box 23.5.
Previously, treatment options were invasive and hence thorough justification by extensive investigation was necessary. Following a dramatic improvement in the treatment options in the last two decades, owing mainly to the availability of phosphodiesterase (PDE)-5 inhibitors, more extensive specialist evaluation of vascular and neurological causes (Box 23.5) is now reserved for a limited subset of patients.
For example, penile arterial blood pressure may be investigated. Penile systolic blood pressure is measured with a Doppler stethoscope and recorded as a ratio to that of the brachial artery. Low penile:brachial ratios (< 0.6) suggest an arterial cause for impotence. Continuous arterial blood pressure recordings can be achieved using a small cuff around the base of the penis attached via a transducer to a recorder. If the arterial supply is impaired, the recording will show small amplitude pulses with abnormal waveforms.
Normal men experience nocturnal erections during rapid eye movement (REM) sleep and their absence suggests an organic cause for impotence. Nocturnal tumescence testing is ideally carried out in a sleep laboratory and should be repeated on three or more occasions to maximize diagnostic accuracy. Other techniques include pudendal arteriography and duplex ultrasonography, but a detailed discussion of these is beyond the scope of this book.
Techniques for the investigation of neurological causes include biothesiometry, which is a simple test of afferent sensory function. An electromagnetic test probe is placed on the penile skin in various sites and the intensity of vibration increased until it is perceived by the patient. The degree of vibration required for perception can be compared with other sites on the patient’s body.
Treatment of erectile impotence
Treatment options for erectile impotence include oral therapy in the form of PDE-5 inhibitors (sildenafil, vardenafil, and tadalafil), intraurethral alprostadil, intracavernosal injection of vasodilators, vacuum devices and surgery (bypass and implants).
The advent of the PDE-5 inhibitors has greatly simplified the treatment of impotence. The available agents have similar efficacy, with sildenafil and vardenafil possessing similar pharmacokinetic profiles and tadalafil exhibiting a longer duration of action. Phosphodiesterase-5 inhibitors should be avoided in patients using, or likely to use, nitrates because of potentiation of their hypotensive effect.
Intracavernosal injection of vasodilatory agents such as alprostadil and papaverine, and intraurethral alprostadil are useful second-line treatment options, local penile pain being the limiting side-effect of the latter. Self-injection can result in successful erections in patients without a vascular cause of impotence. The injection method needs to be adequately taught and should not be used more than once a week to reduce the risk of potential side-effects, such as corporeal fibrosis and priapism.
Variable results have been seen with vacuum devices, which act by increasing corporeal blood flow and are used in conjunction with a penile ring.
Penile prostheses, either semi-rigid or malleable rods inserted into the corpora, or self-inflatable prosthetic devices have been used with varying degrees of success. Mechanical failure, the need for manual inflation from an internal fluid reservoir and the length of waiting lists for the procedure in the UK have resulted in diminished use of this treatment option.
Further reading
American Association of Clinical Endocrinologists. Medical guidelines for the evaluation and treatment of male sexual dysfunction: a couple’s problem – 2003 update. Endocr Pract. 2003. ;9:77–95. https://www.aace.com/pub/pdf/guidelines/sexdysguid.pdf [Accessed October 2013].
Well-structured approach to male sexual dysfunction.
World Health Organization. Laboratory manual for the examination of human semen and semen-cervical mucus interaction. 5th ed. 2010. http://www.who.int/reproductivehealth/publications/infertility/9789241547789 [Accessed October 2013].
A useful volume that attempts to standardize tests and investigation in common use throughout the world.
APPENDIX 23.1 PROTOCOLS FOR ENDOCRINE INVESTIGATIONS
(i) Gonadotrophin releasing hormone stimulation test
In the investigation of gonadotrophin deficiency, assessment of the responses to exogenous GnRH may be useful. There is no need for the patient to fast unless the test is being combined with assessment of other anterior pituitary hormone responses to insulin-induced hypoglycaemia.
Gonadotrophin releasing hormone (100 μg) is given intravenously at time 0 and samples taken at 0, 20 and 60 min. Normally, LH and FSH concentrations both rise, though the degree of increase is dependent on the method used for measurement. The peak response may be seen at either 20 or 60 min.
The interpretation of the GnRH test is largely based on the basal values. In a patient with delayed puberty, a normal response or a response in which the LH peak is greater than that of FSH, suggests that the patient is about to go into puberty. The response to GnRH may be suppressed by intervening illness.
Reference
Besser GM, Ross RJM. Are hypothalamic releasing hormones useful in the diagnosis of endocrine disorders? In: Edwards IR, Lincoln CRW, editors. Recent advances in endocrinology and metabolism. Edinburgh: Churchill Livingstone; 1989, pp. 135–158.
(ii) Clomifene test
The clomifene test may be helpful in distinguishing gonadotrophin deficiency from weight-related hypogonadism and idiopathic delayed puberty. Side-effects include visual disturbances, symptoms of oestrogen deficiency and depression, which may be severe enough to warrant discontinuation of the test.
Clomifene 50 mg is given daily for 5 days. Serum LH and FSH are measured on days 0 and 7; in addition, in females, progesterone concentration is measured on day 21 to confirm ovulation. In a normal response, LH and FSH concentrations rise to outside the reference ranges or to double the basal values. A lack of response suggests gonadotrophin deficiency due to pituitary or hypothalamic disease. Patients with anorexia nervosa may show no response. Prepubertal children show no response and children in early puberty may actually show a reduction in gonadotrophin concentrations during the test.
Reference
Andersen DC, Marshall JC, Young JL et al. Stimulation tests of pituitary–Leydig cell function in normal male subjects and hypogonadal men. Clinical Endocrinology 1972; 1:127–40.
(iii) Human chorionic gonadotrophin stimulation test
In the differential diagnosis of male hypogonadism, a stimulation test with hCG may be useful. There are no specific precautions and the test does not have to be carried out after fasting.
Human chorionic gonadotrophin is injected i.m. in a dose of 2000 IU on days 0 and 2 and serum testosterone measured on days 0, 2 and 4. A normal response is defined as a rise in serum testosterone to within the reference range. A failure of any rise in testosterone suggests an absence of functioning testicular tissue. If the testes are not palpable in the scrotum or inguinal canals, a rise in testosterone suggests the presence of intra-abdominal testes. In gonadotrophin deficiency with normal testes, the low basal testosterone concentration should triple after hCG.
Reference
Andersen DC, Marshall JC, Young JL et al. Stimulation tests of pituitary-Leydig cell function in normal male subjects and hypogonadal men. Clinical Endocrinology 1972; 1:127–40.