CHAPTER 23. DEHYDRATION
Kim K. Kuebler and Valarie A. Pompey
The issues surrounding dehydration and the implementation of hydration in the palliative and end-of-life care setting to date have been somewhat controversial. The traditional standard of medical care has been to provide routine hydration, whereas the traditional hospice model has promoted the concept of not administering parenteral fluid during the dying or terminal phase of incurable illness, based on the experience that artificial hydration causes potentially uncomfortable symptoms in the dying patient (Walker, 2002). Evidence can be found to support both approaches of care. It is important, however, for the clinician to consider dehydration as a contributing factor in the exacerbation of patient symptoms (Walker, 2002). The use of hydration can be a simple and powerful intervention when combating various symptoms arising from the concomitant manifestations that result from dehydration. Symptoms such as delirium, agitation, somnolence, and dizziness can be resolved with simple, cost-effective hydration. At end-of-life, the benefits of artificial hydration must be balanced by the potential burdens of providing fluids, such as increased secretions, the discomfort of needle sticks, and the physical barrier between patient and family caused by adding tubes. The decision to provide or withhold artificial hydration is based on careful evaluation of the patient’s condition and goals. Artificial hydration should not be routinely administered or routinely denied.
DEFINITION AND INCIDENCE
A discussion of the definition and incidence of dehydration must include the symptoms that frequently accompany and are influenced by dehydration. Dehydration may cause a cluster of many symptoms, such as delirium, xerostomia, agitation, myoclonus, somnolence, dizziness, constipation, and fatigue (Huang & Ahronheim, 2002; Waller & Caroline, 2000). Many of these symptoms are discussed elsewhere in this text; the focus of this practice protocol is on briefly describing the role of dehydration in associated symptoms that ultimately influence patient-related quality of life.
Dehydration should be considered under the broader rubric of fluid deficit and defined as the overall reduction of water content within the human body, but particularly from within the intracellular space (Sarhill, Walsh, Nelson et al., 2001). Dehydration is always hypernatremic, while fluid depletion, which is loss of intravascular water and sodium deficit, may be isotonic, hypertonic/hypernatremic, or hypotonic/hyponatremic (Sarhill et al., 2001; Sarhill, Mahoud, Christie et al., 2003). Dehydration is a common condition that occurs in the dying and terminally ill patient as a result of diminished fluid intake, nausea/vomiting, cachexia, anorexia, and diaphoresis (Huang & Ahronheim, 2002). Dehydration may also be the result of drying medications such as opioids, anticholinergics, and diuretics. If not resolved, it can increase the incidence of pressure ulcers, confusion, and renal failure, resulting in an accumulation of active drug metabolites. Accumulation of drug metabolites can further prompt multiple symptoms, such as restlessness, myoclonus, seizures, and hyperalgesia (Walker, 2002).
When describing dehydration, the clinician should also consider the terms fluid deficit, hypovolemia, and volume depletion. Dehydration does not adequately describe, for example, fluid deficit, which is defined as the loss of water with or without accompanying electrolytes, particularly sodium (Beers & Berkow, 2000; Sarhill et al., 2001; Sarhill, 2003). Volume depletion occurs from loss of extracellular fluid (especially intravascular) and is accompanied by a normal, decreased, or increased plasma serum level (Beers & Berkow, 2000; Leaf, 1984; Sarhill et al., 2001). Dehydration occurs when intracellular water is lost. This leads to transmembrane water migration from the intravascular compartment under osmotic pressure and increased plasma sodium concentration (Sarhill et al., 2001; Sarhill et al., 2003).
ETIOLOGY AND PATHOPHYSIOLOGY
To dehydrate is to lose predominantly intracellular water (Feig & McCurdy, 1977; Sarhill et al., 2001). Total body water averages 60% of body weight in young adult males and 50% in young adult females (Sarhill et al., 2001). The total body water decreases with age and accounts for 50% in elder men and 45% in elder women (Sarhill et al., 2001). Because serum sodium and its associated anions account for more than 90% of the solute in extracellular fluid, the plasma sodium concentration is a good indicator of plasma osmolarity (Guyton & Hall, 2001).
In isotonic fluid deficit, there is a depletion of both sodium and water. The serum sodium level is within normal limits (135 to 148 mEq/L) despite a fluid volume deficit. Examples of this deficit can be seen in blood loss and loss of gastrointestinal fluids such as diarrhea, vomiting, or nasogastric suctioning (Sarhill et al., 2001). In hypernatremic dehydration, there is a water loss alone, and in fluid volume deficit, there is water loss in excess of serum sodium. Therefore, serum sodium is concentrated (≥145 mEq/L) as a result of lower fluid volume. Hyponatremic fluid deficit occurs with excessive loss of sodium along with some fluid volume loss, leading to a serum sodium level of less than 130 mEq/L (Guyton & Hall, 2001).
As disease becomes debilitating and the dying process approaches, patients lose their ability or desire to drink fluids. The lack of fluid intake may lead to an isotonic depletion of water and sodium or, possibly, a tendency toward hypernatremic dehydration. In studies of persons experiencing dehydration at end-of-life, more than half had normal or near-normal serum sodium concentrations (Burge, 1993; Ellershaw, Sutcliffe, & Saunders, 1995; Waller, Hershkowitz, & Adunsky, 1994). It must be noted, however, that the results of these studies cannot be generalized because of small sample sizes, potentially biased selection criteria, and missing data (Viola, Wells, & Peterson, 1997).
Patients with dehydration may demonstrate low blood pressure with dizziness and syncope, decreased skin turgor, dry mucous membranes, low-grade fever, decreased urine output, and weight loss. A complaint of thirst is common although not universal. Because the serum is more concentrated, laboratory data may show elevated hemoglobin, hematocrit, and blood urea nitrogen levels (Guyton & Hall, 2001).
Many of the patients in the studies noted did not have isotonic volume depletion but appeared to have a hypertonic/hypernatremic dehydration. Hypernatremia is possible with a prolonged decrease in fluid intake, as may be seen in the terminally ill; it also occurs in the presence of excessive diaphoresis and with diabetes insipidus. The signs and symptoms are the same as those of isotonic dehydration, except that thirst is almost universal, because even slight increases in sodium concentration (as little as 2 mEq/L above normal) activate the thirst mechanism (Guyton & Hall, 2001; Sarhill et al., 2001; Sarhill et al., 2003). As the sodium level increases, anxiety and restlessness may also be present.
The sensation of thirst is important to fluid balance. The presence of thirst or dry mouth has been reported in 25% to 64% of cancer patients initiated to a palliative care service (Morita, Tsunoda, Inoue et al., 1999; Ventafridda, DeConne, Ripamonti et al., 1990). The incidence of thirst is increased to 61% to 87% in the final week of life (Conill et al., 1997; Ellershaw et al., 1995). In dehydration, patients experience thirst due to either dry mucous membranes or increased serum sodium levels and are stimulated to drink to replace the fluid loss. It is important to note, however, that the presence of thirst does not necessarily indicate dehydration. Many medications, such as opioids, phenothiazines, antihistamines, antidepressants, and anticholinergics, can cause the sensation of thirst despite adequate hydration. Thus, thirst alone is not a good indicator of a patient’s hydration status.
The elderly are at an increased risk for dehydration, in part because of a decrease in the sense of thirst that accompanies the normal aging process. A lack of thirst may also be the result of a coexistent inability to respond physiologically to thirst from disease or disability (Morita, Tei, Tsunoda et al., 2001; West, 1993). In addition, the elderly are susceptible to the development of dehydration or hypovolemia as a result of chronic use of medications such as diuretic therapy, which can be responsible for significant water and vital electrolyte depletion (Sarhill et al., 2001).
Confusion, agitation, and restlessness are often associated with dehydration and may result from one of several of the following mechanisms. Dehydration directly affects the body’s blood volume and circulatory reserve and can cause confusion and restlessness in non–terminally ill patients as well as those with an advanced illness (Fainsinger, Mac Cachern, Miller et al., 1994; Fainsinger & Bruera, 1994; Pereira & Bruera, 1997; Sarhill et al., 2001). In addition, the decreased circulatory volume may lead to decreased renal perfusion and eventual renal failure. In persons receiving opioids, poor renal perfusion may cause accumulation of opioid metabolites, leading to confusion, myoclonus, and nausea (Bruera, Franco, Maltoni et al., 1995; Caraceni & Grassi, 2003). Constipation, pyrexia, and electrolyte imbalance result from dehydration and can further contribute to disorientation, agitation, and neuromuscular irritability (Fainsinger & Bruera, 1994; Steiner & Bruera, 1998).
Providing fluids to persons who are dehydrated may alleviate many of the symptoms of dehydration and assist in flushing waste products and medication metabolites from the circulation. Providing fluids to persons whose kidneys cannot eliminate the additional fluid may lead to discomfort from fluid overload, with symptoms of edema, ascites, pulmonary congestion, and nausea and vomiting.
ASSESSMENT AND MEASUREMENT
The determination of a patient’s hydration status is difficult in patients who have multiple problems associated with advanced diseases. Consideration of the following factors, as well as the signs and symptoms of dehydration identified in Box 23-1, may assist the clinician to discern an appropriate diagnosis for dehydration:
Box 23-1
▪ Confusion
▪ Restlessness
▪ Delirium
▪ Myoclonus
▪ Seizures
▪ Constipation
▪ Nausea and vomiting
▪ Decreased glomerular filtration rate (may also be the result of renal failure)
▪ Presence of decubitus ulcers
▪ Higher-than-normal systolic blood pressure
▪ Tachycardia at rest
▪ Thready pulse
▪ Dry mouth, multiple tongue furrows
▪ Delayed capillary refill
▪ Orthostatic blood pressure changes
▪ Increased body temperature
▪ Weight loss
▪ Hypoactive deep tendon reflexes
▪ Flattened neck veins
▪ Severely restricted oral intake
▪ Decreased urine output in patients who do not have preexisting renal failure
▪ Poor skin turgor, dry mouth, and postural hypotension, noting that dehydration is not the only cause of these symptoms
▪ Changes in laboratory findings such as elevated urea, creatinine, hematocrit, sodium, and plasma protein levels
It is important to note that the presence of edema is not a good indicator of the patient’s hydration status. Edema in the advanced cancer population is often the result of low serum albumin level or tumor blockage of the venous or lymphatic systems. Patients taking corticosteroids may also experience edema. Despite excessive fluid in the interstitial spaces, there may be inadequate intravascular fluid.
HISTORY AND PHYSICAL EXAMINATION
History
▪ Review history of the terminal illness and any coexisting medical conditions that may affect urinary elimination or the ability to ingest fluids.
▪ Review intake and output. An estimate from the patient or caregiver may provide sufficient information, or the clinician may instruct the patient and family caregivers to record intake and output.
▪ Review history of any recent infection causing fever.
▪ Review history of any condition causing fluid loss, such as nausea or vomiting, diarrhea, or fistulas or/draining wounds.
▪ Review patient reports of headache, fatigue, muscle weakness, or anorexia, noting onset and any correlation with decreased fluid intake.
▪ Assess for the onset and severity of any mental status changes, such as restlessness, confusion, or delirium.
▪ Assess the use of diuretics, including frequency of administration, last dose, and urine output after last dose.
Physical Examination
▪ Skin and mucous membranes
Body temperature
Skin condition, noting skin turgor and any dryness or pruritus
Alterations in skin integrity, such as decubitus ulcers, draining wounds, or fistulas
Oral mucosa, noting dryness and presence of furrows or fissures
▪ Cardiovascular system
Heart rate, noting strength and any tachycardia
Blood pressure, including measurement of orthostatic changes
Capillary refill
Neck veins, noting any flattening
▪ Genitourinary system
Urinary output and urinary retention
Pain or discomfort on urination
▪ Gastrointestinal system
Nausea or vomiting
Diarrhea or constipation
▪ Neurological system
Deep tendon reflexes
Presence of myoclonus and/or seizures
Mental status changes
▪ Musculoskeletal system
Fatigue
Muscle weakness
DIAGNOSTICS
Diagnostic procedures provide data that assist in the evaluation and monitoring of hydration and renal status. The following diagnostic procedures should be performed when the data from them influence the course of treatment (Sarhill et al., 2001).
▪ Blood pressure
▪ Hematocrit and plasma albumin
▪ Plasma sodium
▪ Plasma pH
▪ Urine osmolarity
▪ Blood urea nitrogen–to–plasma creatinine ratio
▪ Urinary output (normal is 1 ml/kg of body weight per hour, or approximately 1500 ml/day)
INTERVENTION AND TREATMENT
Once the evaluation is complete and the determination is made that dehydration exists, the decision should be made whether to provide hydration. As mentioned previously, decisions about providing hydration at end-of-life remain controversial. However, the clinician should consider the patient’s overall medical condition and goals as well as the perspectives of family members when choosing specific interventions. Providing fluids may be directed by the patient’s and family’s cultural, religious, or moral convictions (Kedziera, 2001).
Many concerns remain regarding the benefits and risks of providing hydration at end-of-life. There is little research involving dying patients to support either position. Traditionally, hospice providers have argued against hydration in the terminally ill because it interferes with the natural dying process and may cause discomfort or complications, including the following (Dalal & Bruera, 2004; Vena, Kuebler, & Schrader, 2005):
▪ Cardiopulmonary overload leading to increased secretions, edema, cough, and/or choking
▪ Increased risk of aspiration
▪ Increased need for suctioning
▪ Incontinence
▪ Discomfort associated with repeated needle sticks
▪ Increased risk for infection related to intravenous lines and central venous catheter devices
▪ Increased risk for thrombosis related to central venous catheter lines
However, questions remain. Does providing hydration impede the dying process? Should providing fluids to the dying be considered a basic comfort measure that ensures a dignified end by reducing concomitant symptoms? Data do not support that the provision of hydration prolongs the dying process to a meaningful degree (Vena et al., 2005). Some potential advantages of hydration include the following:
▪ Prevention or alleviation of neurotoxicity from medications commonly used at end-of-life
▪ Alleviation of uncomfortable symptoms such as constipation, nausea and vomiting, thirst, and dry mouth (xerostomia)
▪ Prevention or alleviation of uncomfortable symptoms of confusion, agitation, and neuromuscular irritability (Dalal & Bruera, 2004; Vena et al., 2005).
Methods for the replacement of fluids, when the oral route is no longer feasible, include enteral via feeding tubes, rectal via proctoclysis, and parenteral via intravenous or subcutaneous (hypodermoclysis [HDC]) infusions (Kedziera, 2001).
Enteral Hydration
Enteral hydration is the preferred route for nutrition and hydration because it is safer, simpler, and less costly than parenteral routes, and it provides a normalized physiological response to food and fluid (Dalal & Bruera, 2004). One major disadvantage with enteral hydration is that as the patient become weaker, there is a risk of aspiration. Common types of enteral “lines” include nasogastric, gastrostomy, and jejunostomy. Some disadvantages that may occur as death approaches include the following:
▪ Discomfort
▪ Potential for a brief hospitalization for line placement
▪ Agitation in an already confused patient
▪ Complications associated with insertion
▪ Infection
▪ Aspiration
▪ Potential for the need to restrain patients to prevent them from pulling on tubes
Proctoclysis
Proctoclysis is the administration of fluids via the rectal route and may be a viable alternative in the home care setting when life expectancy is limited to days. Fluid replacement by proctoclysis is relatively risk free, inexpensive, and easy to administer. When choosing this route, thought should be given to whether family members are willing to assume the physical care associated with this approach (Kedziera, 2001). Disadvantages with protoclysis include the following:
▪ Abdominal cramping and discomfort
▪ Rectal leakage
▪ Reluctance of family members to administer fluids
▪ Immobility due to length of time needed to administer fluids (typically 6 to 8 hours).
Intravenous Hydration
In the acute care setting, hydration is considered routine and involves the utilization of the intravenous route (Lawlor, 2002). Disadvantages to use of this route may include the following (Kuebler & McKinnon, 2002; Lawlor, 2002):
▪ Difficulty finding venous access
▪ Invasive and uncomfortable
▪ Phlebitis
▪ Need for frequent site change
▪ Infection and thrombosis in central lines
▪ Decreased mobility
▪ Cost
▪ Complexity for use in the home setting
Hypodermoclysis
HDC is the administration of fluids via a subcutaneous infusion. This intervention is performed through the use of a butterfly needle (typically a 25-gauge needle) to infuse isotonic fluids into the subcutaneous tissue (Frisoli, de Paula, Feldman et al., 2000; Lawlor, 2002). HDC was widely used in the 1940s and 1950s and slowly fell out of favor with the introduction of hypertonic and electrolyte-free solutions. These fluids resulted in severe adverse reactions, so this intervention was abandoned for many years (Frisoli et al., 2000; Lawlor, 2002).
In the palliative care setting, HDC offers many advantages to patients who might otherwise have difficulty reversing dehydration by oral replacement. It is believed that HDC offers far more benefits of fluid replacement than the traditional intravenous route due to the following factors (Kuebler & McKinnon, 2002; Lawlor, 2002; Moriarty & Hudson, 2001):
▪ No need for venous access
▪ Easy for family members to administer using a subcutaneous injection (butterfly needle)
▪ May be stopped and started without a concern of thrombus development
▪ No need for hospitalization
▪ Subcutaneous sites last for several days
▪ Simple and easy to maintain
▪ Greater patient mobility and comfort
▪ Inexpensive
▪ Less likely to cause edema or fluid overload
In the absence of large volume losses, 1 liter of fluid per 24 hours is usually sufficient to maintain renal function in the patient population with advanced cancer and to prevent potential problems related to overhydration (Kuebler & McKinnon, 2002; Lawlor, 2002). The process of initiating HDC is outlined in Box 23-2.
Box 23-2
Select an insertion site. Preferred sites include the upper chest (avoid breast tissue), upper back, abdomen, back of upper arms, and upper thighs. Seek fatty areas.
Cleanse the skin over the selected site with alcohol or chlorhexidine.
Gently pinch a well-defined amount of tissue and insert a small needle (25- or 23-gauge butterfly) at a 45-degree angle into the subcutaneous space.
Dress the site with a transparent dressing.
Select a hypotonic or isotonic solution such as ⅔, ⅓, or plain normal saline solution, with or without hyaluronidase.
Begin infusions slowly (50 to 75 ml/hr) to determine tolerance. Most patients tolerate 50 to 100 ml/hr
As death approaches, the clinician should evaluate the benefit versus burden of providing hydration. Less noninvasive choices may be ideal and allow family members to continue to participate in care giving until the end. Simple and effective techniques that are often forgotten include the use of the following (Kedziera, 2001):
▪ Water or emollients to moisten lips
▪ Small, frequent sips of fluid (including sports replacement drinks) or ice chips
▪ Fine mist spray to moisten mouth
▪ Fans or air conditioners in hot humid weather to decrease amount of insatiable loss
Because of the lack of conclusive and comprehensive scientific data, considerations on whether to start or stop hydration should include the patient’s right to autonomy. Ethical and moral precepts of “due no harm,” the “doctrine of double effect,” and the maintenance of comfort and dignity when death is imminent should help guide clinicians in assisting patients and families to make choices that ultimately result in a “good death.”
PATIENT AND FAMILY EDUCATION
The prevention and management of dehydration are important in the palliative setting because they can help reduce untoward symptoms. Establishing a patient-specific plan of care is an important opportunity to learn what the patient’s and family’s expectations are as they relate to providing hydration in the dying process. These conversations provide the clinician with important information on how to plan the patient’s care as well as to determine if there is a need for additional disciplines to support the patient and family during the terminal phase. Hydration is often an important issue for family members, who need education and support to understand the benefits and burdens of this intervention.
Patients who experience acute dehydration as a result of vomiting, diarrhea, or polyuria often experience distressing thirst and would benefit from rehydration. However, patients and families who prefer to avoid exogenous hydration for whatever reason should be respected for their informed decisions. The aim of hydration in the palliative care setting is comfort, not the return to a normal fluid and electrolyte balance (Twycross, 1997). Patients and their families should be helped to understand that hydration is a temporary intervention to help relieve distressing symptoms that interfere with the quality of life.
EVALUATION AND PLAN FOR FOLLOW-UP
Ongoing assessment is essential for patients receiving hydration in order to discern their level of comfort. If symptoms improve with hydration and the patient’s quality of living is improved, the intervention is maintained. If symptoms do not improve or the patient shows signs and symptoms of fluid overload, hydration is either lessened or discontinued. Clinicians should keep an open mind about the utilization of hydration and recognize the options for fluid replacement (e.g., intravenous, HDC). Respecting the patient’s and family’s wishes on providing hydration may be the most important intervention offered during the terminal phase. Comfort is the goal in palliative care, and hydration is an easy intervention to address the multiple issues that are associated with dehydration.
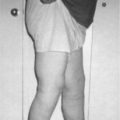
Mr. B., a 75-year-old man, presents to the clinic for his follow-up evaluation after completing his radiation therapy for metastatic prostate cancer to bone. He complains of fatigue, weakness, dyspnea, and a poor appetite. The clinician performs a physical examination and finds that Mr. B. is febrile with an oral temperature of 101.5 °F; an increased heart rate of 122; slow, shallow breaths with a respiratory rate of 14; and his blood pressure is 188/94 mm Hg. Further evaluation reveals poor skin turgor, pale mucous membranes, delayed capillary refill, and diminished pedal pulses. The clinician orders a complete blood cell count and a comprehensive metabolic profile that includes measuring serum calcium. Mr. B. provided the clinician with a history of his intake of food and fluid over the past 48 hours, which included several cups of black coffee, cola, and soups. The clinician prescribes a nutritional consultation for Mr. B. and sends him to the infusion suite for a liter of normal saline while she waits for his laboratory results. She further investigates Mr. B.’s home situation and learns that he had been taking care of his wife, who recently underwent exploratory surgery. The clinician contacts the local home care agency to follow-up with Mr. B. at home to evaluate his physical status and to provide an infusion of another liter of normal saline the following day.
REFERENCES
In: (Editors: Beers, M.; Berkow, R.) The Merck manual of geriatrics ( 2000)Merck Research Laboratories, Rahway, N.J, pp. 561–568.
Bruera, E.; Franco, J.; Maltoni, M.; et al., Changing pattern of agitated impaired mental status in patients with advanced cancer: Association with cognitive monitoring, hydration and opioid rotation, J Pain Symptom Manage 10 (4) ( 1995) 287–291.
Burge, F., Dehydration symptoms of palliative care cancer patients, J Pain Symptom Manage 8 (7) ( 1993) 454–464.
In: (Editors: Caraceni, A.; Grassi, L.) Delirium: Acute confusional states in palliative medicine ( 2003)Oxford University Press, New York, pp. 132–133.
Conill, C.; Verger, E.; Henriquez, I.; et al., Symptom prevalence in the last week of life, J Pain Symptom Manage 14 (6) ( 1997) 328–331.
Dalal, S.; Bruera, E., Dehydration in cancer patients: To treat or not to treat, J Support Oncol 2 (6) ( 2004) 467–487.
Ellershaw, J.; Sutcliffe, J.; Saunders, C., Dehydration and the dying patient, J Pain Symptom Manage 10 (3) ( 1995) 192–197.
Fainsinger, R.; Bruera, E., The management of dehydration in terminally ill patients, J Pain Symptom Manage 10 (3) ( 1994) 55–59.
Fainsinger, R.; MacCachern, T.; Miller, M.; et al., The use of hypodermoclysis for rehydration in terminally ill cancer patients, J Pain Symptom Manage 9 (5) ( 1994) 298–302.
Feig, P.; McCurdy, D., The hypertonic state, N Engl J Med 297 (26) ( 1977) 1444–1454.
Frisoli Jr, A.; de Paula, A.P.; Feldman, D.; et al., Subcutaneous hydration by hypodermoclysis: A practical and low cost treatment for elderly patients, Drugs Aging 16 (4) ( 2000) 313–319.
Guyton, A.; Hall, J., Pocket companion to the textbook of medical physiology. 10th ed ( 2001)Saunders, Philadelphia.
Huang, A.; Ahronheim, J., Issues in nutrition and hydration, In: (Editors: Berger, A.; Portenoy, R.; Weissman, D.) Principles and practice of palliative care and supportive oncology2nd ed. ( 2002)Lippincott Williams & Wilkins, Philadelphia, pp. 956–967.
Kedziera, P., Hydration, thirst and nutrition, In: (Editors: Ferrell, B.R.; Coyle, N.) Textbook of palliative nursing ( 2001)Oxford University Press, New York, pp. 156–163.
Kuebler, K.; McKinnon, S., Dehydration, In: (Editors: Kuebler, K.; Berry, P.; Heidrich, D.) End-of-life care: Clinical practical guidelines ( 2002)Saunders, Philadelphia, pp. 243–251.
Lawlor, P.G., Delirium and dehydration: Some fluid for thought?Support Care Cancer 10 (6) ( 2002) 445–540.
Leaf, A., Dehydration in elderly (editorial), N Engl J Med 311 (12) ( 1984) 791–792.
Moriarty, D.; Hudson, E., Hypodermoclysis for rehydration in the community, Br J Commun Nurs 6 (9) ( 2001) 437–443.
Morita, T.; Tei, Y.; Tsunoda, J.; et al., Determinants of the sensation of thirst in terminally ill cancer patients, Support Care Cancer 9 (3) ( 2001) 177–186.
Morita, T.; Tsunoda, J.; Inoue, S.; et al., Contributing factors to physical symptoms in terminally-ill cancer patients, J Pain Symptom Manage 18 (1999) 338–346.
Pereira, J.; Bruera, E., In: The Edmonton aid to palliative care ( 1997)Division of Palliative Care, University of Alberta, Edmonton, Edmonton, Canada, pp. 58–60.
Sarhill, N.; Mahoud, F.A.; Christie, R.; et al., Assessment of nutritional status and fluid deficits in advanced cancer, Am J Hospice Palliat Care 20 (6) ( 2003) 465–473.
Sarhill, N.; Walsh, D.; Nelson, K.; et al., Evaluation and treatment of cancer-related fluid deficits: Volume depletion and dehydration, Support Care Cancer 9 (6) ( 2001) 408–419.
Steiner, N.; Bruera, E., Methods of hydration in palliative care patients, J Palliat Care 14 (2) ( 1998) 6–13.
Twycross, R., Dehydration, In: Symptom management in advanced cancer ( 1997)Radcliffe Medical Press, Oxon, UK, pp. 170–172.
Vena, C.; Kuebler, K.; Schrader, S., The dying process, In: (Editors: Kuebler, K.; Davis, M.; Moore, C.) Palliative care practices: An interdisciplinary approach ( 2005)Elsevier Mosby, St. Louis, pp. 340–341.
Ventafridda, V.; DeConne, F.; Ripamonti, C.; et al., Quality of life during a palliative care programme, Ann Oncol 1 (6) ( 1990) 415–420.
Viola, R.; Wells, G.; Peterson, J., The effects of fluid status and fluid therapy on the dying: A systematic review, J Palliat Care 13 (4) ( 1997) 41–52.
Walker, P., Hydration, In: (Editors: Elsayem, A.; Driver, L.; Bruera, E.) The M.D. Anderson symptom control and palliative care handbook2nd ed. ( 2002)The University of Texas Health Science Center at Houston, Houston, pp. 77–81.
Waller, A.; Caroline, N., Handbook of palliative care in cancer. ( 2000)Butterworth Heinemann, Boston.
Waller, A.; Hershkowitz, M.; Adunsky, A., The effect of intravenous fluid infusion on blood and urine parameters of hydration and on state of consciousness in terminal cancer patients, Am J Hospice Palliat Care 11 (6) ( 1994) 22–27.
West, C., Ischemia, In: (Editors: Carrieri-Kohlman, V.; Lindsey, A.; West, C.) Pathophysiological phenomena in nursing ( 1993)Saunders, Philadelphia, pp. 1–45.