CASE 23 Coronary Perforation
Cardiac catheterization
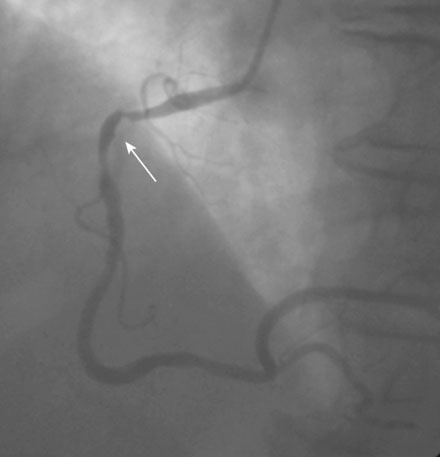
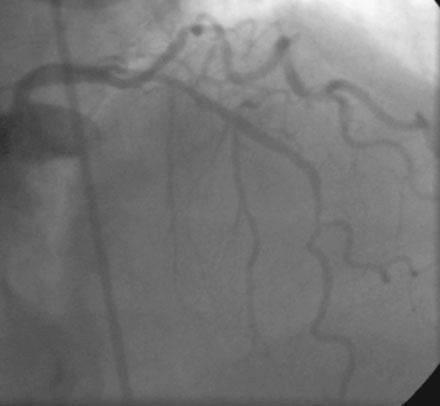
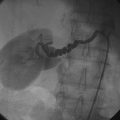
Coronary angiography revealed multivessel coronary disease with severe disease in the proximal segment of the right coronary artery and severe disease of the midportion of the left anterior descending coronary artery (Figures 23-1, 23-2 and Video 23-1). The operator judged the right coronary artery suitable for percutaneous coronary intervention; however, the left anterior descending artery provided several challenges. Not only did the vessel appear small in caliber, there was also marked tortuosity with diffuse disease surrounding the severely stenosed segment. Prior to the catheterization, the patient dismissed the option of bypass surgery and agreed only to percutaneous revascularization. The patient confirmed this after she was presented with the catheterization results, and the operator proceeded with a multivessel intervention on the right coronary artery and left anterior descending artery.
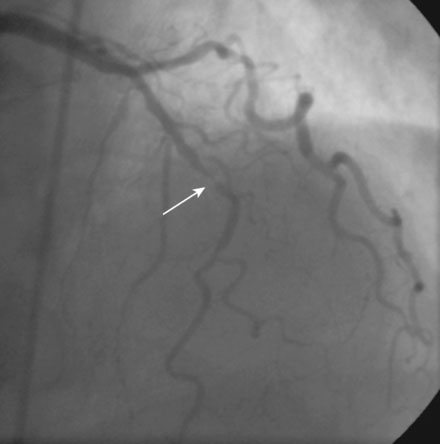
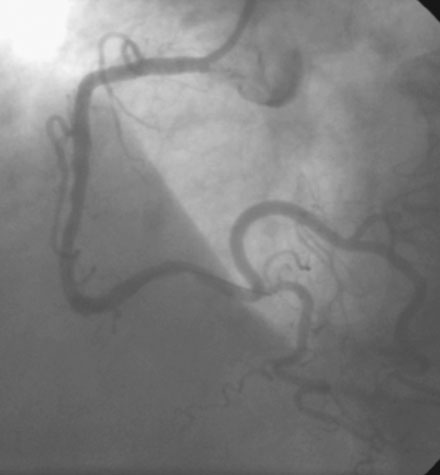
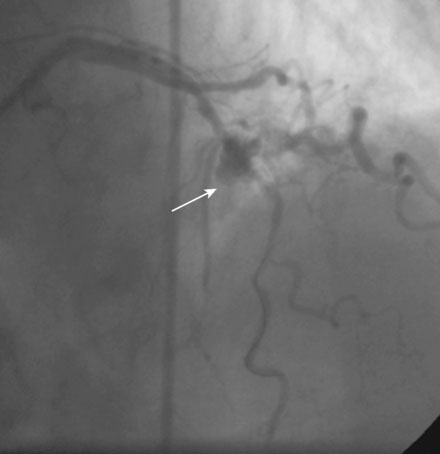
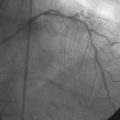
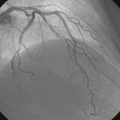
Following administration of a bolus and infusion of the direct thrombin inhibitor bivalirudin, the operator began with the right coronary artery. This lesion was first dilated with a 3.0 mm diameter by 15 mm long compliant balloon and a 3.5 mm diameter by 28 mm long sirolimus-eluting stent was deployed successfully with an acceptable angiographic result (Figure 23-3). The operator then turned to the left anterior descending artery. A 6 French, 45 C-curve guide catheter was engaged and a 0.014 inch floppy-tipped guidewire advanced to the distal portion of the vessel. The 3.0 mm by 15 mm compliant balloon used to predilate the right coronary lesion was advanced to the lesion and inflated to 8 atmospheres for 30 seconds (Figure 23-4). The operator deflated the balloon and observed contrast extravasation at the balloon angioplasty site (Figure 23-5 and Video 23-2). The vessel was immediately tamponaded using the same balloon inflated to 6 atmospheres and the infusion of bivalirudin was discontinued. The patient developed transient hypotension and bradycardia. This corrected quickly with 1 mg of atropine and a 500 cc bolus of fluid. An urgent echocardiogram revealed a small pericardial effusion. The balloon remained inflated for 15 minutes and repeat angiography confirmed successful sealing of the perforation. The operator noted some difficulty advancing the previously-inflated balloon to the lesion because of vessel angulation. Subsequently, two short bare-metal stents (2.5 mm diameter by 13 mm long distally and a 2.5 mm diameter by 12 mm long proximally) were deployed to 12 atmospheres and covered the diseased segment of the mid-LAD. Postdeployment, the stents appeared well-deployed with no further evidence of contrast extravasation (Figure 23-6 and Video 23-3). The patient left the catheterization laboratory hemodynamically stable with minimal chest discomfort.
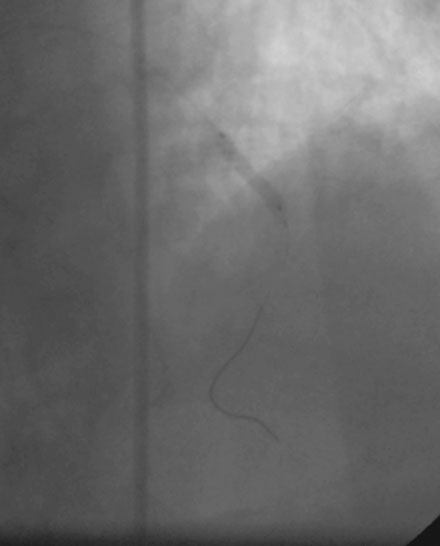
FIGURE 23-4 Balloon angioplasty was performed to predilate the lesion in the left anterior descending artery.
Discussion
The ultimately favorable outcome in this case emphasizes several important concepts in the management of a patient with a coronary perforation. Based on the classification system described by Ellis and colleague the Type II perforation present in this case would be expected to result in tamponade in 10% to 15% of cases.1,2 Rapid recognition and management by the operator prevented this. The operator acted quickly, rapidly repositioning and inflating the balloon at the site of the perforation while simultaneously discontinuing the infusion of bivalirudin. This quick response allowed only a small amount of blood to enter the pericardium, thus preventing tamponade. Even a small delay might have caused enough blood to accumulate in the pericardial space to cause dramatic hemodynamic deterioration requiring patient resuscitation and a potentially less favorable outcome. Note that the brief period of hypotension and bradycardia occurring immediately after the perforation likely represented a vagal reaction, a common sequelae of an acute perforation. In this case, the brief period of hypotension and bradycardia responded immediately to atropine and fluid bolus.
1. Ellis S.G., Ajluni S., Arnold A.Z., Popma J.J., Bittl J.A., Eigler N.L., Cowley M.J., Raymond R.E., Safian R.D., Whitlow P.L. Increased coronary perforation in the new device era: Incidence, classification, management and outcome. Circulation. 1994;90:2725-2730.
2. Fasseas P., Orford J.L., Panetta C.J., Bell M.R., Kenktas A.E., Lennon R.J., Holmes D.R., Berger P.B. Incidence, correlates, management, and clinical outcome of coronary perforation: Analysis of 16,298 procedures. Am Heart J. 2004;147:140-145.