CASE 22 Saphenous Vein Graft Rupture
Cardiac catheterization
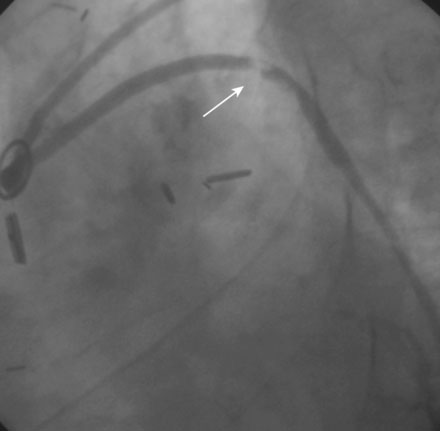
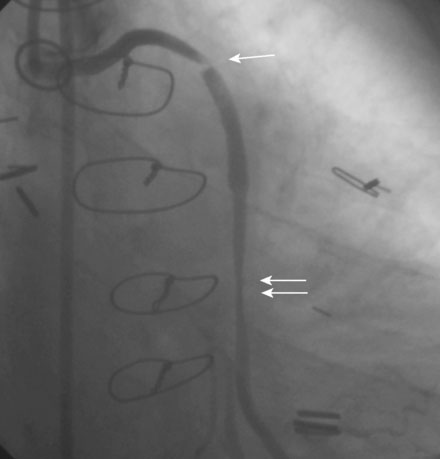
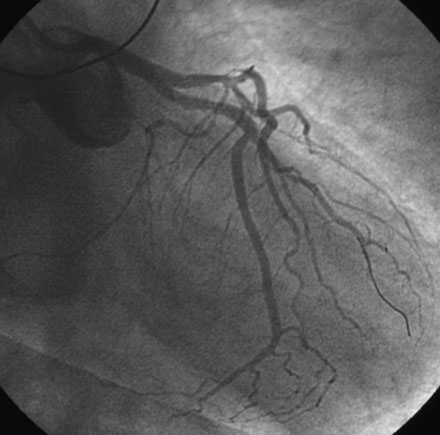
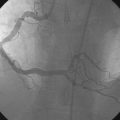
Angiography found wide patency of both internal mammary artery grafts and a severe proximal stenosis and moderately severe, more distal stenosis in the body of the 14-year-old vein graft to the obtuse marginal artery (Figures 22-1, 22-2 and Videos 22-1, 22-2). The operator planned to stent both the proximal and midgraft lesions with distal embolic protection afforded by the PercuSurge Guardwire (Medtronic) temporary balloon occlusion and aspiration system.1 An 8 French, right Judkins guide catheter was inserted in the vein graft. The operator chose unfractionated heparin and eptifibatide for procedural anticoagulation, and the Guardwire was positioned distally in the vein graft. The occlusion balloon was inflated to allow distal embolic protection. After first dilating with a 3.5 mm diameter balloon, a 4.5 mm diameter by 18 mm long bare-metal stent was deployed in the proximal lesion (Figure 22-3). The operator then positioned and deployed a 4.0 mm diameter by 28 mm long bare-metal stent in the midgraft lesion. Aspiration was performed, the distal occlusion balloon deflated, and angiography performed. To the operator’s horror, free-flowing contrast was observed extravasating from a large defect in the midportion of the vein graft within the stented segment (Video 22-3). The patient developed marked hypotension with arterial pressure falling to 50/30 mmHg. To staunch the hemorrhage, a 3.5 mm perfusion balloon was positioned and inflated in the vein graft at the site of the defect, eptifibatide was discontinued, and 30 mg of protamine was administered intravenously. This greatly decreased the amount of contrast extravasation, and the perfusion balloon allowed distal perfusion; however, there remained evidence of ongoing hemorrhage by angiography (Video 22-4). Fluid resuscitation and dopamine infusion restored blood pressure, but each time the balloon was deflated for even a few seconds, the patient developed marked hypotension. Since the operator did not feel there was sufficient time to safely exchange the perfusion balloon for a PTFE-covered stent nor felt confident that the large defect could be successfully sealed with this device, cardiothoracic surgery agreed to operate on the patient emergently.
Discussion
A saphenous vein graft perforation may behave differently than one involving a native coronary artery. A saphenous vein graft may be more prone to perforation since, in a native coronary artery, the presence of a thicker muscular layer, as well as a more robust adventitia and an overlying layer of subepicardial fat, may help contain a deep dissection or smaller perforation that might lead to free bleeding if a similar injury involved a vein graft, which lacks these protective layers. Alternatively, in the event of a definite breach of the vessel during intervention, a perforation of a saphenous vein graft may be less likely to cause tamponade. If the lesion involves the proximal segment of the vein graft, a rupture may cause bleeding into the pleural instead of the pericardial space. Furthermore, the existence of pericardial adhesions or the persistence of a pericardiotomy created at the time of bypass surgery might protect the patient from developing tamponade, should the bleeding enter the pericardial space. Although the case presented here bled into the pleural space and the patient did not develop tamponade, the rupture still led to dramatic hemodynamic collapse from free bleeding into the pleural space. In addition, tamponade may still occur in these cases because the pericardium may reflect high onto the ascending aorta, there may be minimal adhesions, and the pericardial opening made during surgery may close.2
Treatment strategies for native coronary artery perforation have been discussed in other cases in this section involving perforation. These basic principles are also applicable to a saphenous vein graft perforation. Similar to native coronary arteries, the outcome of the perforation will depend on the size of the defect.3 The case presented here is an example of a Type III perforation with freely flowing contrast from an exit hole greater than 1 mm in diameter. Simple measures, such as reversal of anticoagulation and prolonged balloon inflation at the site of the perforation, could not be expected to effectively seal such a large defect. A covered stent might have succeeded; however, the operator chose surgery, primarily based on the large size of the defect and the concern that a covered stent might not necessarily seal the rupture. Furthermore, release of the occluding balloon for even a few seconds led to prompt hemodynamic instability. An attempt at a covered stent would have been technically challenging under these circumstances.
As exemplified in this case, emergency surgery is necessary when there is ongoing bleeding from a coronary perforation despite reversal of anticoagulation and institution of measures to treat the perforation, such as prolonged balloon inflation or use of a covered stent graft. Overall, between 10% and 40% of patients with coronary perforation are treated surgically black4–6; surgery is less likely to be necessary when the perforation is small and not associated with tamponade or hemodynamic instability. In this case, a perfusion balloon was used to occlude the bleeding site while allowing distal perfusion of the artery, thereby limiting ischemia during the time required for transport to the operating room. Unfortunately, perfusion balloons are no longer commercially available, thereby removing this strategy as an option in the current era.
1 Baim D.S., Wahr D., George B., Leon M.B., Greenberg J., Cutlip D.E., Kaya U., Popma J.J., Ho K.K.L., Kuntz R.E. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. 2002;105:1285-1290.
2 Lowe R., Hammond C., Perry R.A. Prior CABG does not prevent pericardial tamponade following saphenous vein graft perforation associated with angioplasty. Heart. 2005;91:1052.
3 Ellis S.G., Ajluni S., Arnold A.Z., Popma J.J., Bittl J.A., Eigler N.L., Cowley M.J., Raymond R.E., Safian R.D., Whitlow P.L. Increased coronary perforation in the new device era: Incidence, classification, management and outcome. Circulation. 1994;90:2725-2730.
4 Fasseas P., Orford J.L., Panetta C.J., Bell M.R., Kenktas A.E., Lennon R.J., Holmes D.R., Berger P.B. Incidence, correlates, management, and clinical outcome of coronary perforation: Analysis of 16,298 procedures. Am Heart J. 2004;147:140-145.
5 Fejka M., Dixon S.R., Safian R.D., O’Neill W.W., Grines C.L., Finta B., Marcovitz P.A., Kahn J.K. Diagnosis, management, and clinical outcome of cardiac tamponade complicating percutaneous coronary intervention. Am J Cardiol. 2002;90:1183-1186.
6 Stankovic G, Orlic D, Corvaja N, Airoldi F, Chieffo A, Spanos V, Montorfano M, Carlino M, Finci L, Sangiorgi G, Colombo A: Incidence, predictors, in-hospital, and late outcomes of coronary artery perforations, Am J Cardiol 93:213–216