Section 22 Pain Relief
22.1 General pain management
Introduction
Pain is defined by the International Association for the Study of Pain as ‘An unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.’1 Acute pain is defined as ‘Pain of recent onset and probable limited duration. It usually has an identifiable temporal and causal relationship to injury or disease.’2 However, once a patient presents for medical care, severe acute pain has ceased to serve a useful purpose. Whereas in some conditions the nature and progression of the pain may be helpful in making the diagnosis of the underlying pathology, too great a reliance has been placed upon this feature, thereby allowing the patient to suffer needlessly for prolonged periods.2,3
When severe pain is inadequately relieved it produces pathophysiological and abnormal psychological reactions that often lead to complications. This is important because acute pain is the most common presenting complaint to an emergency department (ED)4 and its management forms part of the daily practice of emergency medicine. It should be considered poor patient care not to treat pain while attempting to arrive at a diagnosis. There can be no greater gift to one’s neighbour than to practise, teach and discover more effective methods to relieve pain and suffering.2,3 Unfortunately, the management of acute pain is often not a specific component of medical training.
Physiology
Pain is one of the most complex aspects of an already intricate nervous system.2 A number of theories have been developed to explain the physiology of pain, but none is proven or complete.
In 1965, the Melzack–Wall ‘Gate Control Theory’ emphasized mechanisms in the central nervous system that control the perception of a noxious stimulus, and thus integrated afferent, upstream processes with downstream modulation from the brain.5 However, this theory did not incorporate long-term changes in the central nervous system to the noxious input and to other external factors that impinge upon the individual.5 Most pain originates when specific nerve endings (nociceptors) are stimulated, producing nerve impulses that are transmitted to the brain. Nociception is the detection of tissue damage by specialized transducers.5
It is now recognized that nociceptor function is altered by the ‘inflammatory soup’ that characterizes a region of tissue injury.5 The final pain experience is subject to a complex series of facilitatory and inhibitory events that precede pain awareness, such as past experience, anxiety or expectation.6 There are two types of nociceptors:7
Once transduced into electrical stimuli, conduction of neuronal action potentials is dependent on voltage-gated sodium channels.2 A number of chemicals are involved in the transmission of pain to the ascending pathways in the spinothalamic tract. These include substance P and calcitonin gene-related peptide, but many others have been identified.2,8,9 Opioid receptors are present in the dorsal horn, and it is thought that encephalins (endogenous opioid peptides) are neurotransmitters in the inhibitory interneurons.7
Phospholipids released from damaged cell membranes trigger a cascade of reactions, culminating in the production of prostaglandins that sensitize nociceptors to other inflammatory mediators, such as histamine, serotonin and bradykinin.7
The threshold for the perception of a painful stimulus is similar in everyone, and may be lowered by certain chemicals such as the mediators of inflammation. The discrete cognitive processes and pathways involved in the interpretation of painful stimuli remain a mystery. The cognitive and emotional reactions to a given painful stimulus are variable among individuals, and may be affected by culture, personality, past experiences and underlying emotional state.2,5,10 In addition, intense and ongoing stimuli further increase the excitability of dorsal horn neurons, leading to central sensitization.2 With increased excitability of central nociceptive neurons, the threshold for activation is reduced, and pain can occur in response to low intensity, previously non-painful stimuli known as allodynia.2 Pain is a complex, multidimensional, subjective phenomenon.10
Assessment of pain and pain scales
Pain scales have been developed because there are no accurate physiological or clinical signs to objectively measure pain. Three scales have become popular tools to quantify pain intensity:11,12 the visual analogue scale (VAS), the numeric rating scale and the verbal rating scale.
Visual analogue scale
The VAS usually consists of a 100-mm line with one end indicating ‘no pain’ and the other end indicating the ‘worst pain imaginable’. The patient simply indicates a point on the line that best indicates the amount of pain experienced. The minimum clinically significant change in patient pain severity measured with a 100-mm visual analogue scale is 13 mm.13 Studies of pain experience that report less than a 13 mm change in pain severity, although statistically significant, may have no clinical importance.13
Verbal rating scale
The use of pain scales has been restricted predominantly to research, where experimental pain is not associated with the strong emotional component of acute pain. In the clinical setting, anxiety, sleep disruption and illness burden are present.9 It is difficult to use a unidimensional pain scale to measure a multidimensional process. Using pain intensity alone will often fail to capture the many other qualities of pain and the overall pain experience. The best illustration of this problem is that the same pain stimulus can be applied to two different people with dramatically different pain scores and analgesic requirements.14 At best the use of pain scales is an indirect reflection of ‘real’ pain, with patient self-reporting still being the most reliable indicator of the existence and intensity of pain.15
Nevertheless, pain scales are simple and easy to use and are now routine in EDs, with some recommending that they should be a standard part of the triage process.4
General principles
Patients in pain should receive timely, effective and appropriate analgesia, titrated according to response.2 Thus, there is essentially no role for the intramuscular route for parenteral analgesia, which simply delays the onset of analgesia. The following points should be stressed:
Specific agents
Opioids
The term ‘opioid’ refers to all naturally occurring and synthetic drugs producing morphine-like effects. Morphine is the standard opioid agonist against which others are judged.16 These drugs are the most powerful agents available in the treatment of acute pain. A number of specific opioid receptors have been identified. They are responsible for a variety of effects, including analgesia, euphoria, respiratory depression and miosis (μ receptor); cough suppression, sedation (κ); dysphoria, hallucinations (σ); nausea and vomiting, and pruritus (δ).7 Opioids act on injured tissue to reduce inflammation in the dorsal horn to impede transmission of nociception, and supraspinally to activate inhibitory pathways that descend to the spinal segment.9
Unfortunately, many doctors use opioids inappropriately and there are particular concerns regarding the risks of respiratory depression and inducing iatrogenic addiction. Less than 1% of patients who receive opioids for pain develop respiratory depression.17 Tolerance to this side effect develops simultaneously with tolerance to the analgesic effect. If the opioid dose is increased so that at least half the pain is relieved, the chance of respiratory depression is small. Further, naloxone will reverse the effects of opioids. In relation to fears of addiction, large studies have shown that inducing this following opioid analgesia use is exceedingly rare.18
Side effects
All potent opioid analgesics have the potential to depress the level of consciousness, protective reflexes and vital functions. It is mandatory that these are closely monitored during and after administration.7 Specific side effects include:
Route of administration
Opioids may be administered by many routes, including oral, subcutaneous, intramuscular, intravenous, epidural, nebulized, intrapleural, intranasal, intra-articular and transdermal. All may have a role in a specific clinical situation.4 There is a good rationale for the use of the intravenous route in moderate-to-severe pain4 and titration of intravenous opioids remains the standard of care for acute severe pain.
Morphine
The standard intravenous morphine dose is 0.1–0.2 mg/kg or more with a duration of action of 2–3 h. This should be initiated as a loading dose of opioid to provide rapid initial pain relief aiming for an optimal balance between effective pain relief and minimal side effects. This means tailoring the approach to each individual patient. Thus, a young fit healthy man with renal colic may require an initial bolus of 0.1 mg/kg morphine, followed by further increments of 2.5–5 mg. Conversely, a frail elderly patient may only tolerate 1.0–2.5 mg morphine total to begin with. There may also be considerable inter-individual variation in response to analgesia. Procedural pain may require higher-dose opioid analgesia, which has been found to be well-tolerated and safe.19 Appropriate monitoring and resuscitation equipment should be available to maximize safety.
Rapid pain relief and titration to effect are obvious advantages. Intramuscular administration results in unreliable and variable absorption, and older routine practices such as prescribing ‘75 mg pethidine i.m.’ are deplorable and take no account of an individual’s requirements.7 Oral opioids tend to be underused in the ED, but are effective for all levels of pain.
Special considerations
Pethidine
Pethidine should be used with caution in patients with renal failure, as there is increased risk of central nervous system toxicity due to the toxic metabolite, norpethidine. Norpethidine causes tremor, twitching, agitation and convulsions.16 Also pethidine is contraindicated in patients receiving monoamine oxidase (MAO) inhibitors, as they interfere with pethidine metabolism, increasing the likelihood of toxicity.20 Finally, pethidine may trigger the serotonin syndrome if used concomitantly with selective serotonin re-uptake inhibitors (SSRIs). Pethidine has approximately one-eighth the potency of morphine and causes the same degree of bronchospasm and increased biliary pressure as morphine.2 Its use is declining and should continue to be discouraged in favour of other opioids.2
Fentanyl
Allergic reactions are extremely rare with opioids. Fentanyl does not release histamine, making it ideal for treating patients with reactive airways disease. There are advantages in using fentanyl for brief procedures in the ED because of its short half-life. The intravenous dose of fentanyl is 1–2 μg/kg or more with a duration of action of 30–60 min. High doses of fentanyl may produce muscular rigidity, which may be so severe as to make ventilation difficult, but which responds to naloxone or muscle relaxants. Intranasal fentanyl is an effective analgesic in the ED and in the pre-hospital setting.2
Codeine
Codeine is the most commonly used oral opioid prodrug. Unfortunately, up to 6–10% of the Caucasian population, 2% of Asians, and 1% of Arabs have poorly functional cytochrome P450 2D6 (CYP2D6), which may render codeine largely ineffective for analgesia in these patients, although some analgesic efficacy may occur via alternate cytochrome P450 pathways.
Prescribed alone in doses as high as 120 mg, codeine has been demonstrated to be no more effective than placebo in both the adult and geriatric populations, while causing increasing gastrointestinal side effects such as nausea, vomiting and constipation with increasing doses.4 It is frequently given in combination with paracetamol or aspirin.
Tramadol
Tramadol is a new opioid, with novel non-opioid properties.21 Its efficacy lies between codeine and morphine. It has a relative lack of serious side effects such as respiratory depression, and the potential for abuse and psychological dependence is low.21 Other side effects such as nausea, vomiting, dizziness and somnolence may be troublesome, and there is a risk of seizures.21,22 Thus, it should be avoided or used with caution in patients who are taking other drugs that reduce the seizure threshold such as tricyclic antidepressants and SSRIs. Also the concomitant administration of tramadol with monoamine oxidase inhibitors, or within 2 weeks of their withdrawal, is contraindicated.21
The role of tramadol in emergency medicine is yet to be defined. One review concluded that tramadol does not offer any particular benefits over existing analgesics for the majority of emergency pain relief situations,22 with oral doses having equivalent analgesic effects in mild-to-moderate severity acute pain compared with currently available analgesics.22 Intravenous tramadol is less effective than intravenous morphine.22
Non-opioid analgesics
Simple analgesics
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) are either non-selective cyclo-oxygenase (COX) inhibitors or selective inhibitors of COX-2 (COX-2 inhibitors). NSAIDs are effective analgesic agents for moderate pain, specifically when there is associated inflammation.4 As with opioids, there are multiple routes of administration available. Unfortunately, their use in acute severe pain is limited by the length of onset time of 20–30 min. There is no clear superiority of one agent over another.
There is up to a 30% incidence of upper gastrointestinal bleeding when NSAIDs are used for over 1–2 weeks. The risk of bleeding in the elderly for short (3–5 days) acute therapy appears to be minimal.4 NSAID use in pregnancy (especially late) is not recommended. Ibuprofen is considered the NSAID of choice in lactation.
NSAIDs have a spectrum of analgesic, anti-inflammatory and antipyretic effects and are effective analgesics in a variety of pain states.2 Unfortunately, significant contraindications and adverse effects limit the use of NSAIDs, many of these being regulated by COX-1.2 NSAIDs are useful analgesic adjuncts and hence NSAIDs are therefore integral components of multimodal analgesia.2 NSAID side effects are more common with long-term use. The main concerns are renal impairment, interference with platelet function, peptic ulceration and bronchospasm in individuals who have aspirin-exacerbated respiratory disease.2 In general, the risk and severity of NSAID-associated side effects is increased in elderly people.2
Ketorolac is a parenteral NSAID that is equipotent to opioids, with ketorolac and morphine equivalent in reducing pain. There is a benefit favouring ketorolac in terms of side effects, when ketorolac is titrated intravenously for isolated limb injuries.23,24 However, the utility of ketorolac in acute pain is limited due to a prolonged onset of action and a significant number of patients (25%) who exhibit little or no response.25 There is also benefit using ketorolac for acute renal colic.23,26 A combination of morphine and ketorolac offered pain relief superior to either drug alone and was associated with a decreased requirement for rescue analgesia in patients with renal colic.27 Rectal NSAIDs are an effective alternative to parenteral NSAIDs in the treatment of renal colic.
Paracetamol
Paracetamol is an effective analgesic for acute pain2 and has useful antipyretic activity.28 The addition of an NSAID further improves efficacy.2 Paracetamol inhibits prostaglandin synthetase in the hypothalamus, prevents release of spinal prostaglandin, and inhibits inducible nitric oxide synthesis in macrophages.28
Indications for paracetamol include mild pain, particularly of soft tissue and musculoskeletal origin, mild procedural pain, supplementation of opioids in the management of more severe pain allowing a reduction in opioid dosage, and as an alternative to aspirin.28 Paracetamol has no gastrointestinal side effects of note and may be prescribed safely in patients with peptic ulcer disease or gastritis.4 Aspirin has the risk of gastrointestinal side effects, such as ulceration and bleeding. It also has an antiplatelet effect, which lasts for the life of the platelet.
Paracetamol is rapidly absorbed with a peak concentration reached in 30–90 min.28 The recommended adult dose is 0.5–1 g every 4–6 h to a generally accepted maximum of 4 g per day.28 Paracetamol has a low adverse event profile and is an excellent analgesic, especially when used in adequate dose. Chronic use of paracetamol alone does not seem to cause analgesic nephropathy.28 It can be used safely in alcoholics and patients with liver metastases.28,29
Combination drugs
Non-opioid agents, e.g. paracetamol, NSAIDs and paracetamol/codeine combinations, are all useful analgesics for mild-to-moderate pain. A systematic review found that paracetamol–codeine combinations in single dose studies produce a slightly increased analgesic effect (5%) compared with paracetamol alone.30 However, none of the studies reviewed were based in the ED. In multidosage, paracetamol–codeine preparations have significantly increased side effects.30 However, other reports state that the combination of paracetamol 1000 mg plus codeine 60 mg has a number needed to treat of 2.2.2 NSAIDs have a higher rate of serious adverse effects.
Other analgesic agents
Nitrous oxide
Nitrous oxide is an inhalational analgesic and sedative which, in a 50% mixture with oxygen (Entonox®), has equivalent potency to 10 mg morphine in an adult.7 The Entonox® delivery system uses a preferential inhalational demand arrangement for self-administration, which requires an airtight fit between the mask/mouthpiece and face. As the patient holds the mask/mouthpiece their grip will relax if drowsiness occurs, the airtight seal will be lost and the gas flow stops, thereby avoiding overdosage.
Sumatriptan
Sumatriptan is a 5HT1 receptor agonist that is effective for the treatment of acute migraine in a high proportion of patients. The dose is 50 mg orally, or 6 mg subcutaneously if the patient is vomiting. Ideally, it should be taken at the onset of headache, but is still effective when the headache is established. In about one-third to one-half of patients the headache returns within 24 h, but is almost always responsive to a second tablet.31
Acute migraine headache requires a stepwise approach to the use of pharmacological agents. Moderate-to-severe migraine may warrant the use of specific antimigraine medications such as ergotamine or sumatriptan, unless contraindicated.2 The combination of aspirin (900 mg) and metoclopramide is as effective as sumatriptan in the treatment of migraine, is better tolerated and also cheaper.2 Intravenous prochlorperazine is more effective than metoclopramide or rectal prochlorperazine, although is unlicensed for this delivery mode.2 The use of opioids is not recommended.2
Ketamine
Ketamine is an N-methyl-D-aspartate (NMDA) antagonist. It is a unique anaesthetic that induces a state of dissociation between the cortical and limbic systems to produce a state of dissociative anaesthesia, with analgesia, amnesia, mild sedation and immobilization. It does not impair protective airway reflexes, and random or purposeful movements are frequently observed in patients after administration. Side effects include hypersalivation, vomiting, emergence reactions, nightmares, laryngospasm, hypertension, tachycardia and increased intracranial pressure.32,33
Unfortunately, there are many potential contraindications to ketamine use including upper or lower respiratory infection, procedures involving the posterior pharynx, cystic fibrosis, age younger than 3 months, head injury, increased intracranial pressure, acute glaucoma or globe penetration, uncontrolled hypertension, congestive cardiac failure, arterial aneurysm, acute intermittent porphyria and thyrotoxicosis.33 Despite this, ketamine is used increasingly in the EDs as part of procedural sedation (see Ch. 22.3). It is also an effective analgesic especially for opioid resistant pain.
Pain relief in pregnancy
Non-pharmacological treatment options should be considered where possible for pain management in pregnancy, because most drugs cross the placenta.2 Use of medications for pain in pregnancy should be guided by published recommendations.2 Paracetamol is regarded as the analgesic of choice.2 NSAIDs are used with caution in the last trimester of pregnancy and should be avoided after the 32nd week.2 The use of NSAIDs is associated with increased risk of miscarriage.2 Overall, the use of opioids to treat pain in pregnancy appears safe.2
Chronic pain
Chronic pain ‘commonly persists beyond the time of healing of an injury and frequently there may not be any clearly identifiable cause.’2 Patients with chronic pain attend the ED with exacerbations of their chronic pain. They are often taking multimodal therapies prescribed by a pain specialist. The main difference between acute and chronic pain is that in chronic pain central sensitization is the main underlying pathophysiology.34 It is important to avoid a judgemental attitude to these patients as there is a risk of overlooking serious pathology.
Co-analgesics in the setting of chronic pain, especially ketamine, are of particular value in those with poor opioid responsiveness.2 These patients appear to benefit from several days of a ketamine infusion. Other agents may be useful for neuropathic pain.
The other issue with chronic pain is to be aware of adjuvant therapies for decreasing the likelihood of chronic pain developing. For example, early management of acute zoster infection may reduce the incidence of post-herpetic neuralgia.2 Aciclovir given within 72 h of onset of the rash accelerates the resolution of pain and reduces the risk of post-herpetic neuralgia.2 Amitriptyline 25 mg daily in patients over 60 years for 90 days, started at the onset of acute zoster, reduces pain prevalence at 6 months post-zoster infection.35
The acute abdomen
Traditionally, it has been held that pain relief masks the clinical signs of pathology in the acute abdomen. However, evidence from randomized controlled trials clearly shows that the early administration of opioids in patients with an acute abdomen does not reduce the detection rate of serious pathology and may facilitate diagnosis. The effect of analgesia on physical signs cannot be used as a diagnostic test.36–38
Likely developments over the next 5–10 years
1 International Association for the study of pain. Pain terms: a list of definitions and notes on usage. Pain. 1979;6:249-252.
2 Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute pain management: Scientific evidence, 2nd edn. Canberra: Australian Government National Health and Medical Research Council, 2005.
3 Bonica J. Pain management in emergency medicine. Norwalk: Appleton & Lange, 1987.
4 Ducharme J. Emergency pain management: a Canadian Association of Emergency Physicians (CAEP) consensus document. Journal of Emergency Medicine. 1994;12:855-866.
5 Loeser JD, Melzack R. Pain: an overview. Lancet. 1999;353(9164):1607-1609.
6 Paris P, Uram M, Ginsburg M. Physiological mechanisms of pain. Norwalk: Appleton & Lange, 1987.
7 Nolan J, Baskett P. Analgesia and anaesthesia. Cambridge: Cambridge University Press, 1997.
8 Besson JM. The neurobiology of pain. Lancet. 1999;353(9164):1610-1615.
9 Carr DB, Goudas LC. Acute pain. Lancet. 1999;353(9169):2051-2058.
10 Turk D, Melzack R. The measurement of pain and the assessment of people experiencing pain. New York: Guildford Press, 1992.
11 Ho K, Spence J, Murphy MF. Review of pain-measurement tools. Annals of Emergency Medicine. 1996;27(4):427-432.
12 Turk DC, Okifuji A. Assessment of patients’ reporting of pain: an integrated perspective. Lancet. 1999;353(9166):1784-1788.
13 Todd KH, Funk KG, Funk JP, et al. Clinical significance of reported changes in pain severity. Annals of Emergency Medicine. 1996;27(4):485-489.
14 Fatovich D. The validity of pain scales in the emergency setting. Journal of Emergency Medicine. 1998;16:347.
15 Acute Pain Management Guideline Panel. Acute pain management: operative or medical procedures and trauma: clinical practice guideline, 1992. Washington DC
16 McQuay H. Opioids in pain management. Lancet. 1999;353(9171):2229-2232.
17 Miller R. Analgesics. New York: Wiley, 1976.
18 Porter J, Jick H. Addiction rare in patients treated with narcotics. New England Journal of Medicine. 1980;302(2):123.
19 Barsan WG, Tomassoni AJ, Seger D, et al. Safety assessment of high-dose narcotic analgesia for emergency department procedures. Annals of Emergency Medicine. 1993;22(9):1444-1449.
20 Meyer D, Halfin V. Toxicity secondary to meperidine in patients on monoamine oxidase inhibitors: a case report and critical review. Journal of Clinical Psychopharmacology. 1981;1(5):319-321.
21 Bamigade T, Langford R. The clinical use of tramadol hydrochloride. Pain Reviews. 1998;5:155-182.
22 Close BR. Tramadol: does it have a role in emergency medicine? Emergency Medicine Australasia. 2005;17(1):73-83.
23 Rainer TH, Jacobs P, Ng YC, et al. Cost effectiveness analysis of intravenous ketorolac and morphine for treating pain after limb injury: double blind randomised controlled trial. British Medical Journal. 2000;321(7271):1247-1251.
24 Jelinek GA. Ketorolac versus morphine for severe pain. Ketorolac is more effective, cheaper, and has fewer side effects. British Medical Journal. 2000;321(7271):1236-1237.
25 Catapano MS. The analgesic efficacy of ketorolac for acute pain. Journal of Emergency Medicine. 1996;14(1):67-75.
26 Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. British Medical Journal. 2004;328(7453):1401.
27 Safdar B, Degutis LC, Landry K, et al. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Annals of Emergency Medicine. 2006;48(2):173-181.
28 Therapeutic Guidelines Ltd. Therapeutic Guidelines: Analgesic. North Melbourne: Therapeutic Guidelines Ltd, 2002.
29 Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. American Journal of Therapeutics. 2000;7(2):123-134.
30 de Craen AJ, Di Giulio G, Lampe-Schoenmaeckers JE. Analgesic efficacy and safety of paracetamol-codeine combinations versus paracetamol alone: a systematic review. British Medical Journal. 1996;313(7053):321-325.
31 Goadsby P. Sumatriptan and migraine: breakthrough therapy. Current Therapeutics. 1992;33:11-18.
32 Terndrup T. Pain control, analgesia and sedation. St Louis: Mosby Year Book, 1992.
33 Green SM, Johnson NE. Ketamine sedation for pediatric procedures: Part 2, Review and implications. Annals of Emergency Medicine. 1990;19(9):1033-1046.
34 Siddall PJ, Cousins MJ. Persistent pain as a disease entity: implications for clinical management. Anesthesia and Analgesia. 2004;99(2):510-520.
35 Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. Journal of Pain Symptom Management. 1997;13(6):327-331.
36 Thomas SH, Silen W, Cheema F, et al. Effects of morphine analgesia on diagnostic accuracy in Emergency Department patients with abdominal pain: a prospective, randomized trial. Journal of the American College of Surgeon. 2003;196(1):18-31.
37 Attard AR, Corlett MJ, Kidner NJ, et al. Safety of early pain relief for acute abdominal pain. British Medical Journal. 1992;305(6853):554-556.
38 Zoltie N, Cust MP. Analgesia in the acute abdomen. Annals of the Royal College of Surgeons of England. 1986;68(4):209-210.
22.2 Local anaesthesia
Local anaesthesia
Local anaesthetic agents should always be considered for patients presenting to the emergency department (ED) with pain, either to supplement other analgesia or for definitive pain relief. This is particularly appropriate where the pain is quite localized, as in certain fractures and wounds. They may also be used topically mainly in children, and prior to arterial blood gas puncture and insertion of large intravenous cannulae, where contrary to popular perception, they do not increase the likelihood of failing.1,2
Pharmacology
Amino ester and amino amide local anaesthetics
Local anaesthetics are available in single or multidose vials, with or without dilute adrenaline at 1:200 000 (containing 5 μg adrenaline per millilitre) to prolong the duration of action. Antioxidants such as sodium bisulphite or metabisulphite are added to adrenaline-containing solutions and preservative such as methylparaben to multidose vials, and are implicated in some allergic reactions to the anaesthetics. True allergy to local anaesthetics is extremely rare when verified by progressive challenge testing, and is usually to the amino esters.3
The duration of action of local anaesthetics is related to the degree of protein binding, vasoactivity, concentration and possibly pH, although the addition of adrenaline is the most practical way to prolong their effect. Table 22.2.1 gives typical maximum safe doses and duration of action of commonly used agents. Solutions containing adrenaline should not be injected near end arteries, such as in the fingers, toes, nose or penis, even though surprisingly this well-established dogma is not supported by the literature. Normal blood flow is restored to the digit within 60–90 min of inadvertent injection of local anaesthesia with adrenaline (epinephrine) at standard commercial dilutions, without any evidence of harm.4
Table 22.2.1 Maximum recommended safe dose and duration of action of common local anaesthetics
| Drug | Dose (mg/kg)* | Duration (h) |
|---|---|---|
| Lignocaine | 3 | 0.5–1 |
| Lignocaine with adrenaline | 7 | 2–5 |
| Bupivacaine | 2 | 2–4 |
| Prilocaine | 6 | 0.5–1.5 |
Adverse effects
Systemic toxicity (Table 22.2.2)
Table 22.2.2 Features of systemic local anaesthetic toxicity (in order of increasing plasma levels)
| Circumoral tingling |
| Dizziness |
| Tinnitus |
| Visual disturbance |
| Muscular twitching |
| Confusion |
| Convulsions |
| Coma |
| Apnoea |
| Cardiovascular collapse (highest plasma levels) |
The management of systemic toxicity includes immediate cessation of the drug, airway maintenance, supplemental oxygen and incremental doses of an intravenous benzodiazepine such as midazolam 0.05–0.1 mg/kg for seizures. Major reactions may require endotracheal intubation, fluids, vasopressors and inotropic support. As reactions occur immediately or within minutes after local anaesthetic use, medical expertise, resuscitation equipment and monitoring facilities must always be available.
Topical agents
Some agents such as EMLATM (eutectic mixture of local anaesthetics including 2.5% lignocaine and 2.5% prilocaine) are used topically, particularly to decrease the pain of insertion of cannulae or for lumbar puncture and suprapubic catheter insertion in children. EMLATM takes up to one hour for maximal effect and paradoxically is a venoconstrictor making vessel puncture harder, thus a superior alternative for cannula insertion is 4% amethocaine (AnGelTM), which has a quicker onset and is a vasodilator.5 Likewise, a mixture of 1:1000 adrenaline, 4% lignocaine and 0.5% amethocaine with the acronym ALA (or known as LET in North America standing for lidocaine, epinephrine and tetracaine) may be used inside small wounds instead of, or to reduce the pain of, injecting local anaesthetic prior to closure, again in children or adolescents.
Specific nerve blocks
The following nerve blocks are contraindicated in uncooperative patients, those with local sepsis in the injection zone and in the rare patient with true local anaesthetic allergy. Care must be taken not to exceed the recommended maximum local anaesthetic doses (see Table 22.2.1), and monitoring facilities, resuscitation equipment and medical expertise must be available at all times.
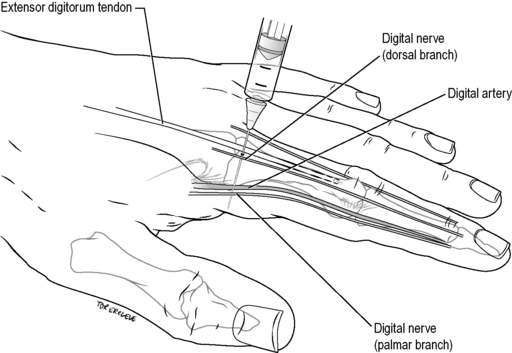
Digital nerve block (‘ring block’)
Technique
Use 2% plain lignocaine. Inject 1–1.5 mL using a 25-gauge needle into the palmar aspect of the base of the finger or toe, approaching vertically from the dorsum. Withdraw the needle until subcutaneous and rotate slightly until pointing to the extensor surface of the digit, and inject a further 0.5 mL (Fig. 22.2.1). Perform the same procedure on the other side of the digit. Allow at least 5 min for the block to work.
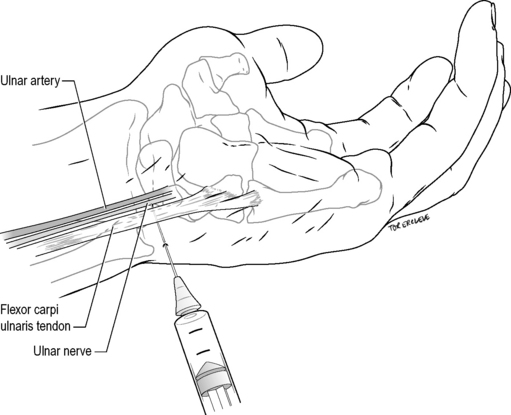
Ulnar nerve wrist block (lateral approach)
Technique
Identify the flexor carpi ulnaris tendon at the proximal palmar crease. Introduce a 25-gauge needle on the ulnar aspect of the tendon, directed horizontally and laterally for 1–1.5 cm under the tendon. Inject 4 mL of 1% lignocaine. Withdraw the needle until subcutaneous then inject 5 mL of 1% lignocaine fanwise to the dorsal midline, to block superficial cutaneous branches (Fig. 22.2.2).
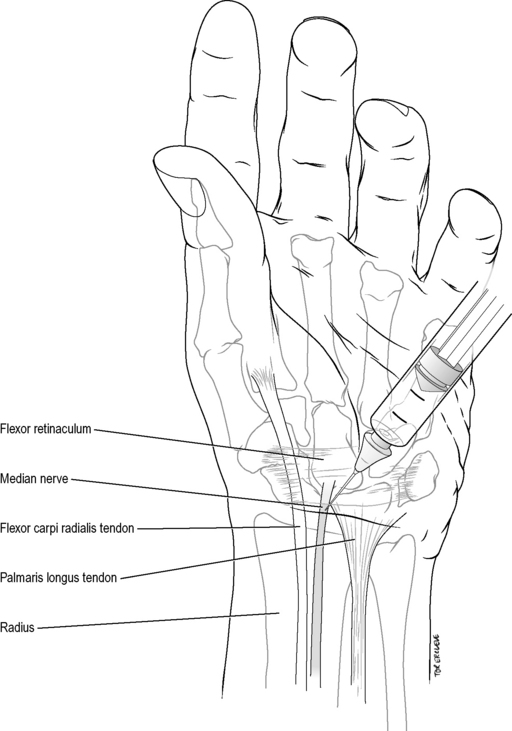
Median nerve wrist block
Technique
Identify the tendons of the flexor carpi radialis and palmaris longus at the proximal wrist crease. Introduce a 25-gauge needle vertically 0.5–1 cm lateral to the palmaris longus (or 0.5 cm medial to the flexor carpi radialis in the 10% of individuals lacking a palmaris longus). Inject 5 mL of 1% lignocaine when the needle gives as it penetrates the flexor retinaculum or paraesthesiae are elicited, at a depth usually of no more than 1 cm to the skin (Fig. 22.2.3). Avoid injecting into the nerve itself, as it may lie more superficial than this.
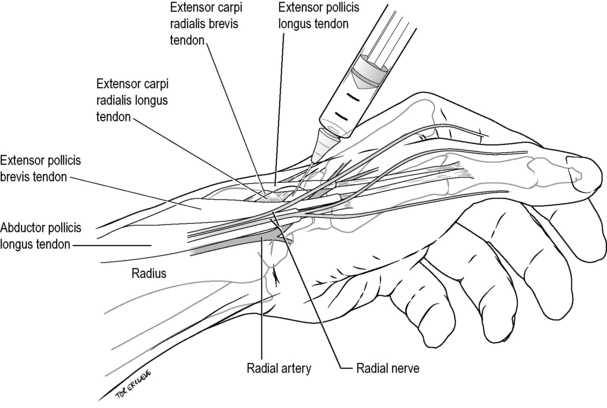
Radial nerve wrist block
Femoral nerve block
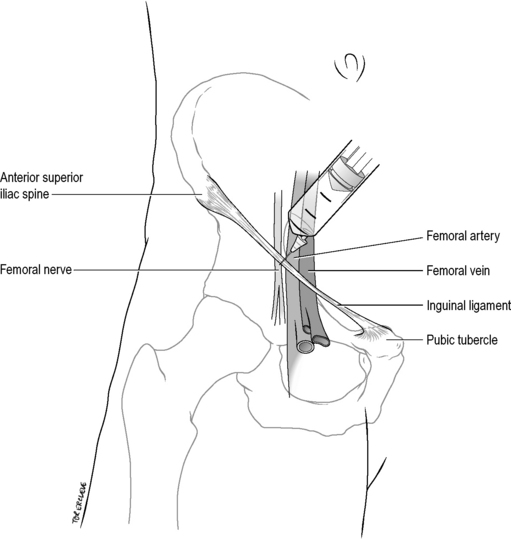
Technique
Palpate the femoral artery below the midpoint of the inguinal ligament, which extends from the pubic tubercle to the anterior superior iliac spine. Insert a 21-gauge needle 1 cm lateral to this point, perpendicular to the skin. Advance until paraesthesiae are elicited down the leg and withdraw slightly, aspirate to exclude intravascular placement, and inject 10 mL of 0.5% bupivacaine (50 mg). Alternatively, feel for a give as the needle punctures the fascia lata, aspirate, then inject 10 mL of 0.5% bupivacaine fanwise laterally away from the artery (Fig. 22.2.5). Allow up to 15–30 min for onset of maximal anaesthesia.
Foot blocks at the ankle
Technique
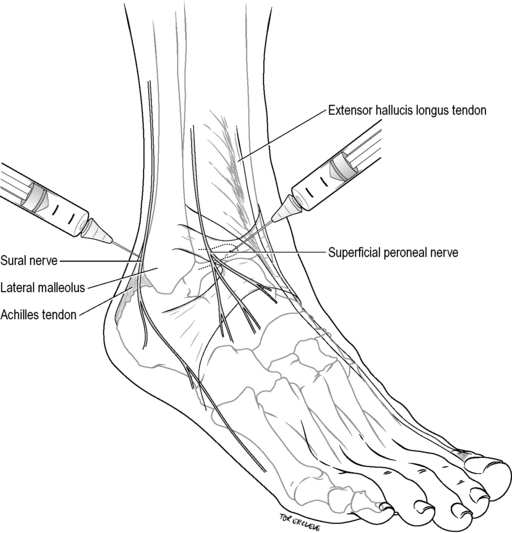
Sural nerve
The sural nerve is blocked by injecting 3–5 mL of 1% lignocaine subcutaneously in a band between the Achilles tendon and the lateral malleolus, 1 cm above and posterior to the malleolus (Fig. 22.2.6). It anaesthetizes a small strip on the lateral dorsum of the foot at the base of the little toe to the lateral malleolus, and the posterolateral aspect of the ankle and heel.
Superficial peroneal nerves
Superficial peroneal nerves are blocked by injecting 4–6 mL of 1% lignocaine subcutaneously in a band between the extensor hallucis longus tendon and the lateral malleolus, on the anterior aspect of the ankle (see Fig. 22.2.6). This block anaesthetizes the dorsum of the foot, save for the lateral aspect (see sural nerve above), and interdigital web between the hallux and second toe (see deep peroneal nerve below).
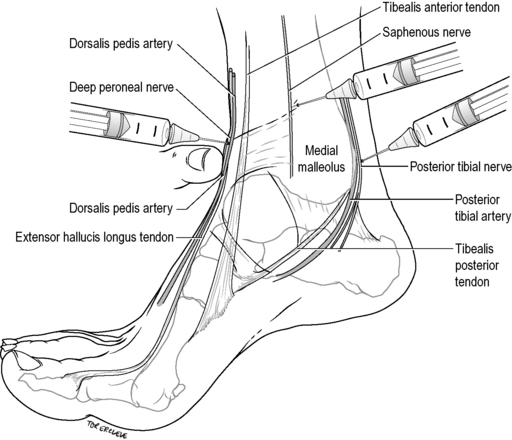
Saphenous nerve
The saphenous nerve is blocked by injecting 3–5 mL of 1% lignocaine subcutaneously above the medial malleolus, laterally until over the tibialis anterior tendon (Fig. 22.2.7). It anaesthetizes the area around the medial malleolus anteriorly and to a lesser degree posteriorly.
Posterior tibial nerve
The posterior tibial nerve is blocked by infiltrating 3–5 mL of 1% lignocaine immediately lateral to the posterior tibial artery as it passes behind the medial malleolus, at a depth of 0.5–1 cm to the skin (see Fig. 22.2.7). It anaesthetizes the sole of the foot, excluding the posterolateral heel (see sural nerve above), via its medial and lateral plantar branches.
Deep peroneal (anterior tibial) nerve
The deep peroneal (anterior tibial) nerve is blocked by infiltrating 1–2 mL of 1% lignocaine just above the base of the medial malleolus, lateral and behind the extensor hallucis longus by the dorsalis pedis pulse at a depth of 0.5 cm (see Fig. 22.2.7). It anaesthetizes the interdigital web between the hallux and second toe.
Intravenous regional anaesthesia or Bier’s block
Technique
Use a specifically designed and maintained single 15 cm adult cuff, placed over cottonwool padding to the upper arm.
Keep the arm elevated and inflate the cuff to 100 mmHg above systolic blood pressure. The radial artery pulse should now be absent and the veins remain empty. If this is not the case, do not inject anaesthetic but repeat the exsanguination procedure and cuff inflation.
Monitor the patient carefully for any signs of anaesthetic toxicity (see Table 22.2.2) over the next 15 min following cuff release, while organizing discharge from the monitored area.
1 Bates D, Cutting P. Local anaesthetic and arterial puncture. Emergency Medicine Journal. 2001;18:378.
2 Murphy R, Carley S. Prior injection of local anaesthetic and the pain and success of intravenous cannulation. Emergency Medicine Journal. 2000;17:406-408.
3 Fisher MM, Bowey CJ. Alleged allergy to local anaesthetics. Anaesthesia and Intensive Care. 1997;25:611-614.
4 Waterbrook A, Germann C, Southall J. Is epinephrine harmful when used with anesthetics for digital nerve blocks? Annals of Emergency Medicine. 2007;50:472-475.
5 Boyd R, Jacobs M. EMLA or amethocaine (tetracaine) for topical anaesthesia in children. Emergency Medical Journal. 2001;18:209-210.
6 Lowen R, Taylor J. Bier’s block the experience of Australian emergency departments. Medical Journal of Australia. 1994;60:108-111.
22.3 Procedural sedation and analgesia
Introduction and rationale
Procedural sedation and analgesia (PSA) is a core competency for the emergency physician, for the performance of brief, but painful procedures, and has become standard emergency medicine practice. PSA refers to the technique of administering sedatives or dissociative agents, with or without analgesics, to induce a state that allows the patient to tolerate unpleasant or anxiety-provoking procedures, while maintaining cardio-respiratory function.1,2
Paediatric patients in particular represent a significant challenge to the emergency physician; children are often frightened when in pain, and their presentation to the hospital disrupts the family’s functioning.3,4 Medical staff underestimate and under-treat pain in children.5 Procedures may have previously been performed with inadequate sedation or ‘oligo-analgesia’ for fear of complications, worry about prolonged recovery time or the perception that with the procedure being brief, the child will not remember it.
Painful procedures in the emergency department (ED) are remembered vividly by children, parents and adult patients. Denial of relief from pain that is proportionate to the expressed need for such relief is an unjustified harm and amounts to substandard and unethical medical practice.6
Underlying principles
Guidelines
The Australasian College for Emergency Medicine (ACEM),7 the American College of Emergency Physicians2 and the Canadian Association of Emergency Physicians8 have all published on the underlying principles for successful procedural sedation and analgesia within the ED. All guidelines cover pre-sedation preparation and assessment, pre-sedation fasting, physician skills, staffing, equipment and setting, patient monitoring, documentation and post-sedation care.
Although the Australasian guidelines were developed conjointly with the Australian and New Zealand College of Anaesthetists (ANZCA), the Joint Faculty of Intensive Care Medicine and Faculty of Pain Medicine, there is no specific recommendation as to choice of agent, despite the potentially confounding information published by ANZCA relating to the use of intravenous anaesthetic agents.9
Depth and duration of sedation
The optimal endpoint of any sedation episode depends on the procedure being performed and the patient’s characteristics. Sedation state classification is now well established ranging from minimal sedation (anxiolysis) through moderate sedation (formerly known as conscious sedation), to deep sedation and general anaesthesia. Dissociative sedation is a separate state induced by ketamine.10 The exact characteristics of respiratory and/or airway reflex depression in relation to the depth of sedation are not well defined.1
Titration of drugs and constant verbal and tactile reassessment of the patient reduce the risk of oversedation.2 Some degree of responsiveness to painful stimuli should indicate preservation of airway reflexes, decreasing the risk of aspiration if vomiting occurs.11
Indications and patient selection
Patient selection is based on the need for sedation for a brief, painful procedure that will usually facilitate early discharge from the ED. These include but are not limited to fracture and dislocation reduction, incision and drainage of abscesses, and cardioversion.12 Inherently less painful but anxiety-provoking procedures in children will also be facilitated by the use of dissociative sedation, for example lumbar puncture, suturing, ocular or auditory canal foreign body (FB) removal, or intravenous cannulation under extreme circumstances.10
Pre-procedure risk assessment
Age
A young patient’s level of anxiety and cooperation will depend upon past medical experiences, anxiety of the parents and the reassurance given by medical staff.10 Elderly patients, whilst mostly cooperative, may have underlying impairment of cardio-respiratory reserve, and are at greater risk of respiratory depression or hypotension.
ASA classification
The American Society of Anesthesiologists Classification (ASA) system13 is used to classify the anaesthetic risk of patients (Table 22.3.1). Patients in ASA Class I and ASA Class II are usually preferred as candidates for procedural sedation in the ED. If an ASA Class III patient requires sedation out of necessity, such as emergency cardioversion, this should not be precluded. The management of respiratory depression becomes a more active issue with increasing ASA class in all age groups.14,15
Table 22.3.1 American Society of Anesthesiologists (ASA) classification
| Class | |
|---|---|
| 1 | Healthy patient, no medical problems |
| 2 | Mild systemic disease, e.g. hypertension |
| 3 | Severe systemic disease, but is not incapacitating |
| 4 | Severe systemic disease that is a constant threat to life |
| 5 | Moribund expected to live <24h irrespective of operation |
Airway assessment
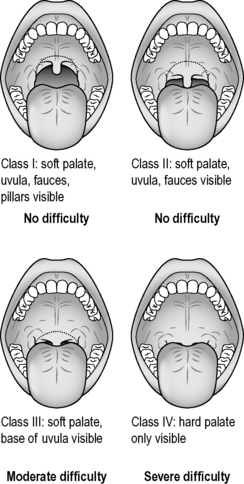
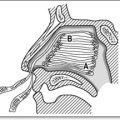
A focused airway assessment with attention to mouth opening, pharyngeal visualization using the Mallampatti score (Fig. 22.3.1), neck movement, thyromental distance and dentition or a known previous troublesome anaesthetic history may signal potential difficulty should active airway intervention be required. An airway assessment checklist predicting difficult endotracheal intubation, should this be needed during or following PSA, is found in Table 22.3.2.
Table 22.3.2 Airway assessment predictors of a difficult endotracheal intubation
| 1 | Mallampatti score III & IV |
| 2 | Inability to open mouth >4 cm |
| 3 | Thyromental distance <6 cm |
| 4 | Limitation of neck movement |
| 5 | Difficulty in protruding lower jaw |
| 6 | History of difficult intubation |
Mallampatti score – see Figure 22.3.1.
Past medical history
Some conditions predispose to gastro-oesophageal reflux such as pregnancy or hiatus hernia. Unstable acute medical or neurological conditions, with the exception of a cardiac arrhythmia requiring cardioversion, may carry too high a risk to proceed with PSA. Allergies to any agent in the past preclude use of that agent, as does an egg/soy allergy to the use of propofol in particular.16
Fasting status
Fasting guidelines
There are no clinical data to support the consensus view regarding prolonged fasting prior to sedation.13,17 In fact prolonged pre-procedural fasting has been shown to increase the rate of vomiting when using ketamine.18,19 The ASA recommends at least 2 h and 6 h from last intake of fluid and food, respectively,13 despite the lack of evidence regarding these conclusions.
Aspiration risk
The risk of aspiration is low with PSA. Fasting status is just one consideration when individualizing decisions about choice of agent, approach to dosing, desired depth of sedation or even formal referral to the operating theatre.11,20 PSA does not involve the use of volatile inhalational anaesthetics, which are particularly emetogenic, as occurs during general anaesthesia.21 In addition ED PSA does not involve pharyngeal manipulation or instrumentation, again a potent stimulus for inducing vomiting.
There is no association between fasting status and adverse events during procedural sedation in the ED for a range of agents including ketamine, midazolam/fentanyl, chloral hydrate, pentobarbital18,22,23 or nitrous oxide.16,24 The proportion of unfasted patients in those studies was 53–71%. Recent data looking specifically at patients undergoing PSA with propofol with respect to fasting status showed no difference in adverse events between fasted and unfasted patients.11 There is only one reported case of aspiration during PSA in the ED literature, which although it probably represents negative reporting bias, is still an extremely low figure.25
Procedural urgency1
The endpoint of sedation in the ED should be tailored to the urgency of procedure and availability of appropriate staff.1,8 Procedures may thus be considered:
Involvement of parents or carer
Parental cooperation is critical to the success of any procedural intervention in children. ED survey data show the vast majority of parents wish to be present for invasive procedures performed on their child in the ED, with a small drop as the invasiveness of the procedure increases, except for full cardiopulmonary resuscitation.27,28
Despite this, more than one-third of parents were asked to leave the room in one study of children undergoing procedures.29 This practice of requesting that parents leave the room when their child undergoes a procedure should be abandoned. Parental presence should be welcomed, but ultimately their decision to stay or go should be supported. PSA in children should include ushering the parents to the bedside, not out of the room, with full explanation to both parents and child what is happening and why.27,30
Documentation
Specific procedural sedation forms or records are recommended. When designed in accordance with current best practice they improve documentation, and may be the focus for educational initiatives and assist in audit, research and quality assurance (QA).31–33 They can also act as a de facto protocol to ensure safe care during procedural sedation. They increase the chances of compliance with guidelines and ensure essential pre-sedation checks and monitoring are performed. They should include provision for recording adverse events, including vomiting, aspiration or respiratory depression as well as any interventions required.11
Choice of agent
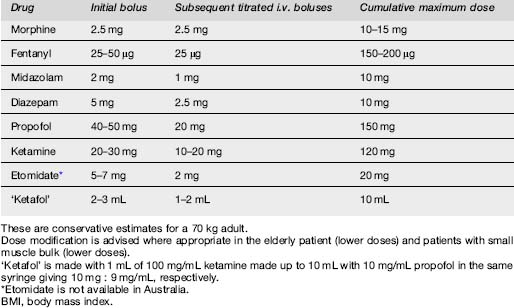
The ideal agent for PSA in the ED should have a profile of rapid onset, short duration of action, rapid recovery, minimal side effects and an amnestic effect. The different classes of drugs used alone or in combination in PSA include sedative hypnotics, analgesics, dissociative sedatives, inhalation agents, and antagonists such as flumazenil and naloxone (Table 22.3.3).
Sedative hypnotics
Benzodiazepines
Midazolam
Midazolam is one of the most commonly used benzodiazepines with amnestic,34 anxiolytic and sedative properties. Side effects are dose dependent. Intravenous dosing for PSA ranges from 0.1 mg/kg in younger children to 0.025–0.05 mg/kg in older children and adults. Other routes of administration include intramuscular and intranasal, although the onset of action is slower.10 Many experienced clinicians have abandoned the use of intranasal midazolam.
Midazolam/opioid combinations were perceived to provide more predictable response and to have a favourable safety profile when compared to propofol, owing to the greater potential with propofol to induce dose-related deep sedation.35 In fact, there is additive respiratory depression with opiate and midazolam-containing combinations for PSA, with prolonged recovery times when compared to propofol.36–38
Diazepam
Diazepam is less potent than midazolam, but there is little or no difference in the propensity of the two drugs to produce respiratory depression.39 Dosing should start at 0.1–0.2 mg/kg with smaller subsequent doses. The antegrade amnestic effect of diazepam is significantly less than that of midazolam.40,41 Diazepam also causes more pain on injection and a lesser degree of early sedation.42 The elimination half-lives of benzodiazepines do not necessarily correspond with their sedative pharmacodynamic effects, so there are no clinically important sedative recovery rate differences between midazolam and diazepam.43,44
Short acting agents
Propofol
Propofol is a non-opioid, non-barbiturate sedative hypnotic that acts at gamma amino-butyric acid (GABA) sites within the central nervous system, providing a rapid onset (<1 min) and short duration (5–15 min) of action facilitating rapid recovery times, with an amnestic effect. Propofol is easily titratable and has some antiemetic properties.17,45 Hypotension is transient when propofol is titrated in euvolaemic patients with normal cardiac function. Propofol has been shown to be safe, when used appropriately, in a wide range of settings including PSA in the ED.2,46,47
The optimum dosing regime for propofol in procedural sedation is yet to be defined. Options vary from single bolus,37,38,48 titration,15,49–52 bolus and infusion36,53 or infusion alone.54–56 Doses recommended include 1 mg/kg initial bolus and 0.5 mg/kg subsequent boluses for PSA in the ED.38,45,57,58 In children initial doses of 2 mg/kg initial bolus have been used.59,60 An alternative is to reduce the dose to 0.5–1.0 mg/kg initial bolus followed by 20 mg boluses.61 Dose reduction is essential in patients over 65 years of age.53 Higher total mg/kg doses are used in children compared with adults.61,62
Sedation times are shorter with propofol and reported respiratory complication rates for propofol are equivalent to midazolam alone,36 midazolam with or without flumazenil, etomidate,37 and midazolam plus fentanyl.38 At excessive doses propofol is associated with greater degrees of oxygen desaturation.48
Respiratory depression is seen in up to 50% of ASA Class 1 and Class 2 patients50,63,64 and 61% in the critically ill (Classes 4 and 5).14 Apnoea may occur but is transient. It may be seen in up to 22% of patients receiving propofol for PSA.11,37,57 Transient hypoxia occurs from 6 to 44% of sedation episodes.14,15,36,37,48–50,52–54,62–64 Supplemental oxygen was not routinely applied during PSA in all studies.49 The use of propofol becomes increasingly safe as familiarity and experience grow.
Etomidate
Etomidate is a non-barbituate hypnotic currently unavailable in Australia. It is often used for rapid sequence induction (RSI) of endotracheal intubation in the UK and USA, as well as for procedural sedation in the ED. It has a rapid onset <30 s with a duration of action of 5–15 min. The starting dose for PSA is up to 0.1 mg/kg with subsequent boluses of 0.05 mg/kg. It has a similar profile in terms of respiratory depression and duration of sedation to propofol, when compared with midazolam, but is more cardiovascularly stable. Propofol is preferred to etomidate as etomidate has a 20% rate of myoclonus, plus emergence phenomena, higher vomiting rates and a theoretical risk of adrenal suppression.65–68
Opiates
The use of opiates before, during and after procedural sedation is common. In addition opiates remain the mainstay of pain control in the ED (see Ch. 22.1). Opiates provide analgesia but have no amnestic or anxiolytic properties.
Dissociative sedative
Ketamine
Ketamine has worldwide use as a dissociative anaesthetic agent, particularly in military situations and third world anaesthesia.70 Ketamine produces a dose-related ‘dissociative anaesthesia’ state between deep sedation and general anaesthesia, by dissociating the thalamocortical and limbic systems. It has a rapid on and offset, with preservation of airway reflexes, although it may cause laryngospasm. As it causes an increase in sympathetic tone it is relatively contraindicated in ischaemic heart disease (IHD) and serious head injury, although conversely it finds favour in the hypotense patient.
Ketamine has become a popular drug in the ED given either intravenously or occasionally intramuscularly, particularly in paediatric procedural sedation. It is safe, with preservation of oropharyngeal reflexes and little or no respiratory depression.6,8,18,71–74 The usual initial intravenous dose is 0.5 mg/kg slowly.
There is some concern with ‘emergence delirium’3,75 also known as ‘emergence phenomena’.72–74,76,77 ‘Emergence delirium’ has been described as either ‘patients are agitated, restless, and combative, and do not seem cognizant of their surroundings. Patients refuse to be comforted, even by their parents,’78 or ‘combative, excited, and disoriented behaviour that requires transient physical restraint’.79 However, ‘emergence phenomena’ may be something as mild as non-distressing visual hallucinations, or transient diplopia.
Atropine reduces hypersalivation and post-procedure vomiting, used with titrated intravenous ketamine for paediatric procedural sedation in the ED.80 However, as hypersalivation per se rarely if ever affects the conduct of the procedure, its use is largely unnecessary.
Combinations utilizing ketamine
Midazolam and ketamine
Midazolam (or another benzodiazepine) has traditionally been used in combination with ketamine, in an effort to decrease the incidence of ‘emergence delirium’, supported by studies reporting lower rates of emergence phenomena/agitation in adults who have received both ketamine and a benzodiazepine, in contrast to ketamine alone. ‘Emergence phenomena’ are fewer in children anyway than in adults, and the rate of emergence reactions in children is not lowered by adding a benzodiazepine.81–84
In addition, recent randomized trials in children show unchanged rates of true emergence delirium with agitation when midazolam is added to ketamine.85–87 Midazolam use has been associated with higher rates of airway and respiratory compromise during procedural sedation in children.13 Midazolam as an adjunctive medication with ketamine for PSA does, however, have lower rates of post-procedural emesis.85,86
Overall the prophylactic use of adjunctive benzodiazepines with ketamine is not recommended. Furthermore midazolam as a sole sedative agent has been reported to have a rate of emergence agitation of up to 42%.88–90
Ketamine and propofol (‘Ketafol’)
The addition of ketamine to propofol (‘Ketafol’) has recently been described for procedural analgesia and sedation, and has been used safely in a number of non-ED settings.91 The combination aims to balance the opposing respiratory and haemodynamic effects of each drug. Additionally, the antiemetic effect of propofol may counteract the vomiting with ketamine, and may minimize the rate of ‘emergence’, although this is unproven.
In a single ED study the combination was safe and resulted in high staff and patient satisfaction. There are some advantages in the use of ketafol over propofol alone. Modest propofol dose reduction was seen, with a reduced respiratory depression profile. Recovery times remained short, and there were no adverse events that altered patient disposition.92 As ketamine is analgesic but not dissociative at low doses, targeted depth of sedation is important when using lower doses of propofol.
Prospective trials to compare this combination of ketafol with other agents alone are awaited, and to find the most beneficial ratio of ketamine to propofol balancing synergy with side effect profile.66 One suggested dilution is to make 1 mL of 100 mg/mL ketamine up to 10 mL with 9 mL of 10 mg/mL propofol in the same syringe, to give a dilution of 10 mg:9 mg/mL respectively.
Preparation and monitoring
Resuscitation area
PSA should always occur in a resuscitation area, with two qualified physician staff; one physician to perform the procedure and one physician to be responsible for the drugs and airway, both assisted by an ED nurse.7 Supplemental oxygen should be given for the majority of cases of PSA in the ED, with the exception of paediatric PSA with ketamine, when the use of supplemental oxygen by mask is unnecessarily upsetting for the child, prior to commencement of sedation.
Equipment and monitoring
Suction, oxygen, airway adjuncts and resuscitation equipment should be prepared and physiological monitoring applied. Monitoring should include pulse oximetry, non-invasive blood pressure, heart rate, ECG rhythm and respiratory rate. End-tidal carbon dioxide monitoring via nasal prongs is increasingly recommended.2,11 Intravenous access is mandatory in all cases. See Table 22.3.4 for essential equipment requirements.
Table 22.3.4 Essential equipment requirements for procedural sedation ‘SOAPMI’
| S | Suction equipment (connected and checked) |
Sedation scoring
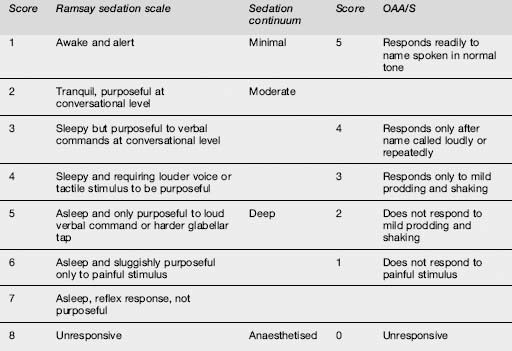
Monitoring is interactive as verbal and tactile stimulation are used to constantly reassess the depth of sedation. Careful dose titration and subjective evaluation of patient responsiveness throughout the procedure are paramount.35,46 The Ramsay Sedation Score,93 or the Motor Component of the Observer’s Assessment of Alertness/Sedation Scale (OAA/S)94 are examples of sedation scores that have been validated for midazolam use. All sedation scoring scales are subject to inter-observer variability and are relatively imprecise, and are not true objective measures of sedation. However, they require little formal training and may be easily incorporated into departmental protocols (Table 22.3.5).
Bispectral EEG analysis
Bispectral EEG analysis (BIS) is not reliably predictive of the conscious state in individual patients.66 Numerical values (0–100) are assigned to a patient’s level of sedation, but they are a poor measure of analgesia and ineffective when used with ketamine. Correlation with OAA/S95,96 and Ramsay Sedation Scores97,98 are poor. Lower BIS scores predict more respiratory depression, but it is unclear whether the use of BIS itself reduces the rate of respiratory depression.63,64,99
Capnography
Capnography detects respiratory depression before clinical examination or pulse oximetry.100–102 Changes in trace character or transient hypercapnia50,51,103 are the earliest warning signs of hypoventilation or impending upper airway obstruction, of particular importance in children or those with reduced respiratory reserve.57 Such early detection may avoid further sedation being given, or result in stimulating the patient or repositioning the airway. Only occasionally are airway adjuncts or bag valve mask ventilation required.11,62 ‘Waiting out’ respiratory depression for a brief time during propofol sedation in a well pre-oxygenated patient is common.58
Post-procedure considerations
Patients should be observed until they have returned to their baseline level of functioning.2,12,13 The exact time of this will depend on the patient, the drugs administered and the reason for the procedure. One study in children suggested a 30-min rule.104 There is no need following ketamine use to darken the room or shield a child from the routine background visual and auditory stimuli of a busy ED in an effort to reduce the likelihood of emergence delirium.
Patients receiving propofol do not need prolonged post-procedure monitoring as re-sedation following propofol use is rare.61 Once the patient can talk, nursing staff have an endpoint for the cessation of physiological monitoring, knowing that a patient is not likely to develop any adverse events after this point.
This stance is supported by a recent Clinical Practice Advisory statement from the USA.12 Nursing allocation can be tailored to these less intensive post-procedure monitoring requirements.105 Written discharge criteria and instructions though do need to be provided (Table 22.3.6).
Table 22.3.6 Recommended adult discharge criteria and instructions following procedural sedation
| 1 | Patient is alert and oriented, or has returned to pre-procedure state |
| 2 | Patient ambulates safely, or has returned to pre-procedure state |
| 3 | Patient is comfortable and has discharge analgesia arranged |
| 4 | Patient is discharged into care of a responsible adult |
| 5 | Driving or the like is banned for a minimum of 8 h |
| 6 | Alcohol or other central nervous system depressants are avoided for 12–24 h |
| 7 | Patients are warned about the potential for post-procedure pain, unsteadiness or dizziness. Seek medical attention if significant or disabling |
Likely developments in the next 5–10 years106
1 Green SM, Roback MG, Miner JR, et al. Fasting and emergency department procedural sedation and analgesia: a consensus-based clinical practice advisory. Annals of Emergency Medicine. 2007;49(4):454-461.
2 Godwin SA, Caro DA, Wolf SJ, et al. Clinical policy: procedural sedation and analgesia in the emergency department. Annals of Emergency Medicine. 2005;45(2):177-196.
3 Dean A. Paediatric sedation in Australasian Emergency Departments. Emergency Medicine (Frem). 1998;10:324-326.
4 Weisman S, Bernstein B, Schechter N. Consequences of inadequate analgesia during painful procedures in children. Archives of Pediatrics & Adolescent Medicine. 1998;152:147-149.
5 Wilson J, Pendleton J. Oligoanalgesia in the emergency department. American Journal of Emergency Medicine. 1989;7:620-623.
6 Walco G, Cassidy R, Schlechter N. Pain, hurt and harm – the ethics of pain control in infants and children. New England Journal of Medicine. 1994;331:541-544.
7 Statement on clinical principles for procedural sedation. Emergency Medicine. 2003;15(2):205-206.
8 Innes G, Murphy M, Nijssen-Jordan C, et al. Procedural sedation and analgesia in the emergency department. Canadian Consensus Guidelines. Journal of Emergency Medicine. 1999;17(1):145-156.
9 Anaesthetists AaNZCo. Guidelines on Conscious Sedation for Diagnostic. Interventional Medical and Surgical Procedures, 2005.
10 Green SM, Krauss B. Procedural sedation and analgesia in children. Lancet. 2006;367:766-780.
11 Bell A, Treston G, McNabb C, et al. Profiling adverse respiratory events and vomiting when using propofol for emergency department procedural sedation. Emergency Medicine Australasia. 2007;19:405-410.
12 Miner JR, Burton JH. Clinical practice advisory: emergency department procedural sedation with propofol. Annals of Emergency Medicine. 2007;50(2):182-187.
13 Practice guidelines for sedation and analgesia by non-anesthesiologists. Anaesthesiology. 2002;96(4):1004-1017.
14 Miner JR, Martel ML, Meyer M, et al. Procedural sedation of critically ill patients in the emergency department. Academic Emergency Medicine. 2005;12(2):124-128.
15 Guenther E, Pribble CG, Junkins EP, et al. Propofol sedation by emergency physicians for elective pediatric outpatient procedures. Annals of Emergency Medicine. 2003;42(6):783-791.
16 Hofer KN, McCarthy MW, Buck ML, et al. Possible anaphylaxis after propofol in a child with food allergy. Annals of Pharmacotherapy. 2003;37(3):398-401.
17 Bahn EL, Holt KR. Procedural sedation and analgesia: a review and new concepts. Emergency Medicine Clinics of North America. 2005;23(2):503-517.
18 Treston G. Prolonged pre-procedure fasting time is unnecessary when using titrated intravenous ketamine for paediatric procedural sedation. Emergency Medicine Australasia. 2004;16(2):145-150.
19 Green SM, Johnson NE. Ketamine sedation for pediatric procedures: Part 2, Review and implications. Annals of Emergency Medicine. 1990;19(9):1033-1046.
20 Green SM. Fasting is a consideration – not a necessity – for emergency department procedural sedation and analgesia. Annals of Emergency Medicine. 2003;42(5):647-650.
21 Green SM, Krauss B. Pulmonary aspiration risk during emergency department procedural sedation – an examination of the role of fasting and sedation depth. Academic Emergency Medicine. 2002;9(1):35-42.
22 Agrawal D, Manzi SF, Gupta R, et al. Preprocedural fasting state and adverse events in children undergoing procedural sedation and analgesia in a pediatric emergency department. Annals of Emergency Medicine. 2003;42(5):636-646.
23 Roback MG, Bajaj L, Wathen JE, et al. Preprocedural fasting and adverse events in procedural sedation and analgesia in a pediatric emergency department: are they related? Annals of Emergency Medicine. 2004;44(5):454-459.
24 Babl FE, Puspitadewi A, Barnett P. Preprocedural fasting state and adverse events in children receiving nitrous oxide for procedural sedation and analgesia. Pediatric Emergency Care. 2005;21(11):736-743.
25 Cheung KW, Watson ML, Field S, et al. Aspiration pneumonitis requiring intubation after procedural sedation and analgesia: a case report. Annals of Emergency Medicine. 2007;49(4):462-464.
26 Roback MG, Wathen JE, Bajaj L, et al. Adverse events associated with procedural sedation and analgesia in a pediatric emergency department: a comparison of common parenteral drugs. Academic Emergency Medicine. 2005;12(6):508-513.
27 Isoardi J, Slabbert N, Treston G. Witnessing invasive paediatric procedures, including resuscitation, in the emergency department: a parental perspective. Emergency Medicine Australasia. 2005;217(3):244-248.
28 Bauchner H, Vinci R, Waring C. Pediatric procedures: do parents want to watch? Pediatrics. 1989;84:907-909.
29 Bauchner H, Waring C, Vinci R. Parental presence during procedures in an emergency room: results from 50 observations. Pediatrics. 1991;87:544-548.
30 Ross D, Ross S. Childhood pain: the school-aged child’s viewpoint. Pain. 1984;20:179-191.
31 Nicol MF. A risk management audit: are we complying with the national guidelines for sedation by non-anaesthetists? Journal of Accident and Emergency Medicine. 1999;16(2):120-122.
32 Law A, Babl F, Priestley D, et al. Pre and post-implementation evaluation of a comprehensive procedural program for children in the emergency department. Journal of Paediatric Child Health. (Supplement 8):2005.
33 Swoboda TK, Munyak J. Use of a sedation-analgesia datasheet in closed shoulder reductions. Journal of Emergency Medicine. 2005;29(2):129-135.
34 Macken E, Gevers AM, Hendrickx A, et al. Midazolam versus diazepam in lipid emulsion as conscious sedation for colonoscopy with or without reversal of sedation with flumazenil. Gastrointestinal Endoscopy. 1998;47(1):57-61.
35 Green SM. Propofol for emergency department procedural sedation – not yet ready for prime time. Academic Emergency Medicine. 1999;6(10):975-978.
36 Havel CJJr., Strait RT, Hennes H. A clinical trial of propofol vs midazolam for procedural sedation in a pediatric emergency department. Academic Emergency Medicine. 1999;6(10):989-997.
37 Coll-Vinent B, Sala X, Fernandez C, et al. Sedation for cardioversion in the emergency department: analysis of effectiveness in four protocols. Annals of Emergency Medicine. 2003;42(6):767-772.
38 Taylor D, O’Brien D, Ritchie P. Propofol versus midazolam/fentanyl for reduction of anterior shoulder dislocation. Academic Emergency Medicine. 2005;12:13-19.
39 Bell GD. Review article: premedication and intravenous sedation for upper gastrointestinal endoscopy. Alimentary Pharmacology and Therapeutics. 1990;4(2):103-122.
40 Tolia V, Fleming SL, Kauffman RE. Randomized, double-blind trial of midazolam and diazepam for endoscopic sedation in children. Developmental Pharmacology and Therapeutics. 1990;14(3):141-147.
41 Sanders LD, Davies-Evans J, Rosen M, et al. Comparison of diazepam with midazolam as i.v. sedation for outpatient gastroscopy. British Journal of Anaesthesia. 1989;63(6):726-731.
42 Wright SW, Chudnofsky CR, Dronen SC, et al. Comparison of midazolam and diazepam for conscious sedation in the emergency department. Annals of Emergency Medicine. 1993;22(2):201-205.
43 Ariano RE, Kassum DA, Aronson KJ. Comparison of sedative recovery time after midazolam versus diazepam administration. Critical Care Medicine. 1994;22(9):1492-1496.
44 Mitchell AR, Chalil S, Boodhoo L. Diazepam or midazolam for external DC cardioversion (the DORM Study). Europace. 2003;5(4):391-395.
45 Symington L, Thakore S. A review of the use of propofol for procedural sedation in the emergency department. Emergency Medicine Journal. 2006;23(2):89-93.
46 Ducharme J. Propofol in the emergency department: another interpretation of the evidence. Journal of Canadian Association Emergency Physicians. 2001;3:311-312.
47 Jackson R, Carley S. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Use of propofol for sedation in the emergency department. Emergency Medicine Journal. 2001;18(5):378-379.
48 Godambe SA, Elliot V, Matheny D, et al. Comparison of propofol/fentanyl versus ketamine/midazolam for brief orthopedic procedural sedation in a pediatric emergency department. Pediatrics. 2003;112(1 Pt 1):116-123.
49 Skokan EG, Pribble C, Bassett KE. Use of propofol sedation in a pediatric emergency department: a prospective study. Clinical Pediatrics (Phila). 2001;40(12):663-671.
50 Miner JR, Biros M, Krieg S, et al. Randomized clinical trial of propofol versus methohexital for procedural sedation during fracture and dislocation reduction in the emergency department. Academic Emergency Medicine. 2003;10(9):931-937.
51 Miner JR, Heegaard W, Plummer D. End-tidal carbon dioxide monitoring during procedural sedation. Academic Emergency Medicine. 2002;9(4):275-280.
52 Bassett KE, Anderson JL, Pribble CG, et al. Propofol for procedural sedation in children in the emergency department. Annals of Emergency Medicine. 2003;42(6):773-782.
53 Frazee BW, Park RS, Lowery D. Propofol for deep procedural sedation in the ED. American Journal of Emergency Medicine. 2005;23(2):190-195.
54 Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Academic Emergency Medicine. 1996;3(3):234-238.
55 Pershad J, Godambe SA. Propofol for procedural sedation in the pediatric emergency department. Journal of Emergency Medicine. 2004;27(1):11-14.
56 Frank LR, Strote J, Hauff SR, et al. Propofol by infusion protocol for ED procedural sedation. American Journal of Emergency Medicine. 2006;24(5):599-602.
57 Green SM, Krauss B. Propofol in emergency medicine: pushing the sedation frontier. Annals of Emergency Medicine. 2003;42(6):792-797.
58 Krauss B, Green SM. Procedural sedation and analgesia in children. 2006;367(9512):766-780.
59 Sacchetti A, Cravero J. Sedation in the emergency department. Pediatric Annals. 2005;34(8):617-622.
60 Barnett P. Propofol for pediatric sedation. Pediatric Emergency Care. 2005;21:111-114.
61 Bell A, Treston G, Cardwell R, et al. Optimisation of propofol dose shortens procedural sedation time, prevents re-sedation and removes the requirement for post procedure physiologic monitoring. Emergency Medicine Australasia. 2007;19:411-417.
62 Burton JH, Miner JR, Shipley ER, et al. Propofol for emergency department procedural sedation and analgesia: a tale of three centers. Academic Emergency Medicine. 2006;13(1):24-30.
63 Miner JR, Biros MH, Heegaard W, et al. Bispectral electroencephalographic analysis of patients undergoing procedural sedation in the emergency department. Academic Emergency Medicine. 2003;10(6):638-643.
64 Miner JR, Biros MH, Seigel T, et al. The utility of the bispectral index in procedural sedation with propofol in the emergency department. Academic Emergency Medicine. 2005;12(3):190-196.
65 Falk J, Zed PJ. Etomidate for procedural sedation in the emergency department. Annals of Pharmacotherapy. 2004;38(7–8):1272-1277.
66 Green SM. Research advances in procedural sedation and analgesia. Annals of Emergency Medicine. 2007;49(1):31-36.
67 Van Kuelen S, Burton J. Myoclonus associated with etomidate for ED procedural sedation and analgesia. American Journal of Emergency Medicine. 2003;21:556-559.
68 Miner JR, Danahy M, Moch A, et al. Randomized clinical trial of etomidate versus propofol for procedural sedation in the emergency department. Annals of Emergency Medicine. 2007;49(1):15-22.
69 Dunn MJ, Mitchell R, Souza CD, et al. Evaluation of propofol and remifentanil for intravenous sedation for reducing shoulder dislocations in the emergency department. Emergency Medicine Journal. 2006;23(1):57-58.
70 Guldner GT, Petinaux B, Clemens P, et al. Ketamine for procedural sedation and analgesia by nonanesthesiologists in the field: a review for military health care providers. Military Medicine. 2006;171(6):484-490.
71 Green SM, Rothrock SG, Lynch EL, et al. Intramuscular ketamine for pediatric sedation in the emergency department: safety profile in 1,022 cases. Annals of Emergency Medicine. 1998;31(6):688-697.
72 Dachs RJ, Innes GM. Intravenous ketamine sedation of pediatric patients in the emergency department. Annals of Emergency Medicine. 1997;29(1):146-150.
73 McCarty EC, Mencio GA, Walker LA. Ketamine sedation for the reduction of children’s fractures in the emergency department. Journal of Bone and Joint Surgery – American volume. 2000;82-A(7):912-918.
74 Howes MC. Ketamine for paediatric sedation/analgesia in the emergency department. Emergency Medicine Journal. 2004;21(3):275-280.
75 Everitt I, Younge P, Barnett P. Paediatric sedation in emergency department: what is our practice? Emergency Medicine (Fremantle). 2002;14(1):62-66.
76 Green SM, Kuppermann N, Rothrock SG. Predictors of adverse events with intramuscular ketamine sedation in children. Annals of Emergency Medicine. 2000;35(1):35-42.
77 Ducharme J. Ketamine: Do what is right for the patient. Emergency Medicine. 2001;13:7-8. (Freemantle)
78 Wells L, Rasch D. Emergence ‘delirium’ after sevoflurane anesthesia: a paranoid delusion? Anesthesia & Analgesia. 1999;88:1308-1310.
79 Uezono S, Goto T, Terui K, et al. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anaesthesia & Analgesia. 2000;91(3):563-566.
80 Heinz P, Geelhoed GC, Wee C, et al. Is atropine needed with ketamine sedation? A prospective, randomised, double blind study. Emergency Medicine Journal. 2006;23(3):206-209.
81 Bovill J, Coppel D, Dundee J. Current status of ketamine anaesthesia. Lancet. 1971;297:1285-1288.
82 Coppel D, Bovill J, Dundee J. The taming of ketamine. Anaesthesia. 1973;28:293-296.
83 Cartwright P, Pingel S. Midazolam and diazepam in ketamine anaesthesia. Anaesthesia. 1984;39:439-442.
84 White P, Way W, Trevor A. Ketamine – its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119-136.
85 Wathen JE, Roback MG, Mackenzie T, et al. Does midazolam alter the clinical effects of intravenous ketamine sedation in children? A double-blind, randomized, controlled, emergency department trial. Annals of Emergency Medicine. 2000;36(6):579-588.
86 Clinical policy for procedural sedation and analgesia in the emergency department. American College of Emergency Physicians. Annals of Emergency Medicine. 1998;31(5):663-677.
87 Sherwin TS, Green SM, Khan A, et al. Does adjunctive midazolam reduce recovery agitation after ketamine sedation for pediatric procedures? A randomized, double-blind, placebo-controlled trial. Annals of Emergency Medicine. 2000;35(3):229-238.
88 Roelofse J, Joubert J, Roelofse G. A double blind randomised comparison of midazolam alone and midazolam combined with ketamine for sedation of pediatric dental patients. Journal of Oral Maxillofacial Surgery. 1996;54:838-844.
89 Davies FC, Waters M. Oral midazolam for conscious sedation of children during minor procedures. Journal of Accident and Emergency Medicine. 1998;15(4):244-248.
90 Massanari M, Novitsky J, Reinstein L. Paradoxical reactions in children associated with midazolam use during endoscopy. Clinical Pediatrics. 1997;36:681-684.
91 Loh G, Dalen D. Low-dose ketamine in addition to propofol for procedural sedation and analgesia in the emergency department. Annals of Pharmacotherapy. 2007;41(3):485-492.
92 Willman EV, Andolfatto G. A prospective evaluation of ‘ketofol’ (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Annals of Emergency Medicine. 2007;49(1):23-30.
93 Habibi S, Coursin D. Assessment of sedation, analgesia, and neuromuscular blockade in the perioperative period. International Anesthesiology Clinics. 1996;34:215-241.
94 Chernik D, Gillings D, Laine H, et al. Validity and reliability of the observer’s assessment of alertness/sedation scale: study with intravenous midazolam. Journal of Clinical Psychopharmacology. 1990;10:244-251.
95 Fatovich DM, Gope M, Paech MJ. A pilot trial of BIS monitoring for procedural sedation in the emergency department. Emergency Medicine Australasia. 2004;16(2):103-107.
96 Overly FL, Wright RO, Connor FA, et al. Bispectral analysis during pediatric procedural sedation. Pediatric Emergency Care. 2005;21(1):6-11.
97 Gill M, Green SM, Krauss B. A study of the bispectral index monitor during procedural sedation and analgesia in the emergency department. Annals of Emergency Medicine. 2003;41(2):234-241.
98 Agrawal D, Feldman HA, Krauss B, et al. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Annals of Emergency Medicine. 2004;43(2):247-255.
99 American Society of Anesthesiologists Task Force on Intraoperative Awareness. Practice Advisory for Intraoperative Awareness and brain function monitoring. Anesthesiology. 2006;104:847-864.
100 Burton JH, Harrah JD, Germann CA. Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices? Academic Emergency Medicine. 2006;13(5):500-504.
101 Anderson JL, Junkins E, Pribble C, et al. Capnography and depth of sedation during propofol sedation in children. Annals of Emergency Medicine. 2007;49(1):9-13.
102 Deitch K, Chudnofsky CR, Dominici P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: a randomized, controlled trial. Annals of Emergency Medicine. 2007;49(1):1-8.
103 McQuillen KK, Steele DW. Capnography during sedation/analgesia in the pediatric emergency department. Pediatric Emergency Care. 2000;16(6):401-404.
104 Newman DH, Azer MM, Pitetti RD, et al. When is a patient safe for discharge after procedural sedation? The timing of adverse effect events in 1367 pediatric procedural sedations. Annals of Emergency Medicine. 2003;42(5):627-635.
105 Holger J, Satterlee P, Haugen S. Nursing use between 2 methods of procedural sedation: midazolam versus propofol. American Journal of Emergency Medicine. 2005;23:248-252.
106 Miner JR, Krauss B. Procedural sedation and analgesia research: state of the art. Academic Emergency Medicine. 2007;14(2):170-178.
107 MacLean S, Obispo J, Young KD. The gap between pediatric emergency department procedural pain management treatments available and actual practice. Pediatric Emergency Care. 2007;23(2):87-93.
108 Paris PM, Yealy DM. A procedural sedation and analgesia fasting consensus advisory: one small step for emergency medicine, one giant challenge remaining. Annals of Emergency Medicine. 2007;49(4):465-467.
109 Cote CJ, Notterman DA, Karl HW, et al. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105(4 Pt 1):805-814.
110 Priestley S, Babl FE, Krieser D, et al. Evaluation of the impact of a paediatric procedural sedation credentialing programme on quality of care. Emergency Medicine Australasia. 2006;18(5–6):498-504.
111 Babl F, Priestley S, Krieser D, et al. Development and implementation of an education and credentialing programme to provide safe paediatric procedural sedation in emergency departments. Emergency Medicine Australasia. 2006;18(5–6):489-497.