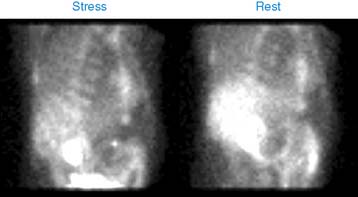
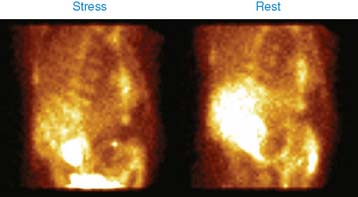
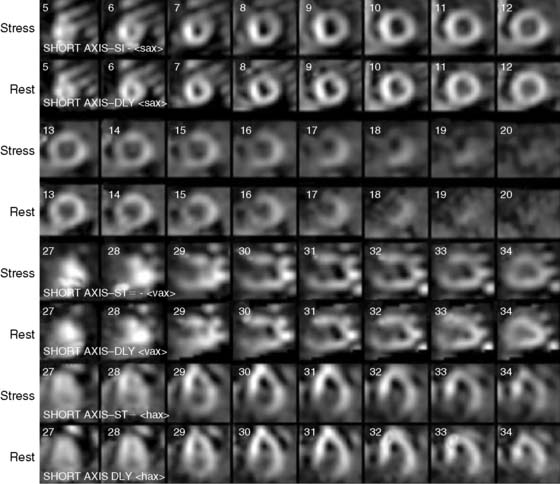
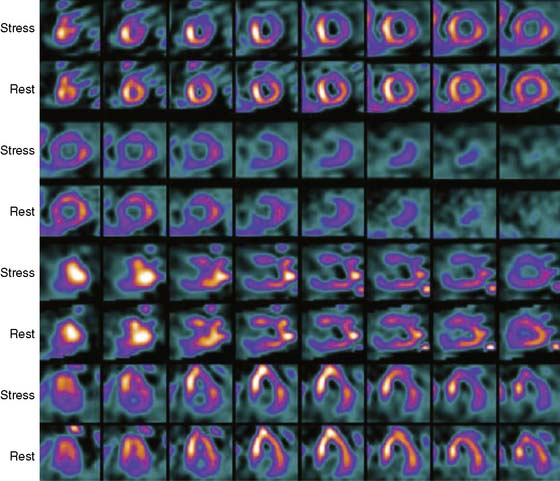
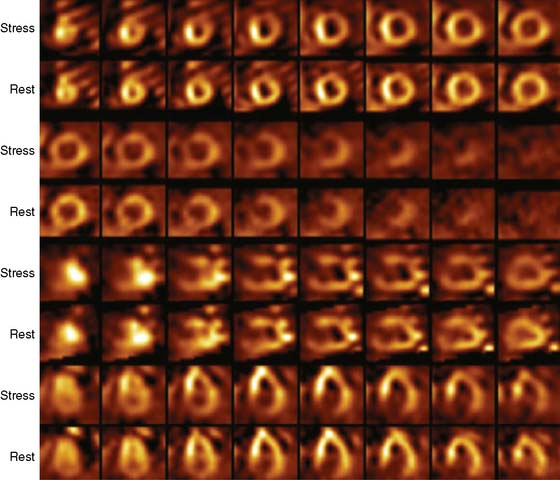
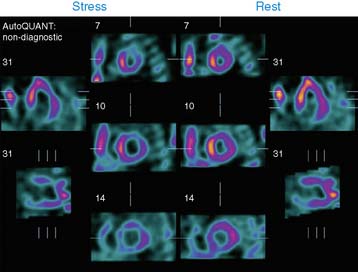
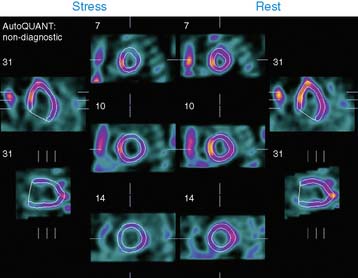
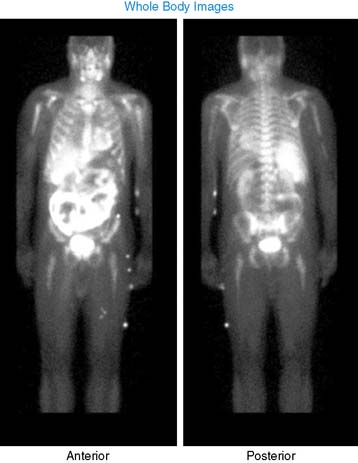
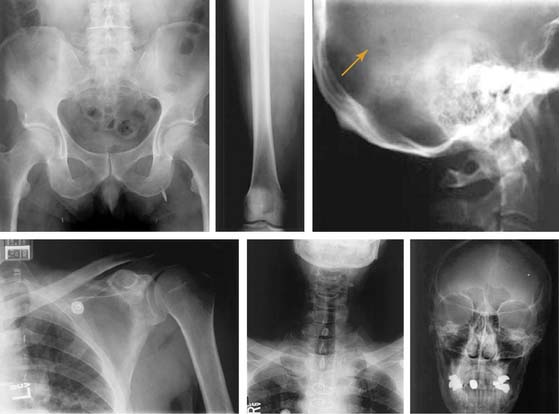
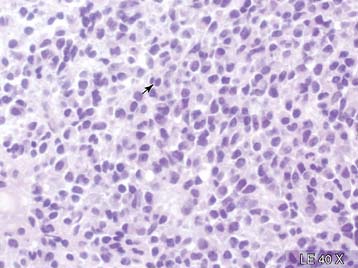
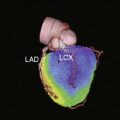
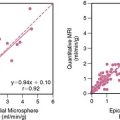
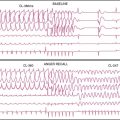
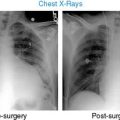
Case 22
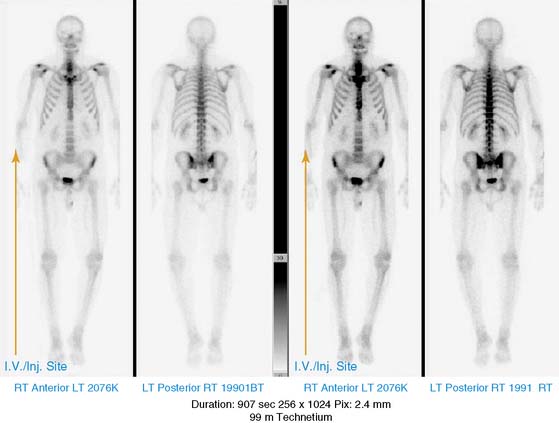
He had a whole-body bone scan, which was negative.
1. Gowda A., Peddington L., Shandilya V., Gavriluke A., Jain D. Abnormal intense skeletal radiotracer uptake on myocardial perfusion imaging with Tc-99m sestamibi. J Nucl Cardiol. 2006;13:427-431.
2. Fonti R., Del Vecchio S., Salvatore M. Bone marrow uptake of 99mTc-MIBI in patients with multiple myeloma. Eur J Nucl Med. 2001;28:214-220.
3. Pace L., Catalano L., Salvatore M. Different patterns of technetium-99m sestamibi uptake in multiple myeloma. Eur J Nucl Med. 1998;25:714-720.
4. Lette J., Cerino M., Demaria S., et al. Serendipitous diagnosis of multiple myeloma during sestamibi myocardial perfusion imaging. Clin Nucl Med. 2002;27:832-833.
5. Fisher C., Vehec A., Kashlan B., et al. Incidental detection of skeletal uptake on sestamibi cardiac images in a patient with previously undiagnosed multiple myeloma. Clin Nucl Med. 2000;25:213-214.
6. Yi A., Jacobs M. Skeletal tetrofosmin uptake in a patient undergoing myocardial perfusion imaging with subsequent diagnosis of multiple myeloma. Clin Nucl Med. 2004;29:327-328.
7. Schomäcker K., Schicha H. Use of myocardial imaging agents for tumour diagnosis—a success story? Eur J Nucl Med. 2000;27:1845-1863.