Hormones and Steroids
Objectives
1. Describe the use of antidiabetic medications.
2. Identify preparations that act on the uterus.
3. Compare and contrast the action of adrenal and pituitary hormones.
5. Compare the actions of various male and female hormones.
6. List the indications for the use of thyroid preparations.
Key Terms
abortifacients (ă-BŎR-tĭ-FĀ-shĕnts, p. 375)
androgens (ĂN-drō-jĕnz, p. 383)
corticosteroids (KŎR-tĭ-kōSTĔR-ŏydz, p. 378)
diabetes mellitus (dī-ă-BĒ-tēz mĕ-LĪ-tĭs, p. 366)
estrogen (ĔS-trō-jĕn, p. 383)
glucometer (GLŪ-kŏ-mēt-ĕr, p. 369)
hormones (HŎR-mōnz, p. 364)
hyperglycemia (hī-pĕr-glī-SĒ-mē-ă, p. 368)
hyperthyroidism (hī-pōr-THĪ-rŏyd-ĭzm, p. 393)
hypoglycemia (hī-pō-glī-SĒ-mē-ă, p. 368)
hypothyroidism (hī-pō-THĪ-rŏyd-ĭzm, p. 393)
incretins (ĭn-krētĭns, p. 367)
insulin (ĬN-sū-lĭn, p. 366)
insulin-dependent diabetes mellitus (IDDM) (dī-ă-BĒ-tēz mĕl-Ī-tĭs, p. 366)
lipodystrophy (lĭp-ō-DĬS-trō-fē, p. 367)
myxedema (mĭk-sĕ-DĒ-mă, p. 394)
non–insulin-dependent diabetes mellitus (NIDDM) (dī-ă-BĒ-tēz mĕl-Ī-tĭs, p.367)
oral hypoglycemic (hī-pō-glī-SĒM-ĭks, p. 373)
oxytocic agents (ŏk-sē-TŌ-sĭk, p. 375)
progesterone (prō-JĔS-tĕr-ōn, p. 383)
sex hormones (HŎR-mōnz, p. 378)
Somogyi effect (SŌM-ō-jē, p. 372)
steroids (STĔR-ŏydz, p. 364)
systemic acidosis (sĭs-TĔM-ĭk ăs-ĭ-DŌ-sĭs, p. 368)
tocolytics (tō-kō-LĬT-ĭks, p. 375)
type 1 diabetes (dī-ă-BĒ-tēz, p. 366)
type 2 diabetes (dī-ă-BĒ-tēz, p. 366)
uterine relaxants (Ū-tĕr-ĭn rē-LĂK-sănts, p. 375)
![]() http://evolve.elsevier.com/Edmunds/LPN/
http://evolve.elsevier.com/Edmunds/LPN/
Overview
This chapter discusses the different hormones and steroids used in medical therapy. Unlike many other categories of medications, many of these are natural or synthetic preparations that replace, increase, or decrease natural chemicals already present within the patient. At times, the body may produce too much of a hormone (for example, in hyperthyroidism), and medication is given to reduce the hormone (such as methimazole, which limits the production of thyroid hormones). In diabetes mellitus, medication is given to replace the hormone insulin when not enough is produced by the pancreas.
Hormones are chemicals that are made in an organ or gland and carried through the bloodstream to another part of the body. Once it arrives, the hormone stimulates that part of the body to increase its activity or secretion. Steroids are a specific chemical group of hormones that have powerful effects on cell sensitization, healing, and development. They are all part of a complex message system of the body, linking together various organs and systems. Lack of one basic hormone stimulates, or signals, the glands to produce more hormone. When the right amount of the hormone is reached, the signal is turned off, and the gland slows production of the hormone. This is called a feedback mechanism and is important in creating stability of the body. If some part of the system does not work properly, failure in one organ system may then cause changes in other hormonal systems.
This chapter is divided into five basic sections. The first section describes insulin and the oral hypoglycemic agents used to treat diabetes mellitus. The various drugs that act on the uterus are presented in the second section. The third section describes the pituitary and adrenocortical hormones, the major steroids that act throughout the body. The fourth section presents the male and female hormones and the different hormones in oral contraceptives. The fifth section describes various drugs used to treat the overproduction and underproduction of thyroid hormones.
Endocrine System
The regulation and coordination of body activities happens in two ways: (1) through nerve impulses carried by the nervous system; and (2) through chemical substances or hormones carried by the blood and lymph. The organs that secrete hormones are called endocrine glands, or glands of internal secretion. All together, these glands make up the endocrine system (Figure 21-1). This system includes the pituitary gland, thyroid gland, parathyroid glands, adrenal glands, pancreas, duodenum, testes, ovaries, and placenta. Sometimes the thymus gland and the pineal body are listed as part of the endocrine system. Endocrine glands are ductless; their secretions go directly into the blood or lymph and are then carried to all parts of the body. In this respect, they are different from exocrine glands (glands of external secretion) such as salivary or sweat glands, whose products go through ducts that open onto a surface.
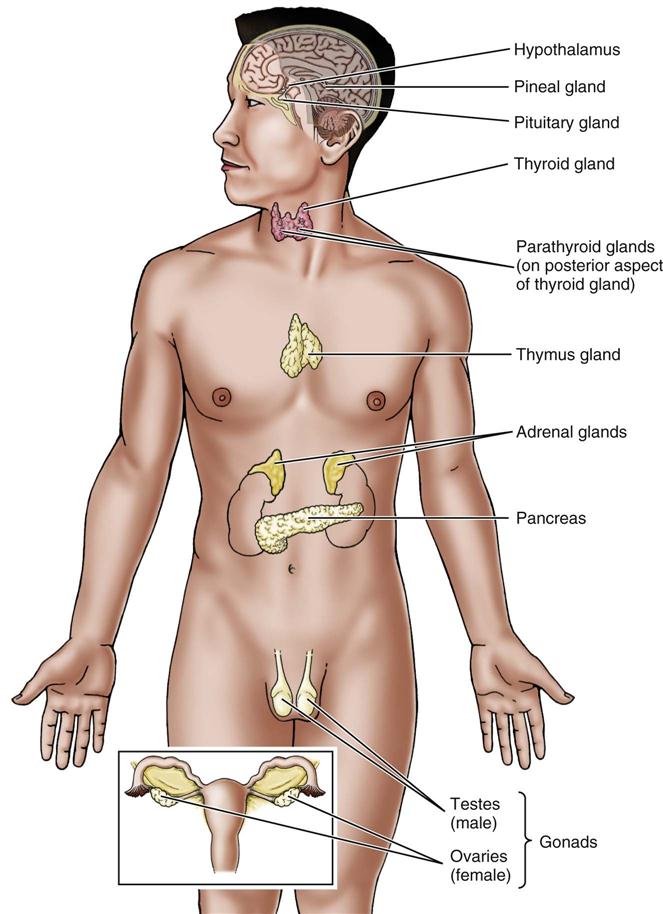
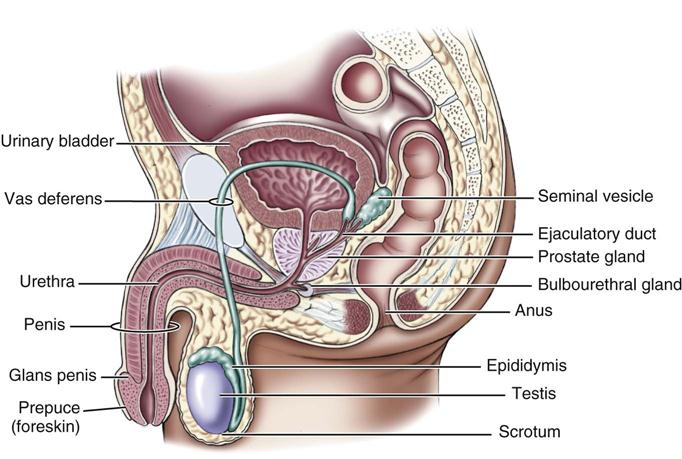
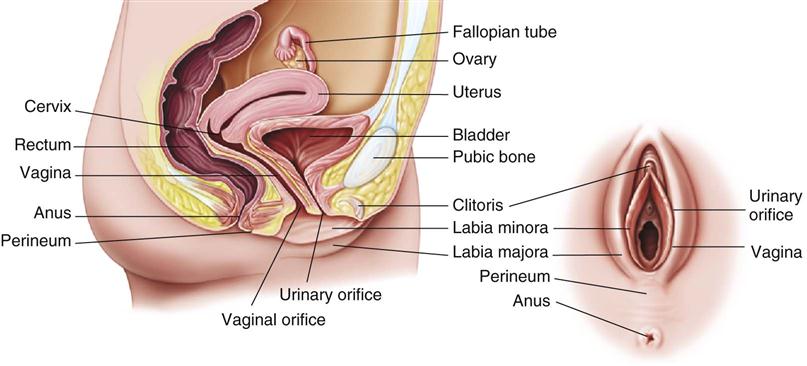
Of special importance are the hormones that affect the reproductive system. The gonads, accessory structures, and genitals of males and females are involved in reproduction and control sexual function and behavior (Figures 21-2 and 21-3). How these reproductive organs develop and function is under the control of hormones.
Antidiabetic Drugs
Overview 
Diabetes mellitus is a chronic disorder of carbohydrate (glucose) metabolism, as well as abnormal fat and protein metabolism. With time, these abnormalities result in microvascular, macrovascular, and neurologic complications. Diabetes mellitus can be described as a catabolic state (a state in which the body breaks down complex compounds into simple substances) that is caused by a relative or absolute lack of insulin, insulin resistance, and impaired or insufficient target cell receptors. Insulin is the hormone necessary for the metabolism and use of glucose in the body and is produced by the beta cells of the pancreas. Insulin helps glucose move into fat and striated muscle cells by turning on a carrier system. The patient with diabetes mellitus has a pancreas that fails to produce enough insulin for the needs of the body.
When there is not enough insulin, glucose is not available for metabolism in the cell, and so it circulates unused and at high levels in the blood. The lack of insulin forces the liver to convert protein and fat to use for energy, increasing the amounts of fatty acids. Some of these fatty acids will convert to cholesterol; over time, this increases the development of atherosclerosis. Acutely, a lack of insulin can increase the production of free fatty acids and increase ketogenesis. Along with an increase in glucagon and other hormones, a decrease in pH can occur, resulting in ketoacidosis. If left untreated, ketoacidosis can result in death.
The two major types of diabetes are type 1 diabetes, formerly known as insulin-dependent diabetes mellitus (IDDM) or juvenile diabetes, and type 2 diabetes, formerly known as non–insulin-dependent diabetes mellitus (NIDDM) or latent-onset diabetes. Patients with type 2 diabetes usually have a pancreas that functions a little and can be encouraged by medication to produce more insulin. Patients with type 1 diabetes usually have little or no production of insulin by the pancreas. These patients must take insulin to control the symptoms of diabetes mellitus. Insulin may also be necessary for some cases of type 2 diabetes, although diet, weight reduction, and oral hypoglycemic agents are usually effective in controlling symptoms.
Insulin replacement and antidiabetic agents are used along with diet, exercise, and lifestyle changes to control blood glucose levels. These agents include insulin and a variety of oral agents from different drug classes.
Insulin
Action
Insulin’s primary effect is to lower blood glucose levels by helping glucose move into target tissues. Once insulin binds to and stimulates an insulin receptor, a series of reactions take place in the cell, making it easier for glucose to pass into the cell. In addition to its role in glucose control, insulin is also very important in fat metabolism. Adequate amounts of insulin inhibit lipoprotein lipase, thereby preventing the release of fatty acids into the blood. Insulin also promotes glucose transport and storage of glucose as triglycerides in fat cells. Thus insulin is an anabolic hormone (one that converts simple substances into more complex compounds) that helps maintain stores of fatty acids, glycogen, and protein.
Uses
Patients with type 1 diabetes do not produce enough insulin and must receive insulin to survive and prevent ketosis. This disorder is thought to be caused by an autoimmune T-lymphocyte attack on the beta cells of the pancreas, leading to destruction of the insulin-producing cells in the individual who has a genetic risk of diabetes.
In type 2 diabetes, tissues are insensitive to insulin, and beta-cell response to glucose is altered. This results in a lack of the circulating insulin that is needed by the body. Unlike type 1 diabetes, ketosis is not likely to occur, because some insulin is present. A nonketotic state with high osmotic pressure may occur in patients with infection or other underlying disease. Lack of tissue sensitivity to insulin, particularly in the muscles and liver, leads to hyperglycemia and insulin resistance. Therefore, higher levels of insulin are necessary to overcome the resistance.
The Diabetes Control and Complications Trial and the Kumamoto Study clearly showed that intensively treated type 1 and 2 patients with diabetes had a delay in the onset and the progress of diabetic complications. The American Diabetes Association consensus statement recommends treatment to produce glucose levels as close to normal as possible.
The best glucose control in type 1 diabetes can be reached with multiple insulin injections. Multiple injection insulin pumps, or continuous subcutaneous insulin delivery devices now allow insulin to be delivered in much the same way as it would be normally in the nondiabetic patient and have made dramatic changes in how insulin is given to patients. Over the last several years, various devices have also been developed to simplify insulin injection. However, the standard insulin syringe and vial of insulin are still used by most patients.
Patients with type 2 diabetes may require insulin because of oral antidiabetic agent failure or to provide an additional glucose-lowering effect when oral agents alone are not adequate. Insulin is also used in patients with type 2 diabetes if the patient has oral agent allergies, liver or renal dysfunction, or is pregnant or contemplating pregnancy. Most patients with type 2 diabetes can be successfully treated with oral antidiabetic medications for years.
Insulin has been produced from various animal sources and by recombinant technology. Animal-source insulins are produced from the pancreas glands of cows and pigs. Synthetic human insulin is prepared using a nonpathogenic strain of Escherichia coli bacteria or Saccharomyces cerevisiae fungus. Since 1999, only pure pork insulin and synthetic insulin have been produced. The advantage of using synthetic human insulin or purified pork insulin is a decrease in the production of antibodies in the diabetic patient. In addition, there is a lower risk of developing lipodystrophy, or shrinkage and loss of the fatty tissue, when insulin is given in the same spot too frequently. Human insulin is also now less expensive than animal-source insulin. However, substituting human insulin is not required when successful treatment has already been achieved with pork insulin.
Insulin lispro, a rapid onset, short-duration insulin, was introduced in the 1990s. This insulin analogue offers quick absorption, an earlier insulin peak, and a faster postpeak decline than regular insulin; its action is more like the body’s natural insulin response.
Use of new drugs known as incretin mimetic agents has also made great changes in how diabetics are treated. Incretins are hormones that are released from the gut postprandially (after eating) and are often in low concentrations in persons with type 2 diabetes. The incretin that has received the most attention is glucagon-like peptide (GLP-1). Incretins stimulate insulin secretion in pancreatic beta cells and have been shown to restore both phases of insulin release. GLP-1 regulates glucose homeostasis. Incretins are also known to:
• Stimulate glucose-dependent endogenous insulin secretion (and perhaps insulin sensitivity).
• Inhibit endogenous glucagon secretion.
• Suppress appetite and induce satiety.
• Reduce the speed of gastric emptying.
• Possibly stimulate islet growth.
• Protect beta cells from cytokine and free fatty acid–mediated injury.
Another drug, exenatide (Byetta), is made from part of the saliva of the Gila monster lizard. It is approved as adjunctive therapy for type 2 diabetes. Exenatide binds to GLP-1 receptors and stimulates insulin secretion when blood sugar is high. It is the first drug that has been shown to restore first-phase insulin secretion, which is missing in persons with type 2 diabetes. It is given as an injection before the morning and evening meals. Adverse effects of exenatide include nausea, vomiting, diarrhea, and upper respiratory symptoms. Many patients lose weight when taking this drug.
Adverse Reactions
Adverse reactions to insulin include local itching, swelling, or erythema (redness or irritation) at the injection site, lipodystrophy, and symptoms of insulin allergy or resistance. The most important adverse reaction is hypoglycemia (serum glucose levels <60 mg/dL), which is caused by taking too much insulin. Symptoms of hypoglycemia include sudden onset of nervousness; hunger; malaise (weakness); cold, clammy skin; lethargy (sleepiness); no urine glucose or acetone; pallor (paleness); diaphoresis (sweating); change in level of consciousness (awareness and ability to respond); and shallow respirations.
Drug Interactions
Insulin needs may be increased by insulin antagonists such as oral contraceptives, corticosteroids, epinephrine, and preparations used for thyroid hormone replacement therapy. Thiazide diuretics may elevate glucose levels. A variety of other drugs, alcohol, and anabolic steroids may increase the hypoglycemic effects of insulin. Insulin promotes the movement of potassium into cells and lowers the serum potassium levels. Propranolol and other beta blockers can mask the signs and symptoms of hypoglycemia.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
A patient whose diabetes mellitus was not previously diagnosed or is poorly controlled or out of control may have a history of polyuria (excretion of a large amount of urine), polydipsia (excessive thirst), polyphagia (excessive uncontrolled eating), weight loss, blurred vision, and fatigue. In severe cases of hyperglycemia, the patient may develop systemic acidosis, a condition in which the basic fluid and electrolyte balance of the body is disturbed, and the blood pH is decreased. Symptoms of systemic acidosis include nausea, vomiting, and changes in level of consciousness.
Ask the patient about signs of pregnancy, infection, and kidney, liver, or thyroid disease, because these conditions alter the requirement for insulin. Find out about any earlier sensitization (allergy to a foreign protein) to beef or pork and whether the patient is taking other drugs that may interact with insulin.
n Diagnosis
What other needs does the patient have? Does the patient need information on weight loss, nutrition, or other knowledge? What other diseases does this patient have that might influence the therapy for diabetes?
n Planning
Successful management of diabetes mellitus depends on the patient understanding the disease. Control and maintenance require that the patient know about the nature of the disease, proper diet and the need for weight control, and the importance of hygiene and exercise. The patient must understand how to do blood and urine testing and how to correctly draw up and inject insulin. A diabetic must know the signs and symptoms of hypoglycemia and hyperglycemia and the appropriate actions to take for each, as well as procedures to follow during illness.
The patient should be shown the proper injection technique, including drawing up, injection, and storage of insulin. Ask the patient to demonstrate how to give the injection.
The patient should be taught about rotation of injection sites to prevent lipodystrophy. Although use of human insulin has reduced the incidence of lipodystrophy, all patients should be encouraged to rotate injection sites regularly to help with absorption. (See Chapter 10, Figure 10-15.)
It may be preferable to have patients use prefilled insulin cartridges and syringes that automatically dispense standard dosages if their vision is bad or they have difficulty understanding. Routine follow-up and evaluation of injection technique is important. The patient should be asked periodically to give a demonstration of the technique on return visits to the clinic or office.
Patients must also be taught how to test the blood glucose level using a glucometer (hand-held testing machine). Have the patient practice using the machine and accurately interpreting the results; the requirements of the specific equipment being used will vary. Provide a booklet or chart in which the patient can record findings. Information on times when the blood should be tested for glucose should be given to the patient as part of written instructions. Times to test blood glucose may vary, based on the type of medication taken and the degree of control required.
Individuals with cerebral vascular disease, coronary disease, or advanced complications may be at higher risk of hypoglycemia and may not benefit from tight glucose control. In general, the goal for the fasting blood sugar (FBS) is less than 120 mg/dL.
The goal for the average preprandial glucose level should be between 80 and 120 mg/dL. The goal for the bedtime glucose level is 110 to 140 mg/dL. Treatment adjustment should occur if the glucose is less than 100 mg/dL or greater than 160 mg/dL.
Insulin allergy (transient local itching, swelling, and erythema at the injection site) commonly develops when therapy is started, particularly with pork insulin. Use of produced by recombinant deoxyribonucleic acid technology has decreased this problem. Insulin resistance (requirements of more than 200 units of insulin per day) is rare and may be caused by infection, inflammatory diseases, obesity, or stress. To make sure that hypoglycemia is avoided, closely monitor the patient with insulin resistance who is being treated with a concentrated insulin injection. Long-acting insulins are not adequate in the treatment and management of acidosis and emergencies.
Administration of insulin by an aerosol inhaler allows some diabetic patients to give up injections. Not all persons with diabetes can use this format.
n Implementation
Techniques for calculation of insulin dosage, preparation of injection, mixing of insulin types, injection sites, and injection technique are all presented in Chapter 10. Refer to this material to review this information.
Insulin is a protein and therefore is inactivated by gastrointestinal (GI) enzymes. Thus insulin is generally given subcutaneously and timed so that it is available in the body when the glucose level rises after eating. The time of administration also depends on the type of insulin preparation. Only regular insulin can be administered intravenously, as is done during ketoacidosis or diabetic coma.
Various substances such as protamine or zinc may be added to delay insulin absorption or turn it into a suspension. Different insulin preparations with different onsets, peaks, and durations of action are required so that patients can individualize their treatment. Premixed insulin products are also available as combinations of neutral protamine Hagedorn (NPH) and regular insulin in ratios of 70/30, 30/70, and 50/50. Information about these products is summarized in Tables 21-1 and 21-2.
![]() Table 21-1
Table 21-1
Insulin Characteristics and Duration of Action
| INSULIN | COLOR | ONSET | PEAK | DURATION |
| Rapid Acting | ||||
| lispro (Humalog) |
Clear | 5-15 min | 30-90 min | 3-4 hr |
| aspart (NovoLog) | Clear | 5-15 min | 1-3 hr | 3-5 hr |
| glulisine (Apidra) | Clear | 5-15 min | 30-90 min | 3-4 hr |
| Short Acting | ||||
| Regular (R) | Clear | 30-60 min | 2-4 hr | 6-8 hr |
| Intermediate Acting | ||||
| NPH (N) | Cloudy | 2-4 hr | 6-10 hr | 10-16 hr |
| Long Acting | ||||
| glargine (Lantus) | Clear | 2 hr | No peak | 20-24 hr |
| detemir (Levemir) | Clear | 1 hr | No peak | 6-24 hr |
| Mixtures | ||||
| 70/30 | Cloudy | 15 min | 30 min-12 hr | 10-16 hr |
| 50/50 | Cloudy | 30 min | 3-5 hr | 10-16 hr |
| Humalog 75/25 | Cloudy | 15 min | 30-90 min | 10-16 hr |
| NovoLog 70/30 | Cloudy | 5-15 min | 1-3 hr | 10-16 hr |
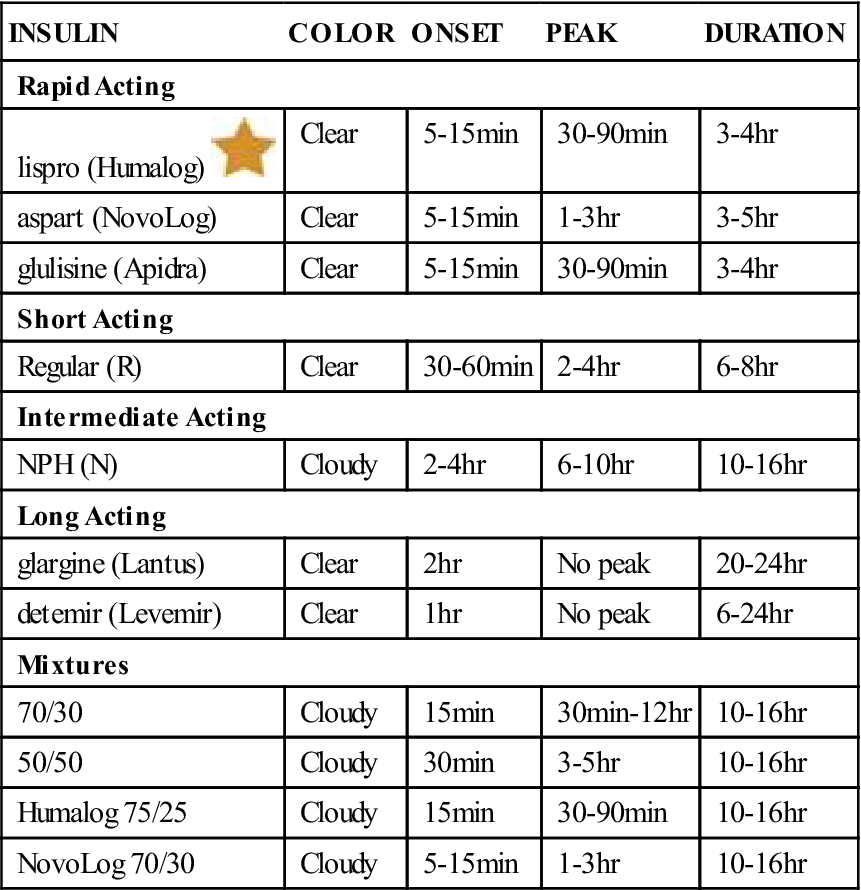
NPH, Neutral protamine Hagedorn.![]() Indicates “Must-Know Drugs,” or the 35 drugs most prescribers use.
Indicates “Must-Know Drugs,” or the 35 drugs most prescribers use.
![]() Table 21-2
Table 21-2
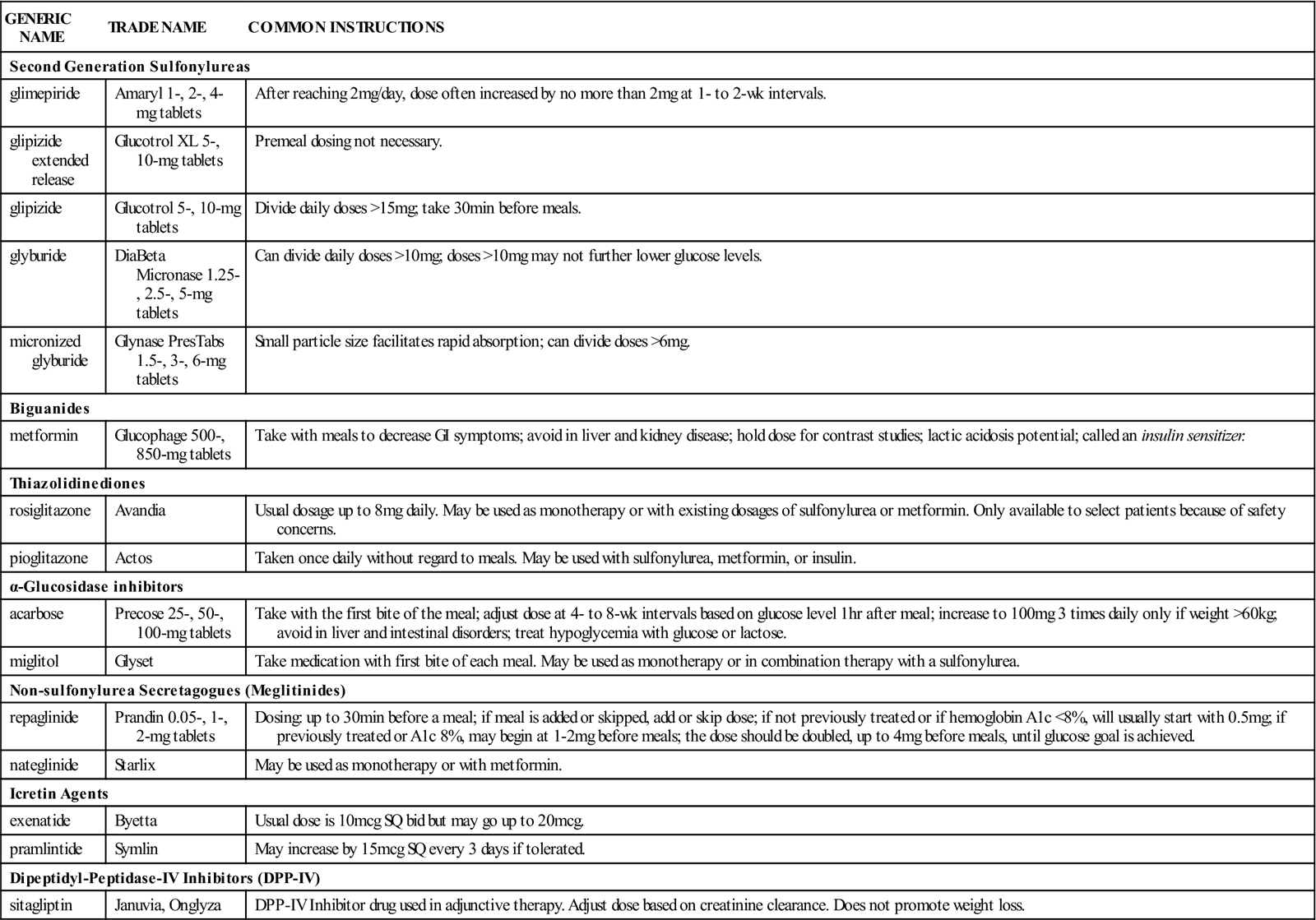
Oral Treatment of Type 2 Diabetes
| GENERIC NAME | TRADE NAME | COMMON INSTRUCTIONS |
| Second Generation Sulfonylureas | ||
| glimepiride | Amaryl 1-, 2-, 4-mg tablets | After reaching 2 mg/day, dose often increased by no more than 2 mg at 1- to 2-wk intervals. |
| glipizide extended release | Glucotrol XL 5-, 10-mg tablets | Premeal dosing not necessary. |
| glipizide | Glucotrol 5-, 10-mg tablets | Divide daily doses >15 mg; take 30 min before meals. |
| glyburide | DiaBeta Micronase 1.25-, 2.5-, 5-mg tablets |
Can divide daily doses >10 mg; doses >10 mg may not further lower glucose levels. |
| micronized glyburide | Glynase PresTabs 1.5-, 3-, 6-mg tablets | Small particle size facilitates rapid absorption; can divide doses >6 mg. |
| Biguanides | ||
| metformin | Glucophage 500-, 850-mg tablets | Take with meals to decrease GI symptoms; avoid in liver and kidney disease; hold dose for contrast studies; lactic acidosis potential; called an insulin sensitizer. |
| Thiazolidinediones | ||
| rosiglitazone | Avandia | Usual dosage up to 8 mg daily. May be used as monotherapy or with existing dosages of sulfonylurea or metformin. Only available to select patients because of safety concerns. |
| pioglitazone | Actos | Taken once daily without regard to meals. May be used with sulfonylurea, metformin, or insulin. |
| α-Glucosidase inhibitors | ||
| acarbose | Precose 25-, 50-, 100-mg tablets | Take with the first bite of the meal; adjust dose at 4- to 8-wk intervals based on glucose level 1 hr after meal; increase to 100 mg 3 times daily only if weight >60 kg; avoid in liver and intestinal disorders; treat hypoglycemia with glucose or lactose. |
| miglitol | Glyset | Take medication with first bite of each meal. May be used as monotherapy or in combination therapy with a sulfonylurea. |
| Non-sulfonylurea Secretagogues (Meglitinides) | ||
| repaglinide | Prandin 0.05-, 1-, 2-mg tablets | Dosing: up to 30 min before a meal; if meal is added or skipped, add or skip dose; if not previously treated or if hemoglobin A1c <8%, will usually start with 0.5 mg; if previously treated or A1c 8%, may begin at 1-2 mg before meals; the dose should be doubled, up to 4 mg before meals, until glucose goal is achieved. |
| nateglinide | Starlix | May be used as monotherapy or with metformin. |
| Icretin Agents | ||
| exenatide | Byetta | Usual dose is 10 mcg SQ bid but may go up to 20 mcg. |
| pramlintide | Symlin | May increase by 15 mcg SQ every 3 days if tolerated. |
| Dipeptidyl-Peptidase-IV Inhibitors (DPP-IV) | ||
| sitagliptin | Januvia, Onglyza | DPP-IV Inhibitor drug used in adjunctive therapy. Adjust dose based on creatinine clearance. Does not promote weight loss. |
Insulin dose depends on the patient’s response. The dosage will be gradually increased or decreased (titrated) to get the best response with the lowest dosage. Generally, the minimal goal of therapy is to avoid extremes of ketoacidosis and hypoglycemia.
The individual presenting with ketones in the blood is usually started on insulin. The goal of therapy is to maintain blood glucose levels as follows: fasting, 90-110 mg/dL; 1 hour after eating (postprandial), less than 180 mg/dL; and 2 hours postprandial, less than 150 mg/dL. There are a variety of recommendations for how insulin might be given daily to achieve these goals:
1. Basal insulin therapy in combination with oral agents
3. NPH/regular insulin before breakfast and before dinner
• Use 0.5 to 1 unit per kilogram of patient’s weight as total daily insulin dose.
• Divide each morning and dinner dose so that two-thirds of the dose is NPH and one-third is regular.
4. Basal-bolus regimens (long-acting insulin in combination with premeal rapid insulin injection)
After the patient is started on a basal dose of long-acting insulin (glargine or detemir), rapid- or short-acting insulin is added before meals. This requires that blood glucose levels be monitored frequently. One option is to add premeal rapid- or short-acting insulin to the largest meal. Then prandial boluses of rapid-acting insulin can be added at other meal times. Rapid- and short-acting insulin can be added as standing doses or on a sliding scale based on blood sugar readings.
This is the most physiologic approach to insulin therapy; however, this regimen can be daunting for many diabetic patients. Consequently, twice-daily premixed insulin (given before breakfast and before dinner) is an acceptable alternative for the patient who is not willing or able to take multiple daily injections.
The insulin vial in use may be stored outside of the refrigerator for 1 month, provided it does not get extremely hot or cold. An extra supply of insulin should be stored in the refrigerator. Insulin should be warmed to room temperature for use, because the injection of cold insulin may irritate the tissues. The expiration date on the bottle should be checked regularly to make sure the insulin is not too old to use safely.
Rapid-acting insulin is used during treatment of ketoacidosis and in other acute situations (infection, surgery) when the patient’s food intake is variable. It is also used in combination with longer-acting insulins to achieve greater control. Regular insulin may be used in divided dose therapy. The dosage is determined by the level of blood glucose. Long-acting insulin is used primarily for patients whose blood sugar level is constantly high at night.
For insulin suspensions, the vial is gently rolled and tipped from end to end before the insulin is drawn up, so that any particles that may have settled out are returned to suspension. Vigorous shaking may result in air bubbles that can make it difficult to accurately draw the insulin. Shaking also breaks down protein molecules in the insulin.
Most patients with diabetes can control their symptoms with 40 to 60 units of insulin per day. Occasionally a patient develops resistance to the insulin or becomes so unresponsive to insulin that several hundred or even thousands of units of insulin may be necessary. Patients who require dosages in excess of 300 to 500 units often have impaired insulin receptors. Concentrated insulin injection allows a larger dose to be given in a smaller amount of fluid. Each milliliter of the concentrated insulin contains 500 units of purified pork, rather than the 100 units in the normal products. Glargine is an insulin product that provides a basal level of insulin for 24 hours.
n Evaluation
The patient’s response to the insulin dose is seen by testing the blood. The nurse, physician, or other health care provider should inform the patient about how frequently to return for checkups, what blood levels are being found at these visits, and what the desired levels should be. The patient must be encouraged to take responsibility for managing his or her own disease.
Patients with type 2 diabetes are frequently overweight. As the patient’s blood sugar level is under control and they begin to lose weight, the dosage of insulin they require is less. The clinician will often reduce the dosages of medications prescribed when weight loss has taken place.
The plan of insulin therapy is to keep blood glucose levels within specific limits and to prevent symptoms of hyperglycemia and hypoglycemia. Patients with home glucometers should be told when to check their blood glucose level, depending on the type of insulin they are taking. Urine ketones should be measured during acute illness or periods of increased glycosuria and in ketosis-prone diabetic patients. The records the patient keeps will provide information regarding control between office visits and should be taken to each visit with the health care provider.
If hypoglycemia occurs, the patient should be taught to eat some form of carbohydrate immediately. The family should also be involved in patient teaching about therapy for hypoglycemia. If the patient is unconscious, honey or corn syrup may be put under the tongue or on the buccal mucosa in the mouth. Additional carbohydrates, such as bread, crackers, or milk, should be provided for the next 2 hours; a sandwich should be eaten if a snack or meal would not be regularly eaten within an hour. Glucagon, a glucose-rich liquid, may be administered by a family member or a care provider to quickly raise blood glucose levels if the patient has accidentally taken too much insulin.
The Somogyi effect (rebound elevation of glucose levels brought on by hypoglycemia) can lead to overtreatment of the patient with insulin when less insulin is actually needed. Patients older than 60 years of age are often sensitive to hypoglycemia. They should be observed for confusion and abnormal behavior, because repeated episodes of hypoglycemia may cause brain damage.
Regular appointments with the clinician will be timed with laboratory blood work to measure blood sugar control. The hemoglobin (Hb) A1c blood test reflects the state of glycemia the patient has experienced for the last 90 days, which is the lifetime of the red blood cell. The goal is to keep the HbA1c level less than 7%, which is equivalent to a blood glucose level of 150 mg/dL. The blood glucose level goes up by approximately 30 points for every 1% increase of the HbA1c (e.g., 8% = 180 mg/dL; 9% = 210 mg/dL). Evaluation of this blood level will tell the clinician about general blood sugar levels, not just the blood sugar level on the day of the appointment.
n Patient and Family Teaching
Oral Hypoglycemics
Action
The primary action of the oral hypoglycemics is to stimulate insulin release by the beta cells of the pancreas. Therefore the patient must have some functioning beta cells if these drugs are to work. These products also increase the peripheral use of insulin and influence other fat and carbohydrate processes.
Uses
The number of classes of oral antidiabetic agents has dramatically increased since the 1980s. They can be used in monotherapy (therapy with one drug) or combined oral agent therapy, or can be combined with insulin to achieve the optimal (best) glucose control in patients with type 2 diabetes. The first available class of oral agents was the sulfonylureas. Sulfonylureas lower serum glucose levels by increasing beta cell insulin production and, to a lesser extent, by decreasing insulin resistance. In the early 1980s, a second generation of sulfonylureas became available, and over time these have replaced the first-generation sulfonylureas. Second-generation sulfonylureas are approximately 1000 times more potent than first-generation agents. Unlike first-generation oral agents, which bind to ionic and nonionic sites, second-generation agents bind only to nonionic sites. This type of binding usually results in fewer interactions with other medications. The major side effect of sulfonylureas is hypoglycemia. These drugs can be used as monotherapy or in combination with insulin, acarbose, or metformin.
The second class of oral agents that became available was the biguanides. The only drug in this class that is still available in the United States is metformin. Metformin use is associated with a very small risk for lactic acidosis, usually in patients who may also have some renal dysfunction. This class of medication lowers glucose levels by decreasing glucose production in the liver, decreasing insulin resistance, and slowing the absorption of glucose from the intestines. As monotherapy, metformin generally does not cause hypoglycemia. Metformin can be used in combination with insulin, and all of the other oral agents.
Alpha-glucosidase inhibitors became available in the 1990s. Acarbose and miglitol are the drugs available in this class. They lower glucose by slowing the breakdown of polysaccharides into simple sugars. As monotherapy, they cannot cause hypoglycemia. Alpha-glucosidase inhibitors can be used with sulfonylureas, insulin, or metformin.
Another class of oral antidiabetic agents, the meglitinides, was released in 1998. The drugs repaglinide and nateglinide, although chemically unrelated to the sulfonylureas, work by stimulating the release of insulin from the beta cells of the pancreas. Their use can result in hypoglycemia. Meglitinides can be used as monotherapy or in combination with metformin.
The thiazolidinedione class of drugs has introduced many new options for treatment of patients with diabetes. These agents increase the body’s response to insulin without increasing insulin secretion. The drugs rosiglitazone (Avandia) and pioglitazone (Actos) have revolutionized the way patients can be treated. Thiazolidinediones have been associated with severe cardiovascular side effects, however, and must be used with care. Currently rosiglitazone is a drug only available to selected patients but pioglitazone is still generally available, although closely monitored.
A whole new class of drugs, the incretins, have also provided new treatment options. The amylin analog drug pramlintide (Symlin), the GLP-1, exenatide, and the DPP-4 inhibitor sitagliptin phosphate (Januvia) are included in this class. Incretins are hormones that are released from the gut postprandially; they often are found in low concentrations in persons with type 2 diabetes. The incretin that has received the most attention is GLP-1. Incretins stimulate insulin secretion in pancreatic β-cells and have been shown to restore both phases of insulin release. GLP-1 regulates glucose homeostasis via multiple complementary actions and along with other incretins is known to:
• Stimulate glucose-dependent endogenous insulin secretion (and perhaps insulin sensitivity).
• Inhibit endogenous glucagon secretion.
• Suppress appetite and induce satiety.
• Reduce the speed of gastric emptying.
• Possibly stimulate islet-cell growth.
• Protect β-cells from cytokine and free fatty acid–mediated injury.
New drugs as well as new treatment delivery systems such as the pump have changed how diabetes is treated.
Adverse Reactions
Hypoglycemia is the most common adverse reaction. Allergic reactions, manifested by urticaria (hives), rash, pruritus (itching), and erythema, may occur at the beginning of therapy, generally temporarily. More common reactions to sulfonylureas include heartburn, nausea, vomiting, abdominal pain, and diarrhea caused by increased gastric acid secretion. Occasionally, sulfonylureas cause hepatotoxicity (damage to the liver) and cholestatic jaundice, with symptoms of jaundice (yellow color of skin, eyes, and mucous membranes), dark urine, and light-colored stools. Leukopenia, agranulocytosis, thrombocytopenia, hemolytic anemia, aplastic anemia, and pancytopenia have also been reported. There are rare reports of disulfiram-like reactions when alcohol is taken with tolbutamide. Lactic acidosis may rarely occur with metformin, and the risk is increased with the use of alcohol. Nausea, vomiting, diarrhea, flatulence, and anorexia are the most common adverse reactions with metformin; these problems tend to improve over time.
Drug Interactions
The hyperglycemic effects of the sulfonylureas and metformin are potentiated (made worse) by oral anticoagulants and various other drugs. Sulfonamide-type antibacterial agents and salicylates displace the sulfonylureas from protein-binding sites, and this leads to high blood levels of the active drug. Barbiturates, sedatives, and hypnotics may have a prolonged effect when taken at the same time as the sulfonylureas because of a decreased rate of elimination from the body. Thiazide diuretics oppose the secretion of insulin from the beta cells and decrease the effectiveness of sulfonylureas. Many of these drugs, when used along with oral contraceptives that contain ethinyl estradiol and norethindrone, may decrease contraceptive effectiveness.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
Try to learn as much as possible about the patient’s health history, including what other drugs the patient is taking that may interact with the oral products, and if the patient is pregnant or has renal insufficiency, impaired liver function, or a history of ketoacidosis. Ask if the patient has any sensitivity (allergy) to sulfa drugs, because he or she may have cross-sensitivity to sulfonylureas (a patient who is sensitive to one type of sulfa drug may be sensitive to all types).
n Diagnosis
Does the patient have any other problems that would interfere with drug therapy? Are there problems with weight, nutrition, vision, or finances? Does the patient have past history or behavior that might cause the nurse to suspect this patient might not be compliant with diet, exercise, medication, and testing requirements?
n Planning
No transition period is necessary when a patient is switched from one oral hypoglycemic to another. Plan the teaching that will be necessary as the nurse works with this patient.
n Implementation
These products are administered orally. The duration of the hypoglycemic effect is the main difference between the various products. The duration of action, dosage range, and approximate doses per day are given in Tables 21-1 and 21-2.
n Evaluation
The patient’s blood glucose levels should be monitored, and the patient should be watched for signs and symptoms of hypoglycemia.
Rashes may develop when sulfonylurea therapy begins, but they generally last only a short time. If they persist, the medication should be stopped. Cholestatic jaundice has been reported in a small number of patients on oral hypoglycemic therapy. Any liver damage that has developed generally goes away when the drug is stopped. Watch for any signs of blood dyscrasias, GI intolerance, or allergic reactions.
n Patient and Family Teaching
Teach the patient and family about diabetes, diet, and exercise, just as the patient is taught about starting to take insulin. Teach patients specifically about nutrition, blood testing, and general precautions to follow. In addition, tell the patient and family the following:
Selected Drugs Used With Pregnancy And Delivery
Overview
Medications used throughout the end of pregnancy and during delivery are a special category of drugs beyond the scope of this text. However, any drug used for the mother also affects the fetus, so paying special attention to drug use is required during the immediate delivery period. Therefore a few of these products are selected for discussion. Excluding anesthetics, most drugs used during the antepartum (before), intrapartum (during), and postpartum (after birth) periods are given primarily for their effect on the uterus. These include tocolytics, oxytocics, uterine relaxants, and abortifacients. These products are used primarily to slow labor at the time of delivery or to help expel the fetus from the uterus to terminate pregnancy.
Action
Abortifacients stimulate or increase uterine contractions and cause the uterus to empty. Oxytocic agents and ergot preparations cause the uterus to contract, helping labor move on to delivery. Oxytocin acts directly on the smooth muscles of the uterus, especially when the mother is at or near full term, to produce firm, regular contractions. They also act on the blood vessels to produce vasoconstriction (narrowing) and on the mammary gland cells in the postpartum phase to stimulate the flow of milk. Because these drugs are given so frequently, most of the information presented here is about oxytocics.
In contrast to abortifacients, oxytocin, and the ergots, the uterine relaxants act on the beta-adrenergic receptors to stop uterine smooth muscle contractions. Tocolytics are agents used to stop preterm labor. They generally act through uterine relaxation.
Uses
Abortifacients are used early in pregnancy to end pregnancy by emptying the uterus. Oxytocics are used for a number of purposes:
• To assist in the delivery of the shoulder of the infant
• To assist in the release of the placenta
• To control postpartum bleeding or lack of muscle tone in the uterus
• To relieve breast swelling or engorgement caused by lack of lactation
• To stimulate uterine contraction after a cesarean section birth or other uterine surgery
The ergots are used to prevent or control hemorrhage after the delivery of the placenta and in the postpartum period.
Uterine relaxants and tocolytics are used when a mother goes into preterm labor and the goal is to delay delivery. Women who show signs of preterm delivery may be treated with a subcutaneous injection and then sent home on an oral maintenance dose. Magnesium sulfate, a common anticonvulsant, has some success as a tocolytic; however, it is not a first-line agent. It has also been used with ritodrine therapy, although with questionable efficacy and an increase in adverse reactions. Hydroxyprogesterone caproate (Makena) is a drug to help reduce the risk of preterm delivery before 37 weeks of pregnancy. This indication is for pregnant women with a history of at least one spontaneous preterm birth, but not for use in women with a twin pregnancy or other risk factors for preterm birth. The Food and Drug Administration has warned that injectable or oral terbutaline, a drug long used off-label to prevent premature birth, should not be used beyond 48-72 hours of preterm labor because of the potential for serious maternal cardiovascular events and death.
Adverse Reactions
Abortifacients may produce severe cramping and pain. Tocolytics often produce visual disturbances, malaise, nausea, and confusion. Oxytocin may produce dysrhythmias (irregular heartbeats), edema (fluid buildup in the body tissues), fetal and neonatal bradycardia (slow heartbeat), anxiety, redness of skin during administration, nausea and vomiting, anaphylaxis (shock), postpartum hemorrhage, cyanosis (blue color to the skin), and dyspnea (uncomfortable breathing).
In the appropriate dosage and in the absence of contraindications, the ergots are fairly safe. The most common adverse reactions are nausea and vomiting. More unusual reactions include allergic reactions, bradycardia, hypotension (low blood pressure), hypertension (high blood pressure), or cerebral-spinal symptoms and spasms. The most common side effects reported with hydroxyprogesterone caproate include pain, swelling, or itching at the injection site; hives; nausea; and diarrhea. Serious adverse reactions are rare.
Excessive doses of oxytocics during labor can produce uterine hypertonicity (extreme muscle tension), spasm, and tetanic contractions and ruptures of the uterus. Smaller overdoses in labor yield a sustained, forceful contraction without rest. Overdose with ergots during labor yields a similar reaction, with cardiovascular and GI symptoms progressing to more dangerous problems.
Drug Interactions
Vasoconstrictors and local anesthetics increase the effects of oxytocics.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
It is important to determine the exact due date for delivery. The patient may be past the anticipated due date for the baby or have a history of engorged breasts. A history of incomplete abortion, cesarean section births, or excessive postpartum bleeding may require use of oxytocics or ergots. Finally, a patient may also want to terminate an unwanted pregnancy early in gestation.
n Diagnosis
What additional problems might this patient have? Is there unreasonable anxiety or fear associated with the delivery? Does the mother have concerns about the health of the child? Have previous experiences been positive? What other medical conditions might make this delivery more risky?
n Planning
The uterine contractions produced by oxytocics should be about the same as those of spontaneous, normal labor.
There are numerous precautions or contraindications to the use of oxytocics. These medications must be given by qualified nurses under the direct supervision of physicians or other health care providers. Inappropriate use of either oxytocic or ergot preparations has caused fetal and maternal death or injury, subarachnoid hemorrhage, and uterine rupture.
n Implementation
Oxytocin is the drug of choice to cause or induce labor in many areas of the country. However, prostaglandins are now preferred in some regions. These are usually given by intravenous (IV) infusion pump.
Ergonovine is now the drug of choice to control postpartum bleeding. It can be given sublingually, intramuscularly, or intravenously in emergency situations. Methylergonovine is the synthetic homologue of ergonovine and has been found to produce fewer vasoconstrictive or hypertensive side effects than ergonovine. It is noted that IV administration of either of these ergot preparations increases the danger of side effects.
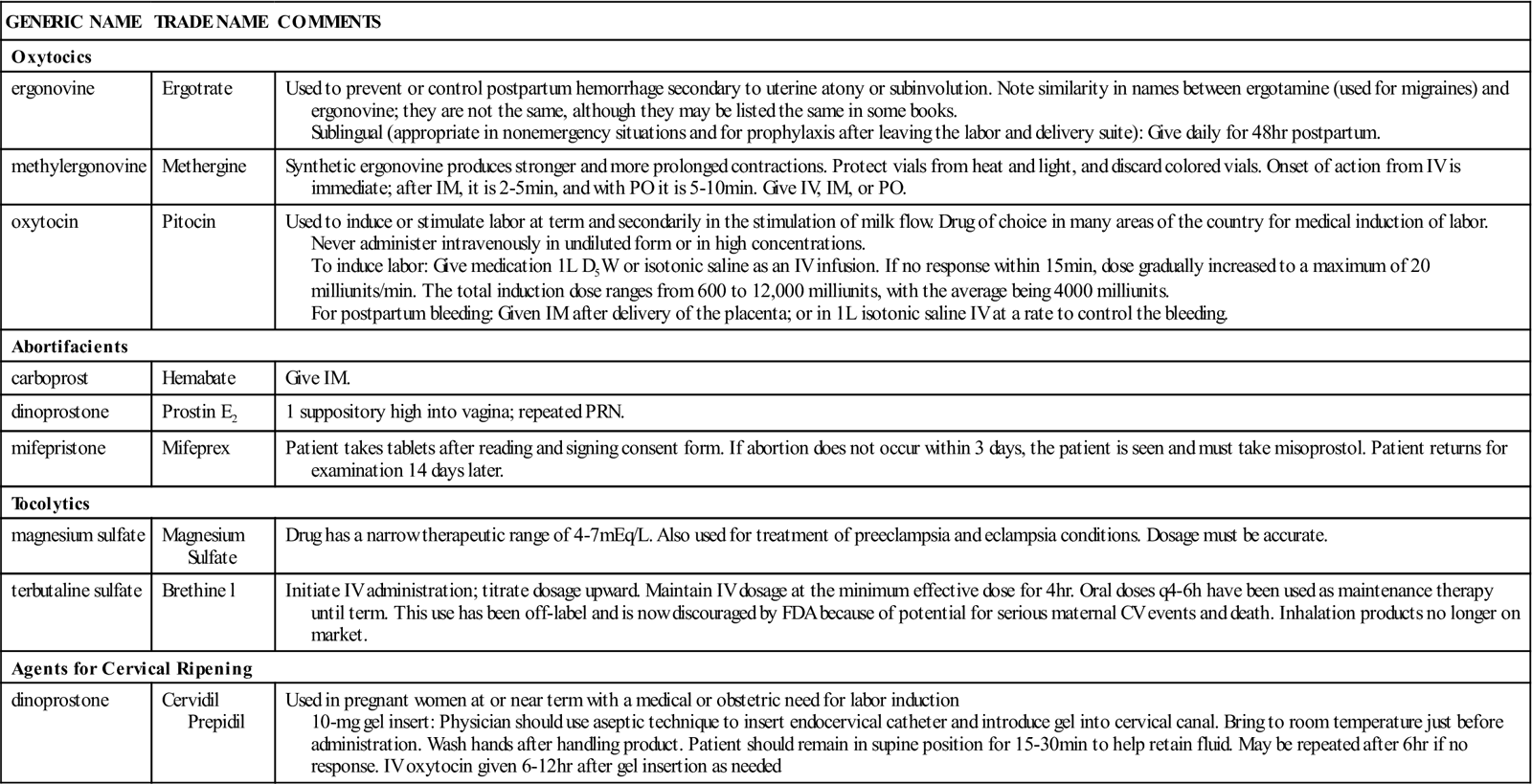
A summary of drugs acting on the uterus is provided in Table 21-3.
![]() Table 21-3
Table 21-3
| GENERIC NAME | TRADE NAME | COMMENTS |
| Oxytocics | ||
| ergonovine | Ergotrate | Used to prevent or control postpartum hemorrhage secondary to uterine atony or subinvolution. Note similarity in names between ergotamine (used for migraines) and ergonovine; they are not the same, although they may be listed the same in some books. Sublingual (appropriate in nonemergency situations and for prophylaxis after leaving the labor and delivery suite): Give daily for 48 hr postpartum. |
| methylergonovine | Methergine | Synthetic ergonovine produces stronger and more prolonged contractions. Protect vials from heat and light, and discard colored vials. Onset of action from IV is immediate; after IM, it is 2-5 min, and with PO it is 5-10 min. Give IV, IM, or PO. |
| oxytocin | Pitocin | Used to induce or stimulate labor at term and secondarily in the stimulation of milk flow. Drug of choice in many areas of the country for medical induction of labor. Never administer intravenously in undiluted form or in high concentrations. To induce labor: Give medication 1 L D5W or isotonic saline as an IV infusion. If no response within 15 min, dose gradually increased to a maximum of 20 milliunits/min. The total induction dose ranges from 600 to 12,000 milliunits, with the average being 4000 milliunits. For postpartum bleeding: Given IM after delivery of the placenta; or in 1 L isotonic saline IV at a rate to control the bleeding. |
| Abortifacients | ||
| carboprost | Hemabate | Give IM. |
| dinoprostone | Prostin E2 | 1 suppository high into vagina; repeated PRN. |
| mifepristone | Mifeprex | Patient takes tablets after reading and signing consent form. If abortion does not occur within 3 days, the patient is seen and must take misoprostol. Patient returns for examination 14 days later. |
| Tocolytics | ||
| magnesium sulfate | Magnesium Sulfate | Drug has a narrow therapeutic range of 4-7 mEq/L. Also used for treatment of preeclampsia and eclampsia conditions. Dosage must be accurate. |
| terbutaline sulfate | Brethine l | Initiate IV administration; titrate dosage upward. Maintain IV dosage at the minimum effective dose for 4 hr. Oral doses q4-6h have been used as maintenance therapy until term. This use has been off-label and is now discouraged by FDA because of potential for serious maternal CV events and death. Inhalation products no longer on market. |
| Agents for Cervical Ripening | ||
| dinoprostone | Cervidil Prepidil |
Used in pregnant women at or near term with a medical or obstetric need for labor induction 10-mg gel insert: Physician should use aseptic technique to insert endocervical catheter and introduce gel into cervical canal. Bring to room temperature just before administration. Wash hands after handling product. Patient should remain in supine position for 15-30 min to help retain fluid. May be repeated after 6 hr if no response. IV oxytocin given 6-12 hr after gel insertion as needed |
n Evaluation
If oxytocin is used during induction of labor, the patient should be monitored for degree of contractions and the development of adverse reactions. The blood pressure and pulse should be checked frequently, and there should be continuous monitoring of the fetal heart rate. Monitor the dilation of the cervix and the progression of contractions. Drastic increases in the frequency, force, and duration of contractions and in resting uterine tone may require the drug to be stopped. The contractions should not be more than 50 mm Hg, the frequency should be no longer than every 2 minutes, and the duration should be no longer than 90 seconds. Both the mother and fetus should be monitored with internal monitoring equipment (fetal scalp electrode and intrauterine pressure catheter).
Watch out for the symptoms of ergotism: vomiting; diarrhea; unquenchable thirst; tingling, itching, and coldness of the skin; a rapid, weak pulse; confusion; and unconsciousness.
Ergonovine might stimulate cramping. If this becomes too uncomfortable, the physician may either decrease the dosage or treat the symptoms.
The most common side effects of ergonovine are nausea and vomiting. These symptoms can sometimes be stopped if the patient is given a phenothiazine antiemetic.
If overdosage of an oxytocic occurs, producing a continuous contraction, the drug must be stopped immediately. It may be necessary to give a general anesthetic to relax the uterus, particularly if the fetus is threatened.
n Patient and Family Teaching
Pituitary And Adrenocortical Hormones
Overview
The pituitary, or “master” gland, lies in the sella turcica in the sphenoid bone in the skull and is connected to the brain by a slender stalk. This area is almost directly between the eyes in the middle of the brain. The anterior (front) portion of the pituitary, or the adenohypophysis, and the posterior (back) portion of the pituitary, or the neurohypophysis, produce hormones that control growth, metabolism, electrolyte balance, water retention or loss, and the reproductive cycle.
The adrenal cortex manufactures the corticosteroids and a small amount of the sex hormones. These hormones are substances that influence many organs, structures, and life processes of the body. The corticosteroids are composed of the glucocorticoids and the mineralocorticoids, and the sex hormones include the androgens and estrogens.
 Pituitary Hormones
Pituitary Hormones
Anterior Pituitary Hormones
Action And Uses
The major anterior pituitary hormones include two gonadotropins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). They are called gonadotropins because they influence the gonads, which are the organs of reproduction. They influence the production of sex hormones, the development of secondary sex characteristics, and the pattern and regularity of the reproductive cycle. An additional anterior pituitary hormone, prolactin, stimulates the production of breast milk after childbirth.
There are a number of sources for gonadotropins that are used clinically. Human chorionic gonadotropin is taken from human placentas and contains FSH and LH. A purified form of FSH and LH, known as menotropins, is taken from the urine of postmenopausal women. These hormones may be given to produce ovulation in women with ovulatory failure, to stimulate production of sperm in men, or to assist in treatment when the testes have failed to descend into the scrotum. Clomiphene is a synthetic nonsteroidal compound that is also used to promote ovulation.
Somatotropic hormone (somatotropin) and adrenocorticotropic hormone (ACTH, or corticotropin) are also produced by the anterior pituitary. Somatotropin comes from human pituitary glands removed at autopsy. This hormone regulates growth during childhood and is given to children who have failed to grow because of a growth hormone deficiency. ACTH stimulates the adrenal cortex to produce and secrete hormones, primarily glucocorticoids. ACTH is used in diagnostic testing and in the treatment of some acute neurologic problems.
Adverse Reactions
Because all of these medications are hormones, their primary adverse reactions include systemic or local hormonal reactions. Menotropins may produce ovarian enlargement, blood inside the peritoneal cavity, and febrile reactions; when it is used to increase fertility, multiple births may be produced. Clomiphene may produce abdominal discomfort, ovarian enlargement, blurred vision, nervousness, and nausea and vomiting. Vasomotor flushes (hot flashes), much like those seen in menopause, may also occur. Chorionic gonadotropin may cause headache, irritability, restlessness, fatigue, and edema. Precocious puberty (onset of sexual development at an early age) may result from its use in treatment for undescended testes.
Somatotropin may provoke antibody stimulation in some individuals, resulting in failure of the drug to produce any growth. ACTH is involved with numerous adverse reactions because it stimulates the adrenal gland. A summary of the most commonly used types of ACTH is provided in Table 21-4.
![]() Table 21-4
Table 21-4
Common Anterior Pituitary Hormones
| GENERIC NAME | TRADE NAME | COMMENTS |
| corticotropin (ACTH) | Acthar | Very rapid absorption and use necessitates administration q6h to maintain desired production; give IM or SC. |
| corticotropin repository | HP Acthar Gel | Slowly absorbed and can be administered in a single daily IM dose. |
| cosyntropin | Cortrosyn | Synthetic subunit of ACTH but exhibits all the pharmacologic properties of natural ACTH. Cosyntropin 0.25 mg is equivalent in action to 25 units natural ACTH and is less likely to produce allergies. Adrenocortical insufficiency testing: Give IM or IV. |
ACTH, Adrenocorticotropic hormone; IM, intramuscular; IV, intravenous; SQ, subcutaneous.
Posterior Pituitary Hormones
Action And Uses
The posterior pituitary gland produces the antidiuretic hormone (ADH) vasopressin, as well as oxytocin, a hormone that stimulates the uterus. Vasopressin regulates the reabsorption of water by the kidneys. This hormone is specifically released whenever the brain senses that the urine is becoming concentrated because the patient has had severe diarrhea or vomiting or has become dehydrated through some other condition.
Vasopressin may be given when the body loses water when it should not do so, as in diabetes insipidus, or when the pituitary fails to secrete vasopressin because of disease or surgical removal. Vasopressin is also used in some GI problems and in the treatment of nighttime bedwetting. Pituitary extract is also given to increase smooth muscle contraction of the digestive tract and blood vessels. Information on vasopressin and desmopressin (DDAVP, the synthetic form) is provided in Table 21-5.
![]() Table 21-5
Table 21-5
| GENERIC NAME | TRADE NAME | COMMENTS |
| desmopressin | DDAVP Stimate |
Synthetic antidiuretic inhalant; drug of choice in patients with mild to moderate diabetes insipidus. Offers prolonged antidiuretic activity without vasopressor or oxytocic side effects. Adults: Give in the evening. Effect noted and increased nightly by 2.5 mcg until satisfactory sleep duration attained. |
| vasopressin | Pitressin Vasopressin tannate |
Water-insoluble derivative of vasopressin with longer duration of action; of use in long-term treatment of diabetes insipidus in children and some adults. Adults: Give IM or SQ, 2 to 4 times daily. |
Oxytocin acts directly on the smooth musculature of the uterus to produce firm, regular contractions, as described in the second section of this chapter.
ACTH usually is reserved for testing and replacement therapy. ACTH stimulates the adrenal cortex to secrete cortisol, corticosterone, aldosterone, and several other weaker substances.
Adverse Reactions
Adverse reactions to small doses of vasopressin include abdominal cramps, anaphylaxis, bronchial constriction, circumoral (around the mouth) pallor, diarrhea, flatus (gas in the intestine), intestinal hyperactivity, nausea, “pounding” headaches, sweating, tremors, urticaria, uterine cramps, vertigo (feeling of dizziness and spinning), and vomiting. Vasopressin given in larger doses may produce death.
ACTH use, particularly over a sustained period, is associated with substantial adverse effects of the cardiovascular, endocrine, GI, musculoskeletal, and ophthalmic systems. The patient must be monitored closely while using these products. Suddenly stopping the medication may worsen symptoms.
Drug Interactions
Oral antidiabetic agents, urea, and fludrocortisone increase the effects of vasopressin, and large doses of epinephrine, heparin, and alcohol decrease the effect. The antidiuretic effect of desmopressin is decreased by lithium, large doses of epinephrine, demeclocycline, heparin, and alcohol. The antidiuretic effect of desmopressin may be increased by chlorpropamide, urea, and fludrocortisone.
ACTH interacts with aspirin, anticholinesterases, diuretics, barbiturates, and hydantoins.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
Learn everything possible about the patient’s health history to determine medication use and the presence of other diseases or conditions that would influence whether it is safe to use pituitary hormones.
n Diagnosis
Patients needing anterior pituitary hormones often have many symptoms that must be dealt with while the primary problems are resolved. These patients often have emotional, financial, and physical problems. Diagnosing problems that bother the patient and helping take care of them will be important in meeting the long-term treatment goals for each individual.
n Planning
There are no oral forms of pituitary hormones. They are given intramuscularly, subcutaneously, intravenously, or intranasally. Patients taking posterior pituitary products must be monitored closely. Additional doses may be required in times of stress.
n Implementation
The dosages of desmopressin are individualized so that the patient has an adequate daily rhythm of water metabolism and adequate duration of sleep. Generally the administration should be at the same time as polyuria or polydipsia and before sleep.
Even though vasopressin is given in an injection, the patient should drink one to two glasses of water at the time of administration to reduce the incidence of adverse effects.
n Evaluation
Monitor the patient taking ADH for a decrease in the frequency and the amount of urination, monitor the specific gravity of the urine, and watch for water intoxication or signs of dehydration.
n Patient and Family Teaching
Tell the patient and family the following:
Adrenocortical Hormones
Action
The adrenal cortex manufactures glucocorticoids, mineralocorticoids, and small amounts of sex hormones. Hydrocortisone and cortisone are two of the many glucocorticoids produced by the adrenal glands. These hormones regulate glucose, fat, and protein metabolism and control the antiinflammatory response and the immune response system. The mineralocorticoids consist of aldosterone and desoxycorticosterone. These hormones work with others to maintain the fluid and electrolyte balance in the body. They conserve sodium and increase the elimination of potassium. They are used in replacement therapy for adrenal insufficiency.
Uses
Glucocorticoids may be given in normal or physiologic doses for replacement of missing hormones in adrenal insufficiency (Addison disease). They are more commonly given in pharmacologic doses to reduce inflammatory, allergic, or immunologic responses and with antineoplastics to treat hematologic and malignant diseases. Examples of when glucocorticoids might be used are acute emergencies, allergic states, collagen diseases, connective tissue disease, diagnostic testing of adrenocortical hyperfunction, edematous states, hematologic and neoplastic diseases, ophthalmologic diseases, respiratory diseases, and miscellaneous conditions such as acute Bell palsy, chronic kidney disease, ulcerative colitis, and thromboembolic disease.
Local steroids might be used for intraarticular (into joints), soft tissue, or intrabursal (into bursae) problems, or for intralesional (into lesions) or subcutaneous dermatologic problems. Steroids might also be used topically for acute and chronic dermatoses, rectal problems, and some eye or ear problems.
Adverse Reactions
The side effects of systemic corticosteroids in pharmacologic doses are predictable exaggerations of the actions of the corticosteroids that are normally produced by the adrenal glands, or the results of reduced function of the hypothalamic-pituitary-adrenal axis. These are not benign drugs. Some adverse reactions are quite common; others are more unusual. Adverse reactions that might develop are listed in Table 21-6.
Table 21-6
Adverse Reactions Associated with Corticosteroids
| BIOLOGIC SYSTEM | POTENTIAL ADVERSE REACTIONS |
| Endocrine | Atrophy of adrenal cortex* (can occur after 10 days); anterior pituitary suppression; diabetes* (catabolism of fat, protein, glycogen, resulting in hyperglycemia); fluid/electrolyte imbalance* (from overlapping mineralocorticoid effect); hypokalemia; muscle cramps; irregular heart rate; redeposition of lipids* (moon face, buffalo hump, truncal obesity, striae, hirsutism, acne); and androgenic effects from sex hormones |
| Gastrointestinal | Gastritis,* peptic ulcer* (unrelated to local irritation of oral tablets); esophagitis; and pancreatitis |
| Immune | Absence of signs of infection*; uninhibited invasion and proliferation of virus, bacteria, fungus; and inhibition of fibroplasia with delayed wound healing |
| Musculoskeletal | Muscle wasting* (catabolism of protein) and osteoporosis |
| Neurologic | Mood changes (euphoria, insomnia, nervousness, irritability); mood swings (psychotic episodes, depression, exaggerated sense of well-being); and EEG changes |
| Ophthalmologic | Induces or aggravates glaucoma by decreasing aqueous outflow; cataracts; optic nerve damage; increased susceptibility to viral or fungal infection; and corneal perforation (when used in conditions that cause cornea to thin) |
| Vascular | Thrombosis, thromboembolism, thrombophlebitis, hypercholesterolemia, and atherosclerosis; these problems are especially prominent with cortisone |
| Miscellaneous | Hypertension; collagen tissue breakdown can activate latent TB by liberating organisms from deposits in pulmonary tissue; hypersensitivity reactions |
Drug Interactions
Corticosteroids increase the effects of barbiturates, sedatives, narcotics, and anticoagulants. They decrease the effects of insulin and oral hypoglycemics, coumarin anticoagulants, isoniazid, aspirin, and broad-spectrum antibiotics. Drugs that increase the effects of steroids are indomethacin, aspirin, and oral contraceptives, especially estrogen. Drugs that decrease the effects of steroids include ephedrine, barbiturates, phenytoin, antihistamines, chloral hydrate, rifampin, and propranolol. Some drugs produce exaggerated side effects when given with steroids. These include alcohol, aspirin and antiinflammatory drugs, amphotericin B, thiazides and other potassium-wasting diuretics, anticholinergics, cardiac glycosides, and stimulants such as adrenalin, amphetamines, and ephedrine. Steroids also interfere with numerous laboratory tests.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
There are many contraindications and precautions to the use of these drugs. Learn everything possible about the patient’s health history, including other diseases, other medications that might interact with corticosteroids, and whether the patient might have an infection or be pregnant.
n Diagnosis
Once a patient has started glucocorticoids, there is a constant need to look for adverse effects, both physical and psychologic. The nurse must be constantly aware of new symptoms that may represent pathologic conditions or disease.
n Planning
Steroids come in many forms. Corticosteroids may be administered by the following routes: oral, inhalation, intranasal, IV, intramuscular, subcutaneous, intrabursal, intradermal, intrasynovial, intralesional, soft tissue injection, topical, and per rectum. Only corticosteroid preparations with specific labels should be used for ophthalmologic or otic administration.
Steroids that are used topically affect only a small part of the body. Steroids that are injected or taken by mouth affect the whole body—they have effects throughout the body’s systems. Although glucocorticoids are highly potent drugs, short-term use of even very large doses is not likely to cause long-term problems. However, intermediate and long-term administration (longer than 6 days of systemic treatment) places the patient at high risk for a large number of serious adverse effects. How much risk is involved and how much benefit the patient will receive must be carefully considered. These medications stop production of steroids by the body, so if the medication is suddenly stopped, the body may be unable to function. The immediate and long-term effects of these drugs vary greatly and depend on the disease, the route of administration, dosage, duration, and frequency and time of administration.
Generic forms of the drugs are much less expensive than brand-name drugs. Generally, prednisone is considered the drug of choice to reduce inflammation and depress the immune system. It is recommended that antacids be taken with or between doses to help reduce the chance of peptic ulcer. Systemic corticosteroids are given orally, except in emergency circumstances or when the patient is unable to take oral medication. The onset of action is 2 to 8 hours, and the effects last for 24 hours. Oral corticosteroids are almost completely absorbed in the GI tract.
When corticosteroids are given orally to patients with functioning adrenal glands, the total dose should be taken first thing in the morning. This is the time when the adrenal glands are normally secreting the most hormones, so the corticosteroid dose will not cause problems with the body’s feedback loop.
For conditions requiring a local injection, a single injection yields sufficient antiinflammatory effects to reduce symptoms in many cases. The slowly absorbed forms (acetate, diacetate, tebutate) of corticosteroids generally give relief for 1 to 2 weeks.
n Implementation
Dosages vary a lot; the dose will be determined for each patient and each problem, based on the diagnosis, severity, prognosis, and estimated length of the disease. Patient response and tolerance will also be considered when deciding on dosage. Individuals may respond better to one form than another, but this is unpredictable. The general rule the physician follows, regardless of route of administration, is to prescribe as high a dose as necessary initially to get a favorable response, then decrease the amount gradually to the lowest level that will maintain the therapeutic effect but not produce complications.
In systemic administration, dosage regimens are of two types: (1) physiologic, for replacement of glucocorticoids in adrenal insufficiency; and (2) pharmacologic, to reduce symptoms.
Corticosteroids cannot be stopped without tapering (slowly reducing) the dose over time. Stopping the drug suddenly leads to steroid withdrawal syndrome, with symptoms of anorexia, nausea and vomiting, lethargy, headache, fever, joint pain, skin peeling, myalgia (widespread muscle pain), weight loss, and hypotension. Abruptly stopping the drug may also result in a rebound of symptoms of the condition being treated.
When corticosteroids are administered for longer than 1 to 2 weeks at pharmacologic doses, pituitary release of ACTH is stopped, and this causes secondary adrenocortical insufficiency. Patients undergoing physiologic, emotional, or psychologic stress may need additional support through larger amounts of steroids. This suppression of ACTH may last up to 2 years after the patient stops taking the drug.
During tapering to maintenance doses or to stop the drug, the patient must be watched carefully and taught the signs of adrenal insufficiency (malaise, hypotension, and anorexia [lack of appetite] are common, but many other symptoms may also occur). If these symptoms occur, or if the patient’s disease flares up, the steroid dose is increased until symptoms go away. Tapering then begins again on a more gradual plan. After shorter steroid courses (1 to 2 weeks), the dosage is reduced by 50% each day. The same scheduled dose intervals are kept.
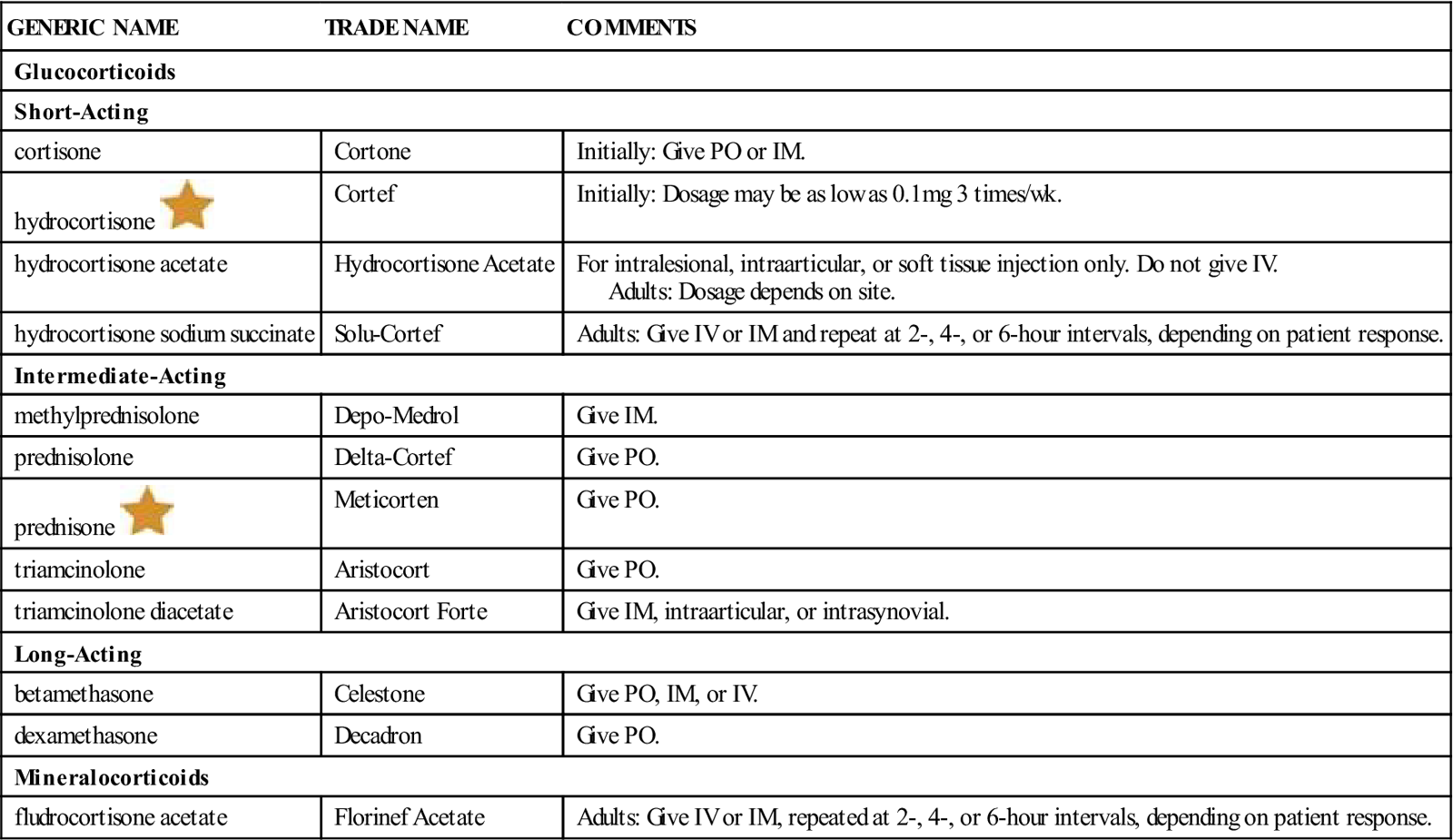
Table 21-7 provides a summary of adrenocortical hormones.
![]() Table 21-7
Table 21-7
| GENERIC NAME | TRADE NAME | COMMENTS |
| Glucocorticoids | ||
| Short-Acting | ||
| cortisone | Cortone | Initially: Give PO or IM. |
| hydrocortisone |
Cortef | Initially: Dosage may be as low as 0.1 mg 3 times/wk. |
| hydrocortisone acetate | Hydrocortisone Acetate | For intralesional, intraarticular, or soft tissue injection only. Do not give IV. Adults: Dosage depends on site. |
| hydrocortisone sodium succinate | Solu-Cortef | Adults: Give IV or IM and repeat at 2-, 4-, or 6-hour intervals, depending on patient response. |
| Intermediate-Acting | ||
| methylprednisolone | Depo-Medrol | Give IM. |
| prednisolone | Delta-Cortef | Give PO. |
| prednisone |
Meticorten | Give PO. |
| triamcinolone | Aristocort | Give PO. |
| triamcinolone diacetate | Aristocort Forte | Give IM, intraarticular, or intrasynovial. |
| Long-Acting | ||
| betamethasone | Celestone | Give PO, IM, or IV. |
| dexamethasone | Decadron | Give PO. |
| Mineralocorticoids | ||
| fludrocortisone acetate | Florinef Acetate | Adults: Give IV or IM, repeated at 2-, 4-, or 6-hour intervals, depending on patient response. |
IM, Intramuscular; IV, intravenous; PO, by mouth.![]() Indicates “Must-Know Drugs,” or the 35 drugs most prescribers use.
Indicates “Must-Know Drugs,” or the 35 drugs most prescribers use.
n Evaluation
All patients receiving systemic corticosteroids should be watched carefully, and the dosage should be adjusted to reflect reduced or increased symptoms, the patient’s response, and any periods of stress in the patient’s life (injury, infection, surgery, and emotional crisis). Patients should be monitored for 1 or 2 years after high-dosage or long-term treatment. To prevent unmonitored steroid use while patients are receiving steroids, they are usually given prescriptions that cannot be refilled.
Corticosteroids hide infection and increase the patient’s risk for infection. Corneal fungal infections are particularly likely to develop with extensive ophthalmologic corticosteroid use. Corticosteroids are particularly dangerous to use in patients with a history of tuberculosis, because the disease can be reactivated. With long-term use, active psychologic disorders may be made worse or hidden disorders made active because steroids affect mood. Long-term use may also produce osteoporosis, leading to vertebral collapse.
Although steroids are often used illegally in sports because they affect muscle size and strength, it is dangerous to take steroids for a long time because of all the damage they can do to the body.
n Patient and Family Teaching
Sex Hormones
Overview
The sex hormones are produced under the influence of the anterior pituitary gland. The male hormone testosterone and its related hormones are called androgens; the female hormones are estrogen and progesterone. Androgens help to develop and maintain the male sex organs at puberty and develop secondary sex characteristics in men (facial hair, deep voice, body hair, body fat distribution, and muscle development). They promote the anabolic or tissue-building processes in the body. Anabolic steroids are synthetic drugs with the same use and actions as androgens. These medications may be given as replacement therapy for testosterone deficiency. Androgen therapy may also be given to women as part of the treatment for estrogen-dependent inoperative metastatic breast carcinoma in patients who are past menopause. Androgens are also used to reduce postpartum breast pain and engorgement. Some postmenopausal women also use low-dose androgen therapy to treat a relative androgen deficiency. This helps reverse some of the masculinizing symptoms of menopause.
In addition to the two naturally occurring female hormones estrogen and progesterone, there are also a number of synthetic estrogen and progesterone preparations. Estrogen is secreted by the ovarian follicle and the adrenal cortex. Estrogens help develop and maintain the female reproductive system and the primary and secondary sex characteristics in women. They also are part of the feedback system to the pituitary, providing signals for the release of the gonadotropins. Estrogens play a role in the fluid and electrolyte balance in the tissues, especially in relation to calcium. They are active in most of the tissue and muscular processes involved in pregnancy and labor.
Progesterone is produced by the corpus luteum in the ovary, by the placenta, and in small amounts by the adrenal cortex. Progesterone is essential for the development of the placenta and helps maintain pregnancy once it occurs. Progesterone also helps prevent pregnancy by inhibiting the pituitary gonadotropins that cause the ovarian follicle to mature and produce ovulation.
Estrogen, progesterone, and combinations of the two hormones are very effective as oral contraceptives. They prevent ovulation and cause a state that mimics pregnancy in the female.
 Male Sex Hormones
Male Sex Hormones
Androgens
Action
The main action of androgens is to develop secondary male sex characteristics. Androgens are anabolic, increasing the building of tissue. Androgens are also antineoplastic when used to treat certain breast cancers in women. Erythropoiesis, or an increase in red blood cell formation, occurs with the administration of androgens.
Uses
Androgens are used in hypogonadism, hypopituitarism, dwarfism, eunuchism, cryptorchidism, oligospermia, and general androgen deficiency in males. They are used to restore a positive nitrogen balance in patients with chronic, debilitating illness or trauma; in treatment of anemia secondary to renal failure and in other blood dyscrasias in which increased erythropoiesis is needed; for palliative therapy (therapy to treat symptoms in terminal cases) for advanced breast cancer in postmenopausal women; and for treatment of endometriosis in younger women. Androgens are also used to suppress milk production. They are commonly misused by body builders and athletes who wish to have bigger and stronger muscles.
Adverse Reactions
Adverse reactions to androgens include edema caused by sodium retention (usually only with large doses), acne, hirsutism (excessive body hair), male pattern baldness, cholestatic hepatitis with jaundice, buccal irritation, diarrhea, nausea, and vomiting. In women, androgens may produce clitoral enlargement and masculinization. In men, androgens may cause a decrease in sperm count, excessive sexual stimulation, gynecomastia (enlargement of the breasts), impotence, and urinary retention. In children, use of androgens may produce precocious puberty. Children may also develop short stature because of premature bone epiphyseal closure.
Drug Interactions
Anabolic steroids may increase the effects of anticoagulants, antidiabetic agents, and other drugs. Corticosteroids given at the same time as androgens increase the possibility of edema. Barbiturates decrease the therapeutic effects of androgens because of increased breakdown in the liver. Androgens may affect the results of many laboratory tests.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
Learn as much as possible about the health history of the patient, including the presence of carcinoma; cardiac, renal, or liver dysfunction; other drugs the patient may be taking; and the possibility of pregnancy.
The male patient may have a history of impotence, reduced libido (sex drive), weight loss, male climacteric, or castration. There may be a history of traumatic castration or failure to develop secondary sex characteristics by 15 to 17 years of age.
n Diagnosis
These patients often have other problems that need to be diagnosed and addressed. In addition to their medical problems, they often have great concern about their sexuality, body image, and self-esteem.
n Planning
When androgens are given for hypogonadism, careful descriptions of secondary sex characteristics and measurements should be recorded for a baseline to monitor the therapeutic effects.
If cholestatic jaundice develops or liver function decreases, the drug should be stopped. Stomatitis (inflammation of the mouth) may result from buccal administration.
n Implementation
Androgens can be taken buccally, sublingually, and through the skin as a patch, depending on the specific drug and the reason for therapy. Dosages vary from 2 to 10 mg daily for replacement therapy. Higher divided doses are given for antineoplastic therapy.
Patients must be taught not to swallow the pill and not to eat, drink, or smoke or chew tobacco until the buccal tablet is absorbed.
Table 21-8 provides a summary of androgen products.
![]() Table 21-8
Table 21-8
| GENERIC NAME | TRADE NAME | COMMENTS |
| danazol | Androxy | Synthetic androgen is used to treat endometriosis, fibrocystic breast disease, and hereditary angioedema through suppression of pituitary gonadotropins and subsequent reduction in menstruation. Endometriosis: Give twice daily for 3-6 mo; may be continued for 9 mo. Used only for those who cannot tolerate other drugs or who fail to respond; therapy begun during menstruation to rule out pregnancy. Fibrocystic breast disease: Give PO twice daily for 4-6 mo; begun during menstruation; used only when pain is severe. |
| fluoxymesterone | Androxy, Halotestin | GI disturbances are more frequent with this product than with other oral androgens. Oral medication used for hypogonadism or breast cancer treatment. |
| methyltestosterone | Android | Patient should not drink, eat, or smoke or chew tobacco until tablet is absorbed buccally. Check mouth each visit for signs of local irritation. Used for male eunuchism, androgen deficiency, undescended testicle after puberty, and female breast cancer. |
| Testred | ||
| Virilon | ||
| testosterone gel | AndroGel | Primary hypogonadism: 1% gel (5 g tube contains 50 mg testosterone) applied in the morning to clean, dry, intact skin of shoulders, upper arms. AndroGel may be applied to abdomen, Testin is not used on abdomen. Application adjusted based on blood levels of testosterone. |
| Testin | ||
| Testosterone cypionate (in oil) |
Depo-Testosterone | Give injection every 2-4 wk. |
| testosterone pellets | Testopel | Use pellets given SQ every 3-6 mo. Each pellet contains 75 mg of testosterone. Number of pellets to be implanted gradually increases as parenteral injection dosage decreases. |
| Testosterone transdermal system | Androderm | 5 mg/day or two systems initially. System applied at night to a clean, dry area of skin on the back, abdomen, upper arms, or thighs. Should not be applied to scrotum. Patches worn for 24 hours. Blood levels tested and drug titrated up or down through use of additional patch. |
| Testoderm | Primary or secondary hypogonadism: Therapy started with a 6-mg/day system applied daily. Patch placed on a clean, dry scrotal area and worn 22-23 hr daily. After 3-4 wk of use, blood levels checked 2-4 hr after applying patch. Patient should achieve adequate blood levels in 6-8 wk or shift to another form of therapy. | |
| testosterone buccal | Striant | Mucoadhesive tablets for replacement therapy: 1 tablet taken morning and night; rounded side placed against gum and held firmly in place for 30 seconds to ensure adhesion. Tablet remains in position until the next tablet is applied. Protect tablet from heat and moisture. |
| Androgen Hormone Inhibitors | ||
| finasteride | Propecia | Used in benign prostatic hyperplasia and prostatic carcinoma; 5 mg once daily. May require 6 mo of therapy or more. Also used in treating male pattern alopecia |
| Proscar | ||
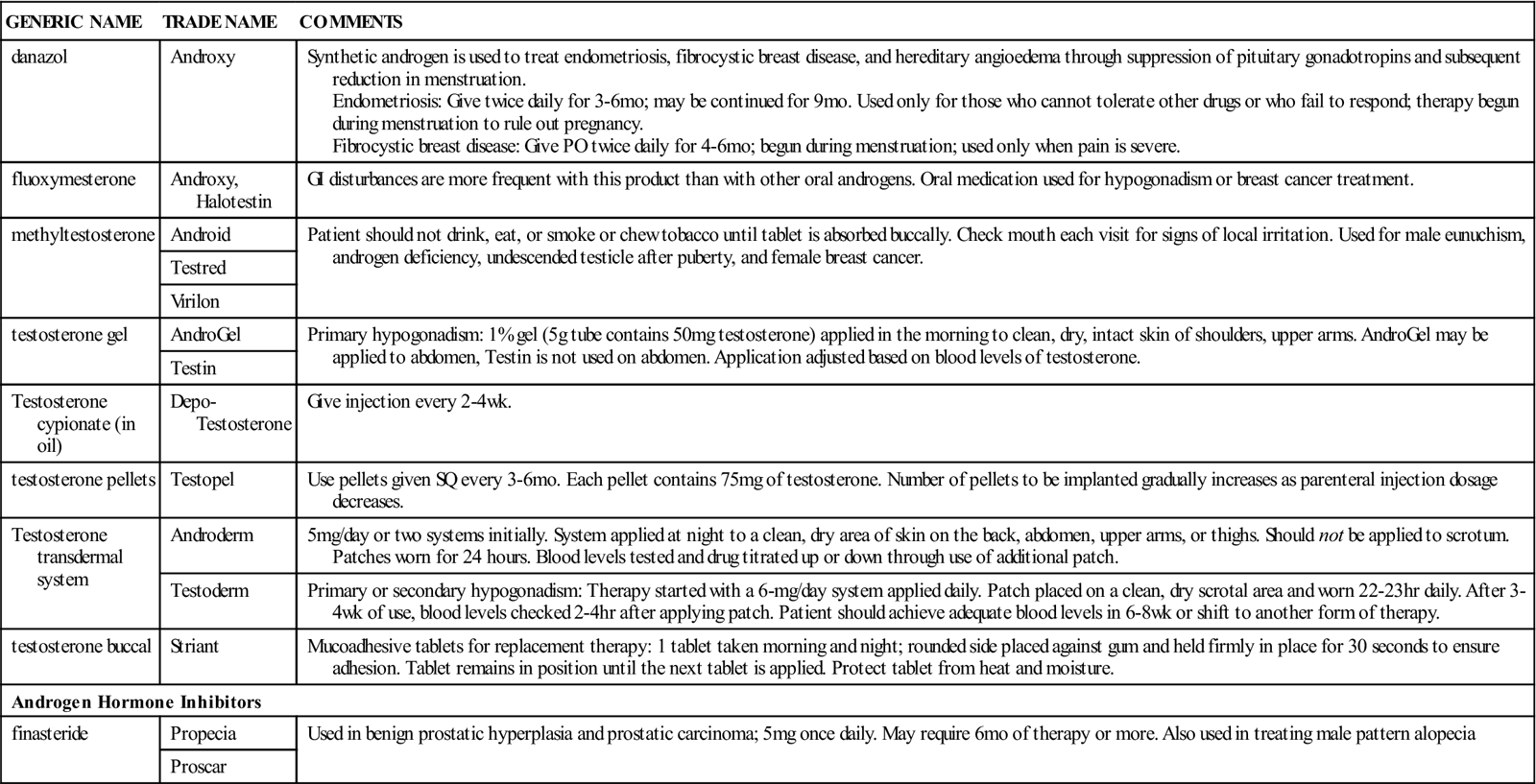
n Evaluation
The therapeutic response may be slow, requiring 3 or more months to affect symptoms. Monitor for the development of secondary sex characteristics and improvement in sexual functioning.
n Patient and Family Teaching
 Female Sex Hormones
Female Sex Hormones
Estrogens
Action
Exogenous estrogens (those produced outside the body) aid in the development of both primary and secondary sex characteristics, including growth and development of the uterine musculature and endothelium, vaginal epithelium, and fallopian tubes; development of breasts; increased cervical mucus and decreased vaginal pH; increased uterine motility; growth of axillary and pubic hair; decreased long bone growth in prepubertal and pubertal girls; and decreased calcium loss from bones. Estrogens suppress the release of gonadotropins (FSH and LH) from the pituitary or hypothalamus through a feedback mechanism. Estrogens are anabolic and cause retention of salt, water, and nitrogen, an increase in serum lipoproteins and triglycerides, and a decrease in cholesterol. They suppress ovulation when given in adequate doses.
Uses
Estrogens are used for hormone replacement therapy in menopause or other conditions in which the natural estrogens are decreased, such as ovarian failure, primary amenorrhea, and oophorectomy. They are used in infertility work-ups, for palliative therapy in prostatic cancer, and in breast cancer that occurs at least 5 years after menopause. After many years of controversy, it has now been demonstrated conclusively that estrogens are effective for prevention of postmenopausal osteoporosis when used with other measures, but they do increase the risk for stroke, heart attack, and breast cancer. Estrogen-progestin combinations also seem to provide a decreased incidence of uterine cancer. Use of these drugs should be determined for each patient after a careful weighing of the risks and benefits.
Adverse Reactions
Adverse reactions to estrogens include edema, hypertension, thrombophlebitis, depression, migraine headaches, skin rash, decreased glucose tolerance, intolerance to contact lenses, abdominal cramps, diarrhea, nausea, vomiting, breast tenderness and enlargement, changes in vaginal bleeding, worsening of estrogen-dependent malignancies, increase in size of uterine fibroids, vaginal candidiasis, and changes in weight and libido.
Drug Interactions
Rifampin and barbiturates may reduce the effects of estrogen. Estrogens may reduce the effects of oral anticoagulants, tricyclic antidepressants, anticonvulsants, and antidiabetic agents. They may potentiate antiinflammatory or glycosuric effects of hydrocortisone and the effect of meperidine. Estrogens alter the results of many diagnostic tests.
Progestins
Action
Progestins cause the uterine endometrium to shed during menses after tissue growth stimulated by estrogen. They maintain the endometrium and vaginal epithelium and decrease uterine motility during pregnancy. Acting with estrogen, they cause the breasts to secrete milk and become more vascular. Some progestins have estrogenic or androgenic effects. Progestins suppress pituitary gonadotropins through a feedback mechanism. They can suppress ovulation, control uterine bleeding caused by hormonal imbalance, increase sodium excretion, and cause a negative nitrogen balance.
Uses
Progestins are used for contraception, control of excessive uterine bleeding caused by hormonal imbalance, treatment of secondary amenorrhea, dysmenorrhea, and premenstrual tension, and control of pain in endometriosis. They may be used in the diagnosis and treatment of infertility. They are also used as palliative therapy for endometrial cancer. When used for contraception, progestin-only preparations are known as “mini-pills.”
Adverse Reactions
Adverse reactions to progestins include fluid retention, thromboembolic events (including pulmonary embolism), dizziness, headache, mental depression, rashes, decrease in glucose tolerance, weight gain or loss, cholestatic jaundice, diarrhea, nausea, vomiting, amenorrhea, breast tenderness or enlargement, decreased libido, galactorrhea, increased vaginal discharge, spotting, and withdrawal bleeding (bleeding that occurs when the drug is stopped). Overdosage produces changes in menses, nausea, vomiting, and withdrawal bleeding.
Drug Interactions
Progestins alter the results of several laboratory tests.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
For patients who have not had menses, ask about primary amenorrhea and sexual infantilism. For women of childbearing age, ask about the possibility of pregnancy, history of ovarian failure, need for contraception, and dysmenorrhea. For patients near the age of menopause, see if they have a history of hot flashes, menstrual irregularities, dyspareunia (pain during intercourse), vaginal discharge, vulvar pruritus, urinary frequency, and history of oophorectomy or hysterectomy.
There is an increased dose-related risk of thromboembolic disease, especially in premenopausal women. Progestins should not be used during pregnancy, especially the first 3 months, because of the risk of congenital anomalies and of vaginal adenosis or vaginal or cervical cancer in female offspring when they reach childbearing age. There is an increased risk of gallbladder disease with long-term use. Postmenopausal estrogen therapy is associated with an increase in the risk of endometrial cancer of 5 to 15 times the normal risk; the level of risk is related to the length of treatment. Administration of estrogen may result in hypercalcemia in patients with breast or bone cancer.
n Diagnosis
Based on the assessment, the nurse should be prepared to recognize and deal with other emotional or physical problems arising from estrogen or progestin use. As women go through their reproductive years, they have different concerns and physical problems. Determine age-related factors that may be of concern to the patient. Individualize therapy as much as possible.
n Planning
Estrogen therapy affects many body systems. When estrogens are used before puberty, short stature and decreased growth can result. Use in adult women can increase the risk for migraine headaches, hypertension, diabetes, and certain benign and malignant tumors. Because estrogens are metabolized in the liver and excreted through the kidneys, renal or hepatic dysfunction can alter their actions. Fibroid tumors of the uterus may increase in size. Because fluid is retained, symptoms of hypertension, asthma, epilepsy, migraine, and heart or kidney dysfunction may be increased. Topical estrogens are readily absorbed and may have systemic effects.
Progestins are especially valuable in women who cannot take estrogen. Progestins often cause menstrual cycle changes, and patients must understand this before taking these drugs if they are to have a successful experience. These medications can be used for contraception in women who are breastfeeding.
n Implementation
Estrogens can be given orally, intramuscularly, or topically. For control of menopausal symptoms, ovarian failure, or postoophorectomy symptoms, they are usually given in cycles of one tablet daily for 3 weeks, followed by 1 week off the drug. Usually, the lowest effective dose is given for the shortest period. High doses or long-term therapy should be tapered gradually.
Natural progestins are poorly absorbed orally; therefore oral progestins are synthetic products. Tablets are given daily. Progestins are quickly metabolized in the liver, but daily doses are effective.
Table 21-9 presents a summary of estrogens and progestins.
![]() Table 21-9
Table 21-9
| GENERIC NAME | TRADE NAME | COMMENTS |
| Estrogens | ||
| conjugated estrogens | Premarin | Contain 50%-65% sodium estrone sulfate and 20%-35% sodium equilin sulfate; these are naturally occurring and extracted from the urine of pregnant mares. Store in closed containers. Medication should be given for 3 wk on a daily basis, with 1 wk off the medication. Used for shortest time possible due to long-term adverse effects on heart. |
| esterified estrogens | Menest | Contain 75%-85% sodium estrone sulfate and 6%-15% sodium equilin sulfate. Store medication in a tightly closed container. Vasomotor menopausal (natural or surgical) symptoms: Adjusted to lowest dose that controls symptoms. |
| estradiol | Climara Delestrogen Estrace Estraderm |
Vasomotor menopausal symptoms, senile vaginitis, kraurosis vulvae, or replacement therapy in hypogonadism or female castration, ovarian failure. Give PO daily, cycled 3 wk on and 1 wk off. Lowest therapeutic dosage should be used. Transdermal system: Use patch 2 times/wk, increasing dose until symptoms resolve. May use 3-wk cycle on patch, 1 wk off. |
| estrogen vaginal creams | Estrace Estring Premarin |
Preparations used vaginally and on vulva to treat atrophic epithelial changes related to low estrogen levels. Can be absorbed systemically and produce side effects. Most effective when used at bedtime. Contain various synthetic estrogens. Usual dose: Use applicator included or rub on topically; lowest dose for shortest time that will control symptoms. |
| estropipate or piperazine estrone | Ogen | Composed of crystalline estrone and piperazine for stability. Prevention of postmenopausal osteoporosis, senile vaginitis, or vasomotor menopausal symptoms: Cycled 3 wk on, 1 wk off; lowest dose that controls symptoms should be used. |
| Estrogen and Progestin Combination | ||
| conjugated estrogens; medroxyprogesterone | Prempro | Moderate to severe vasomotor symptoms associated with menopause, prevention of osteoporosis: Give tablet once daily. Atrophic vaginitis: Vaginal cream daily for 1-2 wk then reduced dose 1-3 times/wk. |
| Premphase | ||
| Estrogen Agonist/Antagonists | ||
| raloxifene | Evista | Use for osteoporosis prevention. |
| Progestins | ||
| medroxyprogesterone acetate | Provera | Duration of action is long and somewhat variable. Secondary amenorrhea, abnormal uterine bleeding caused by hormonal imbalance: Give daily for 5-10 days, beginning on 16th or 21st day of menstrual cycle. Maximum therapeutic effect will be noted with 10 mg/day for 10 days beginning on 16th day of cycle. Withdrawal bleeding should occur 3-7 days after last dose. |
| megestrol acetate | Megace | Used for appetite enhancement in AIDS and cancer patients and for palliative treatment of advanced carcinoma of the breast or endometrium. |
| norethindrone | Micronor Nor-QD Ovrette |
This medication represents the only ingredient in some “mini-pill” contraceptives. Should be taken with meals to reduce nausea and stored in a closed container. Amenorrhea or uterine bleeding caused by hormonal imbalance: Give daily on 5th to 25th day of the menstrual cycle. Endometriosis: Give daily for 2 wk, increasing at 2-wk intervals until a target dose is reached. Continued 6-9 mo or until breakthrough bleeding occurs. Then it can be stopped temporarily. |
| norethindrone acetate | Aygestin | This medication is twice as strong as norethindrone. It is mildly androgenic. Should be taken with meals to reduce nausea and stored in a closed container. Uterine bleeding caused by hormonal imbalance: Give daily on 5th to 25th day of the menstrual cycle. Endometriosis: Give daily for 2 wk, increasing at 2-wk intervals until a target dose is reached. Continued 6-9 mo or until breakthrough bleeding occurs. Then it can be stopped temporarily. The object of therapy is to prevent menstruation |
| progesterone | Crinone | For amenorrhea, abnormal uterine bleeding, AIDS wasting: Give daily for 6-8 days by IM injection. |
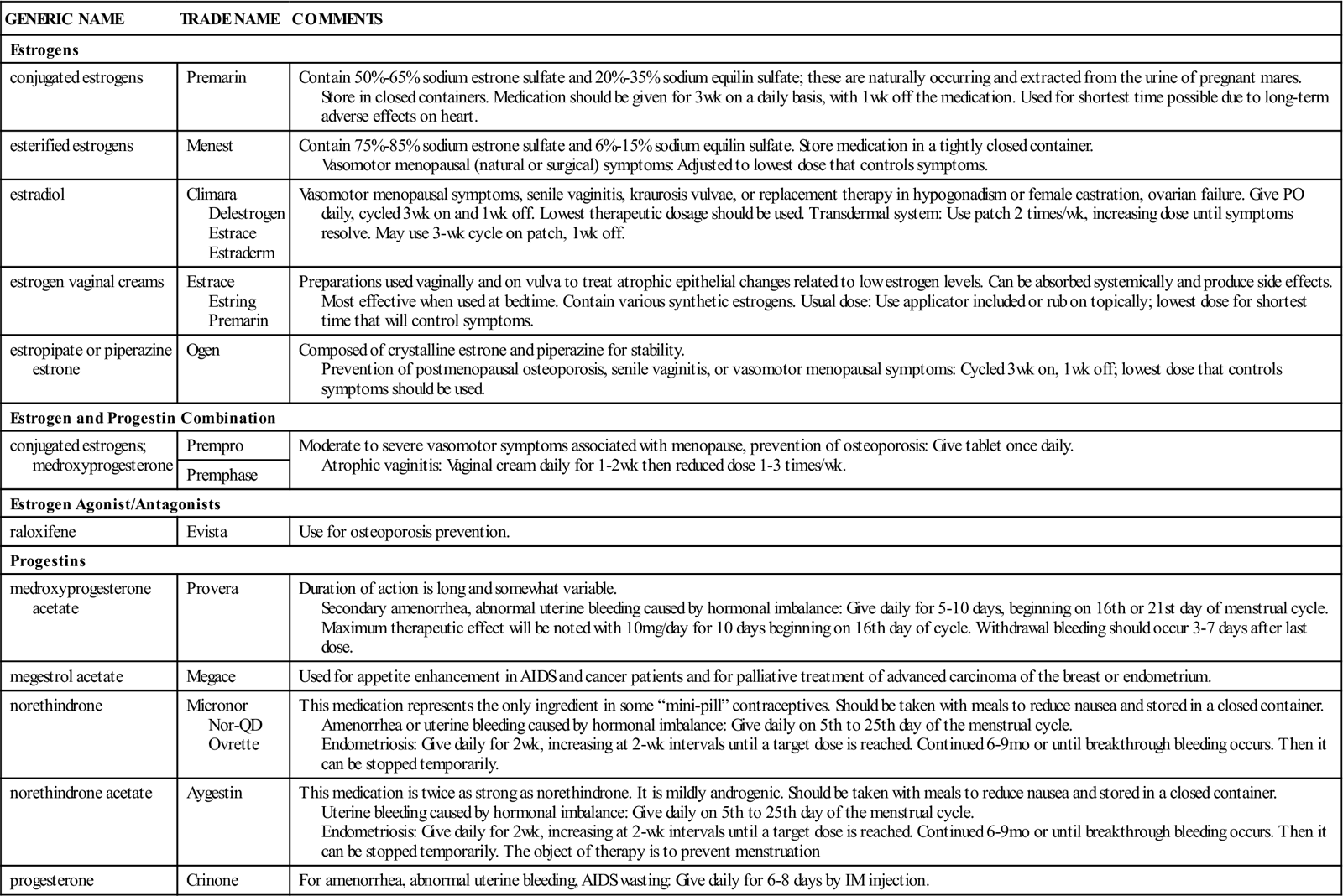
AIDS, Acquired immunodeficiency syndrome; IM, intramuscular; PO, by mouth.
n Evaluation
Patients on female hormone therapy should be monitored regularly, and the nurse should watch for adverse effects. Problems that should raise concern are thrombophlebitis and edema in women and feminizing changes or impotence in men.
Timing and description of any vaginal bleeding should be noted to determine if response is therapeutic or adverse.
n Patient and Family Teaching
Oral Contraceptives
Action
Most oral contraceptives are combination drugs that contain both an estrogen and a progestin. The principal action is to prevent ovulation by inhibiting FSH and LH. The progestin-only “mini-pill” prevents ovulation by the same mechanism but is more variable in suppressing the gonadotropins. The progestins in both types of oral contraceptive pills have several other contraceptive effects: creating thick cervical mucus hostile to sperm, slowing ovum transport by decreasing motility of the fallopian tubes, and blocking implantation. Oral contraceptive pills come in a variety of combinations, including those like Yasmin with very low estrogen content.
Uses
Oral contraceptives are used to prevent pregnancy when a highly effective method is needed and heterosexual activity is regular.
Adverse Reactions
Information on adverse reactions to oral contraceptives is provided in the previous sections on estrogen and progestin. Most adverse reactions are caused by hormonal imbalance. The types of hormonal imbalance and the symptoms they cause include the following:
Emergency Contraception
Any time a woman has unprotected intercourse and does not want to become pregnant, she may use an emergency contraception kit containing two tablets (or take two multiple-tablet doses) of selected combined oral contraceptive pills. Treatment must be started within 72 hours of intercourse, with two doses taken 12 hours apart. Emergency contraceptive pills work primarily by blocking or delaying ovulation or by changing the way sperm or ova are transported, thereby preventing conception. The risk of pregnancy is decreased by 75%. The pills often produce nausea and irregular menstrual bleeding.
Drug Interactions
There may be an increase in breakthrough bleeding and a decrease in contraceptive effectiveness in patients taking antitubercular medication, many antibiotics, barbiturates, and anticonvulsants.
Oral contraceptives may decrease the effectiveness of anticoagulants, antihypertensives, anticonvulsants, tricyclic antidepressants, oral hypoglycemics, and vitamins. When oral contraceptives are given with troleandomycin, the effect may be additive in causing jaundice. Oral contraceptives may alter many laboratory test results.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
To determine the most appropriate type of contraception for a patient, learn as much about the patient’s history as possible, including a thorough menstrual, contraceptive, and reproductive history. This must include any current diseases, the patient’s drug history, and whether the patient smokes. The nurse should make certain the patient is not breastfeeding or pregnant, assess the patient’s sexual activity and knowledge of contraceptive methods, and discover whether there are any contraindications for drug use (Box 21-1).
n Diagnosis
Does the patient have other knowledge deficits or financial, nutritional, or social problems that would interfere with her taking this medication? For example, women who smoke and use oral contraceptives are at much greater risk for venospasm and thromboembolic events. These women should either not be placed on oral birth control (OBC) if they are older than 35 years or should be helped to discontinue smoking.
For women using emergency contraception, do they understand other more effective ways to prevent pregnancy?
n Planning
Although breakthrough bleeding may be a side effect, nonfunctional causes should be investigated. Bleeding irregularities are more common with progestin-only pills.
There is some risk of infertility after oral contraceptives are stopped, especially in women who have had irregular or scanty periods before taking pills.
Research suggests that many women forget to take pills, resulting in many unintentional pregnancies. Discuss with the patient how the pill will fit into her lifestyle in such a way that she will remember to take it.
n Implementation
To be effective, oral contraceptives must be taken at about the same time each day. This is particularly true with progestin-only pills. Taking medication with meals will reduce the nausea common in the first cycles.
All oral combination contraceptives are to be taken for 21 days. Usually, therapy is started either the fifth day after or the Sunday after menstruation starts. Another method of contraception should be used for the first 7 to 10 days of the first cycle. Pills are packaged in a 1-month packet with the days named or numbered. Some preparations contain 28 pills to be taken daily, 7 of which contain an inert substance or iron. Others require the patient to go 7 days without pills before starting another 28-day cycle. During the “resting” phase of the cycle, vaginal bleeding should occur.
Combination pills vary in the type and amount of estrogen and progestin they contain (Box 21-2). All have at least one estrogen and one progestin. Two estrogens, ethinyl estradiol and mestranol, may be used in the different pills. Mestranol is half as strong as ethinyl estradiol. Several progestins are used in combination with them. Some progestins are estrogenic, antiestrogenic, or androgenic in effect. A dose of 50 mcg or less of estrogen is used to start therapy. Less than this dose may cause breakthrough bleeding, but doses of less than 50 mcg are increasingly being prescribed. Yasmin and YAS are low-estrogen formulations containing drospirenone and ethinyl estradiol. YAS may also be effective in preventing mild premenstrual syndrome. New combinations are introduced frequently. For the progestin-only pills, the medication is taken daily on a continuous basis.
Table 21-10 provides a summary of oral contraceptives.
![]() Table 21-10
Table 21-10
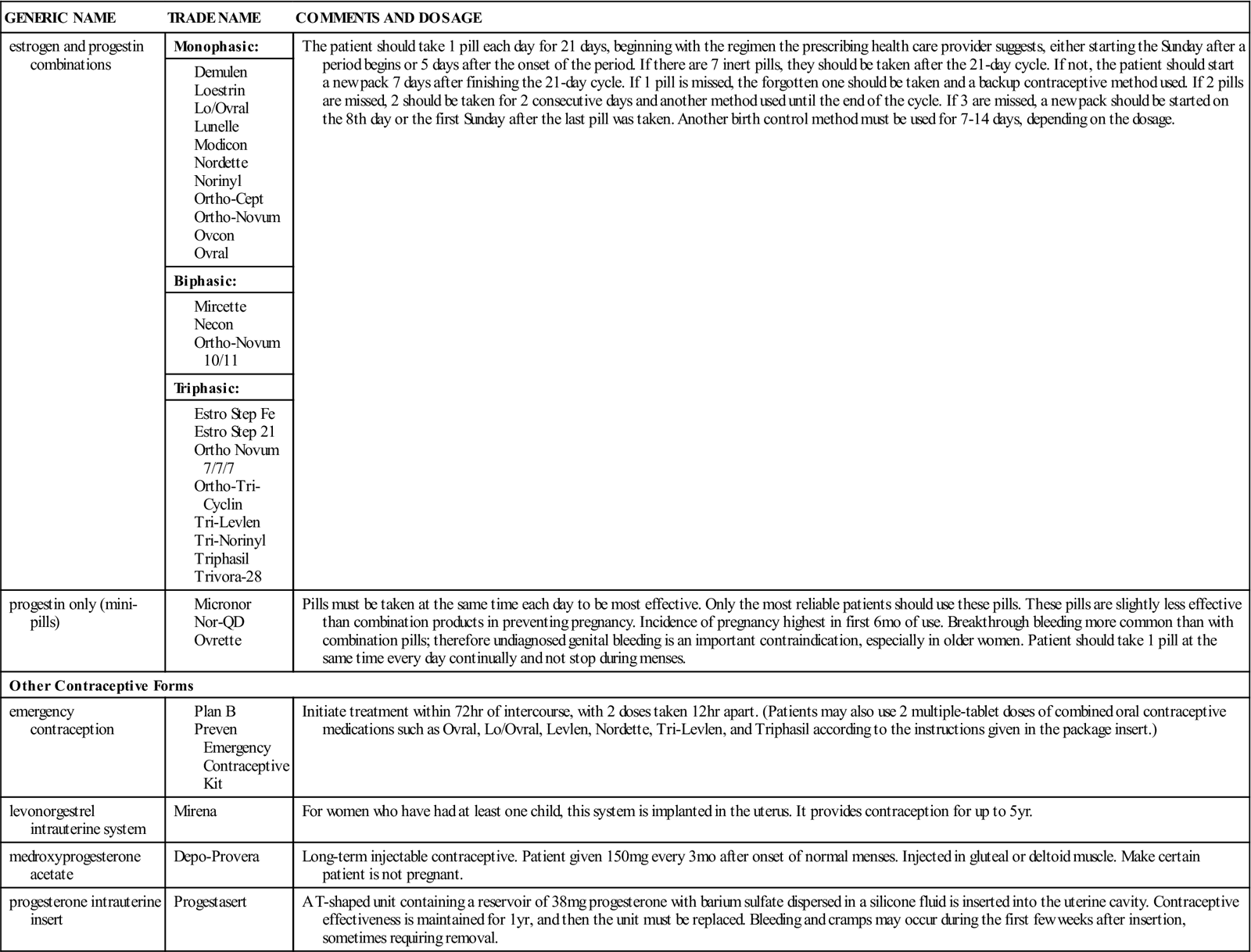
n Evaluation
Monitor for adverse effects, which may vary in severity. They may be a result of the different strengths of estrogen and progestin. Side effects may be avoided by changing to a different combination of estrogen and progestin pill. Some spotting can be tolerated in younger women.
Evaluate the patient’s compliance in taking medications. Patients who have trouble remembering to take other medications are not good candidates for oral contraceptives.
n Patient and Family Teaching
Tell the patient and family the following:
• The patient must return for scheduled checkups.
Each visit to the health care provider should include a history of possible side effects or adverse reactions since the previous visit, a review of whether the patient is taking the pill correctly, and a reminder of signs and symptoms to report.
Thyroid Hormones
Overview
The thyroid gland, located in the neck in front of the trachea, produces the hormones thyroxine (T4) and triiodothyronine (T3), which influence almost every organ and tissue of the body. Although their exact mechanism of action is unknown, their primary action is to control the metabolic rate of the tissues.
The anterior pituitary gland secretes thyroid-stimulating hormone (TSH), which tells the thyroid gland to release the hormones it has stored. When the level of circulating thyroid hormones is high, TSH from the anterior pituitary gland is withheld; when the circulating level falls, this information is also signaled, and TSH is once again released. This type of arrangement is called a feedback mechanism, because physiologic action influences the organ sending the signals.
Two general types of diseases can influence the hormone-producing activity of the thyroid gland. A decrease in the amount of thyroid hormones manufactured or secreted is called hypothyroidism. Symptoms include fatigue, malaise, lethargy, moderate weight gain (around 10 pounds) with minimal appetite, cold intolerance, menorrhagia, dry skin, coarse hair, hoarseness, impaired memory, and constipation. An increase in the amount of thyroid hormones manufactured and secreted is called hyperthyroidism. Symptoms include weight loss, decreased or absent menstruation, rapid or pounding heart, heat intolerance, nervousness, irritability, diarrhea, sweaty skin, insomnia (inability to sleep), fever, or chest pain.
Synthetic hormones, natural hormones, or a combination product may be given to increase the level of thyroid hormone in hypothyroid conditions or given as replacement therapy when the thyroid gland has been surgically removed. In hyperthyroid conditions, other preparations are given that slow the rate of thyroid production. Both thyroid supplements and antithyroid medications are described.
Thyroid Supplements Or Replacements
Action
The main action of the thyroid hormones is to increase metabolic rate. This results in an increase in tissue oxygen consumption, body temperature, heart and respiratory rate, cardiac output, and carbohydrate, lipid, and protein metabolism. In addition, thyroid hormones influence growth and development of the skeletal system, especially ossification in the epiphyses of long bones (the growth center).
Uses
Thyroid hormones are used in replacement therapy to manage hypothyroidism, myxedema, cretinism, or nontoxic goiter caused by deficiency of thyroid hormones, atrophy, congenital defects, and the effects of surgery, antithyroid products, or radiation. They are also used to treat chronic thyroid infections and tumors that depend on thyrotropic hormone.
Adverse Reactions
Adverse reactions to thyroid replacements include dysrhythmias, hypertension, tachycardia, hand tremors, headache, insomnia, nervousness, diarrhea, vomiting, weight loss, menstrual irregularities, rash, glycosuria, hyperglycemia, increased prothrombin time, and increased serum cholesterol levels. Overdosage produces signs of hyperthyroidism.
Drug Interactions
Thyroid preparations may increase the patient’s need for antidiabetic agents. Anticoagulant effects may be exaggerated by thyroid replacement because of increased hypoprothrombinemia. Corticosteroid needs are increased for patients taking thyroid preparations because of increased tissue demands. Effects of tricyclic antidepressants are increased by thyroid hormones. Many other isolated medications may be affected.
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
Try to learn as much as possible about the patient’s health history, including other drugs being taken that may produce drug interactions. Does the patient have diabetes mellitus, cardiovascular disease, adrenocortical insufficiency, or pregnancy? These conditions are precautions to the use of thyroid supplements. The patient may also have a history of hypothyroidism.
On examination, the nurse may find skin changes associated with myxedema, the most severe form of hypothyroidism. These changes include nonpitting edema; doughy skin; puffy face; large tongue; decreased body hair; and cool, dry skin. The thyroid gland may be normal in size, enlarged, or not palpable, depending on the cause of hypothyroidism. Neurologic signs include slow thinking, muscle weakness, slowed relaxation phase of the deep tendon reflexes, dull facial expression, and carpal tunnel syndrome. Cardiac signs include bradycardia and decreased blood pressure.
Laboratory findings in thyroid disease may include reduced free T4 index and elevated serum TSH; other tests may be abnormal.
n Diagnosis
Because thyroid disease may be insidious (hard to notice because of small changes) in onset, the patient may have many symptoms that require therapy at the time of diagnosis. Patients may have become depressed, have gained weight, or have problems with body image and self-esteem that should be addressed. Patients may find it hard to wait for the length of time it takes for thyroid medications to return them to normal functioning and resolve some of these problems.
n Planning
Patients older than 50 years of age are often very sensitive to thyroid hormones, so it is important for them to begin on a small dose. They should be observed for signs and symptoms of cardiovascular disease before the dosage is increased.
n Implementation
All treatment should begin with small doses and be increased gradually by the drug prescriber. A cut in dosage followed by a slower increase in the dose may be necessary when side effects occur. Therapy will often be withdrawn for 2 to 6 days and then restarted at a lower dosage if this happens.
The patient’s age, the presence of cardiac disease, and the severity of symptoms should be considered when thyroid hormone replacement therapy is begun. Titration of dosage (gradual adjustment of dose up or down) to gain the best response with the lowest dosage is the goal of therapy. The usual maintenance dosage in the treatment of hypothyroidism is 0.5 to 2 g as a single daily dose before breakfast.
T4 is the treatment of choice for hypothyroidism because of its purity and long duration of action. Because T4 has a slow onset of action, therapeutic effects may not occur for 3 to 4 weeks. T3, which has a rapid onset, may be given if rapid correction of hypothyroidism is necessary. The equivalent strengths of the various thyroid products vary, and care must be used in changing from one product to another. Patients should take the medication at the same time every day, preferably before breakfast. If medication is taken late in the day, insomnia may result.
Table 21-11 presents a summary of thyroid supplements or replacements.
![]() Table 21-11
Table 21-11
Thyroid Supplements or Replacements
| GENERIC NAME | TRADE NAME | COMMENTS |
| levothyroxine | Levothroid Synthroid Unithroid |
Synthetic preparation; drug of choice because effect is predictable. Increase dosage at 2-wk intervals until therapeutic effect is achieved. |
| liothyronine (T3) | Cytomel Triostat |
Synthetic hormone; has rapid effect and short duration of action, which allow fast dosage adjustment and quick reversibility of overdosage. Therapeutic effects achieved in 24-72 hr and persist up to 72 hr after withdrawal of drug. Mild hypothyroidism: Therapy initiated then dosage increased every 1-2 wk until effects achieved. |
| liotrix | Thyrolar | Liotrix is a combination of synthetic levothyroxine sodium (T4) and liothyronine sodium (T3) in a 4 : 1 ratio. Predictable therapeutic effect is an advantage. Therapy start and then increase at 1- to 2-wk intervals as needed. |
| thyroid, desiccated | Armour Thyroid | Desiccated thyroid contains T4 and T3 thyroid hormones in their natural state. Because these drugs are composed of desiccated animal thyroid glands, the hormonal content is variable, and T3 and T4 levels fluctuate; therefore avoid varying brands. Myxedema without hypothyroidism: Initiate therapy with increases until therapeutic effects are achieved. |
n Evaluation
Response to therapy is not immediate. Most patients begin to feel better within 2 weeks, and the therapeutic results are often seen in 3 months.
Teach patients the signs and symptoms of hypothyroidism and hyperthyroidism so they can determine if they are receiving too much or too little medicine.
If symptoms of overdosage occur, the prescribing health care provider should be consulted promptly. The medication will likely be stopped for several days, and therapy may be started again at a lower dosage.
Periodic blood tests should be done before thyroid hormone therapy is started and periodically once the patient is on a maintenance dose.
n Patient and Family Teaching
Tell the patient and family the following:
Antithyroid Products
Action
Antithyroid products are the main drugs used to treat hyperthyroidism or Graves disease. The principal action of antithyroid products is to stop the new production of thyroid hormones. These agents do not inactivate or inhibit thyroid hormones (T3 and T4) already stored or circulating in the blood.
Uses
Antithyroid products are used to treat hyperthyroidism or to improve hyperthyroidism in preparation for surgery or radioactive iodine therapy.
Adverse Reactions
Adverse reactions to antithyroid products include drowsiness, headaches, neuritis, paresthesias (numbness and tingling), vertigo, epigastric distress, jaundice, nausea, vomiting, skin rash, urticaria, myalgia, edema, alopecia, and lymphadenopathy. Hypothyroidism may occur as a result of prolonged therapy. Agranulocytosis (very low number of white blood cells [WBCs]) is a rare but serious occurrence. In addition, other more serious problems may develop. Propylthiouracil now carries a safety alert for serious liver damage, including liver failure or death in some patients.
Drug Interactions
The effects of anticoagulants are increased or potentiated by propylthiouracil. Caution should be taken when antithyroid drugs are given to patients who are receiving additional drugs known to cause agranulocytosis (e.g., hydantoin).
 Nursing Implications and Patient Teaching
Nursing Implications and Patient Teaching
n Assessment
Try to learn as much as possible about the patient’s health history, including hypersensitivity (allergy) to antithyroid drugs, other medications being taken that could cause drug interactions, and pregnancy or breastfeeding.
The patient may have a history of hyperthyroidism, including nervousness or tremor, weight loss with increased appetite, heat intolerance and excessive sweating, mood swings, and muscle weakness. On physical examination, the nurse may find exophthalmos (bulging eyes); thyroid enlargement; tachycardia; increased blood pressure; tremor; proximal muscle weakness; and warm, moist, smooth skin. Weight loss and the signs of chronic heart failure may be the most obvious signs of hyperthyroidism in older adults.
Laboratory findings may show elevated free T4 index, increased T3, or decreased TSH.
n Diagnosis
Because thyroid disease may be slow in onset, the patient may have many symptoms that require therapy at the time of diagnosis. Patients may be restless or anxious, have eating and sleeping problems, or have problems with concentration and memory that must be addressed. Patients may find it hard to wait for the length of time it takes for thyroid medications to return them to normal functioning and resolve some of these problems.
n Planning
Antithyroid drugs usually remove symptoms of hyperthyroidism if taken correctly for 1 to 2 years. Patient compliance with therapy should be encouraged to help them return to normal thyroid levels.
n Implementation
The therapeutic objective is to correct the hypermetabolic state with a minimum of side effects and without producing hypothyroidism. Clinical response to the antithyroid drugs usually takes 1 to 2 weeks because the drugs do not affect the release of thyroid hormone. Response depends on stopping the production of thyroid hormone in the thyroid gland. This in turn depends on the amount of hormone production materials present in the gland, and the rate of conversion of these materials into the thyroid hormones. Generally, therapy is maintained for 12 to 24 months and then reduced to see if the hyperthyroidism starts again. Titration to gain the best therapeutic response with the lowest dosage is the objective. Table 21-12 provides a summary of antithyroid products.
![]() Table 21-12
Table 21-12
| GENERIC NAME | TRADE NAME | COMMENTS |
| iodine products | Lugol’s Iodine Thyro-Block |
May have daily dose and dose before surgery. |
| methimazole | Tapazole Northyx |
Does not inhibit peripheral conversion of thyroxine to T3. More potent than PTU, and doses are one tenth those of PTU. Acts more rapidly but less consistently than PTU. |
| propylthiouracil | Propylthiouracil | PTU interferes with synthesis of thyroxine and blocks peripheral conversion of thyroxine to T3. May cause hypoprothrombinemia and bleeding. Adults: Give initial dose at 8-hr intervals, with adjustments in dosage made after 2 wk, depending on free T4 levels and symptoms. Therapy continued 6-18 mo before tapering. Watch for signs of liver damage. |
n Evaluation
Laboratory blood tests should be completed before beginning antithyroid therapy and periodically once the patient is on a regular maintenance dosage. Before therapy is started, a WBC count with differential is done; this should be repeated if there is any sign of infection. Serum T4 and TSH levels are monitored initially and after every 2 weeks of therapy until a euthyroid state (normal function of the thyroid gland) is achieved, usually in 3 to 5 months. Once the patient has been euthyroid for 6 to 12 months, a decision may be made to reduce the dosage and see whether the hyperthyroidism is under control. If hyperthyroidism seems to be absent, therapy is stopped.
n Patient and Family Teaching
Tell the patient and family the following:
Complementary And Alternative Therapies
Some herbal preparations may be used by patients for the treatment of hyperglycemia, hyperthyroidism, or hypothyroidism. These products may interact with other medications, as indicated in the Complementary and Alternative Therapies box.

