CASE 2
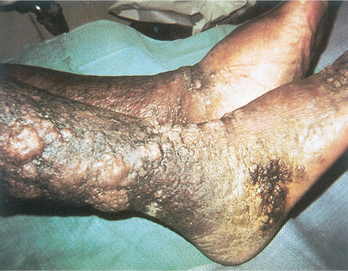
RD is a 1-year-old adopted boy in your practice who has had a surprisingly high (eight) number of severe recurrent viral and fungal infections (e.g., respiratory syncytial virus, Candida albicans) during his first 14 months. Each of these has eventually resolved, albeit very slowly, and the fungal infections have responded to the appropriate medication. You are nevertheless concerned to find the underlying cause of his problem. Recent chest radiographs performed at a local hospital to rule out pneumonia have been returned to you by the radiologist with a note that there was an abnormality, in particular an apparent absence of a thymic shadow. The radiologist has asked if there are any other stigmata of congenital absence of a thymus. As you describe this child’s history, you also mention his physical appearance (eyes widely separated, low ears, cleft palate).
QUESTIONS FOR GROUP DISCUSSION
RECOMMENDED APPROACH
Implications/Analysis of Family History
A family history is not possible because the child has been adopted.
Implications/Analysis of Clinical History
Viral and fungal infections point us immediately to a defect in T cells (Fig. 2-1). Although a defect in phagocytes may result in increased susceptibility to fungal infections, T cells are the key players in both viral and fungal infections. (Recall that the patient in Case 1 had a clinical history of fungal, viral, and bacterial infections.)
Implications/Analysis of Laboratory Investigation
The absence of a thymic shadow indicates a defect during embryogenesis resulting in partial or complete absence of a thymus. When the defect is very severe, T cell maturation cannot occur in the thymus. A deficiency of T cells would explain RD’s clinical history. Additionally, RD’s physical appearance is consistent with that of children diagnosed with this defect.
Additional Laboratory Tests
RD’s blood cell count was low (see Appendix for reference value), whereas the differential showed a profound decrease in lymphocytes (normal pediatric value = 48%). Skin tests to all three antigens were negative, despite the documented infection with Candida albicans. A negative skin test to all three antigens is interpreted as a defect in T cell function. (When the skin test is not definitive, a test that measures polyclonal activation of peripheral blood cells with T cell mitogens [concanavalin A and phytohemagglutinin] is used to assess T cell function.) In this assay, peripheral lymphocytes are incubated with a mitogenic lectin and proliferation (DNA synthesis) is assessed by measuring the amount of 3H-thymidine that is incorporated into the cells. Results of RD’s tests to detect the presence of serum antibodies specific for antigens used in pediatric immunization, DPT (diphtheria toxoid, pertussis, and tetanus toxoid), were all below normal. This is not surprising in that antibody responses to protein antigens require both cognate interaction with T cells as well as T cell–derived cytokines.
THERAPY
Depending on the severity of the defect, pharmacologic intervention may suffice during infections. However, more severe cases require transplantation of functional components of fetal/postnatal allogeneic thymic tissue slices placed into both quadriceps muscles or under a renal capsule. Because thymic aplasia is the result of abnormal development of the third and fourth pharyngeal pouches during embryogenesis, patients also have hypoplasia (or aplasia) of the parathyroids.
T CELL DEVELOPMENT IN THE THYMUS
Role of the Thymus
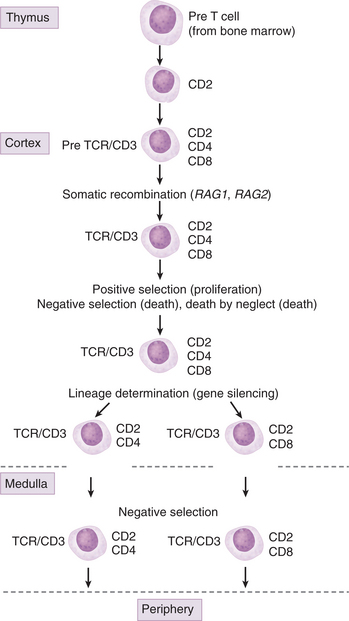
The rationale for transplanting a thymic tissue fragment into patients with DiGeorge syndrome is to provide an organ of differentiation for precursor T cells generated in the bone marrow (Fig. 2-2). To understand the complexities associated with this therapeutic intervention, it is important to know something about how T cells recognize antigenic peptides and the role of the thymus in selecting antigen-specific T cell receptors (TCRs). Each TCR chain consists of a variable and a constant region. Although most T cell clones express T cell receptors constructed with the same constant regions, each clone expresses receptors with a unique variable region. The key event in the construction of a unique TCR variable region is somatic recombination.