CHAPTER 162
Traumatic Brain Injury
David T. Burke, MD, MA; Di Cui, MD
Definition
Traumatic brain injury (TBI) is an insult to the brain from an external physical force and resulting in temporary or permanent impairment, functional disability, or psychosocial maladjustment. TBI occurs twice as frequently in males as in females. The incidence of TBI peaks among those 15 to 24 years old and again among those 75 years and older [1]. TBI usually is a consequence of motor vehicle accidents, falls, violence, and sports. Motor vehicle accidents and violence are more common in a younger population, and falls are more common in aging populations [2,3]. Recent trends have shown that TBI due to motor vehicle accidents is decreasing because of better traffic safety enforcement, whereas the percentage of TBI due to violence has increased, reported to be 7% to 10% [3]. These trends have consequences for the type of brain damage seen; contusion injury tends to be associated with falls, and diffuse injuries are more often seen in high-velocity traffic accidents [2].
In the United States, an average of 1.4 million TBIs occur each year, including 1.1 million emergency department visits, 235,000 hospitalizations, and 50,000 deaths [1,2,4,5]. The financial burden of TBI has been estimated at more than U.S. $60 billion per year [2]. However, routinely reported U.S. national data underestimate the true burden of TBI for several reasons. First, they do not include persons treated for TBI in other settings, including outpatient settings and physicians’ offices. Second, patients seen in military facilities both in the United States and abroad are not recorded. Finally, the number of those who receive medical care but for whom the TBI is not diagnosed or who sustain a TBI and do not seek care is not known [4,5].
The pathophysiologic process of brain injury is usually divided into primary injury, which is the injury to the brain that results at the time of the insult, and secondary injury, which can be thought of as the summation of the biochemical or physiologic damage that develops during a period of hours, days, weeks, and perhaps months after the primary injury. The primary injury is sustained from external forces as a result of direct impact, rapid velocity changes, penetrating injuries, or blast injuries. The resulting injuries include contusion, hematomas, and diffuse axonal injuries. These are often associated with superimposed hypoxic or ischemic injury, often as a result of systemic insult. In patients with mild TBI, there is often a disruption of the sodium channels on axons, which can result in a transient disruption in function and, with it, an increased state of vulnerability to additional trauma [6]. Secondary insults include intracranial hemorrhage, swelling, hypoxia, brain shift, herniation, and numerous neurochemical and cellular events [2]. Although many of the mechanisms of secondary TBI have yet to be elucidated, it is thought that the processes include neurotransmitter release, free radical generation, calcium-mediated damage, inflammatory responses, and mitochondrial dysfunction [2,7].
Symptoms
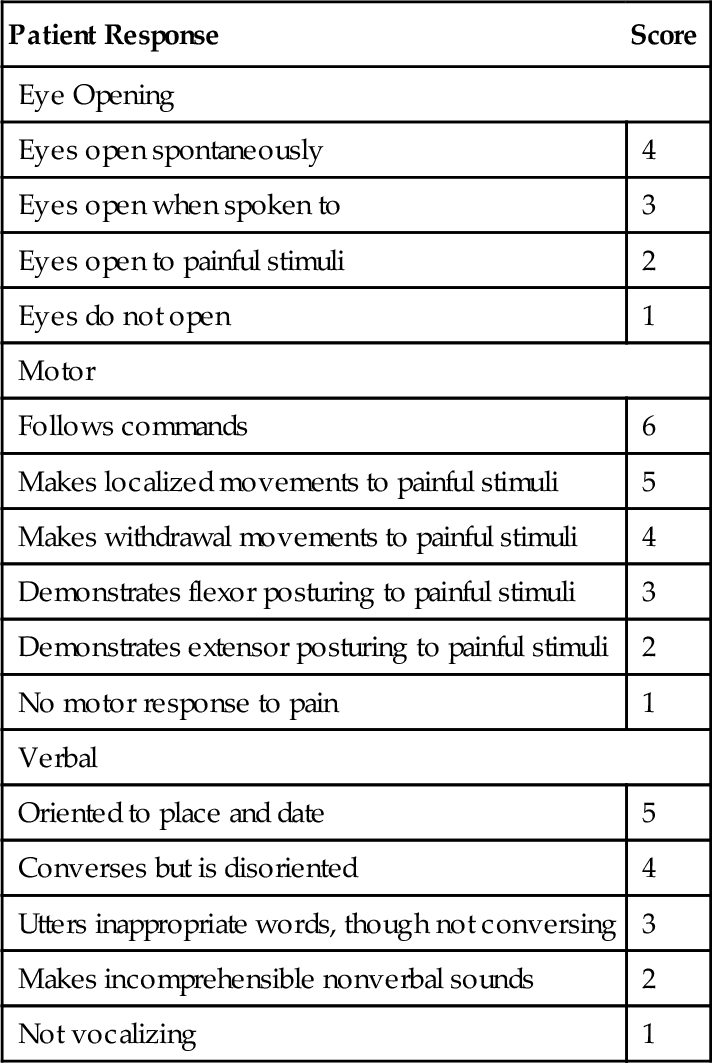
Symptoms may vary according to the severity of the injury and the stage of recovery. The patient’s history should include a detailed summary of the mechanism of injury, comorbid conditions, initial Glasgow Coma Scale score (Table 162.1), length of the coma (if any), and length of post-traumatic amnesia. Glasgow Coma Scale scores, however, can be obscured by confounders such as concurrent spinal cord injury, sedation, intubation, or other related injuries. Extracranial injuries (such as extremity fractures, thoracic or abdominal traumas), which have been reported to occur in about 35% of the cases, are associated with a higher incidence of secondary brain injuries [2,8].
Patients with severe injury and dramatically altered levels of arousal often can offer no subjective symptoms. After the acute phase of recovery, the clinician can expect symptoms to include seizures, contractures, spasticity, altered vision, vertigo or dizziness, and altered sense of smell. These may be the result of cranial nerve injuries or of central processing dysfunction. Symptoms of dysautonomia may still be seen at outpatient follow-up and may be characterized by increased body temperatures, tachycardia, tachypnea, increased posturing or tone, and profuse sweating [9]. Common late symptoms may include memory deficits, higher level executive dysfunction, headaches, difficulty with sleep-wake cycles, labile mood, depression, apathy, difficulty with attention, social disinhibition, sexual dysfunction, anxiety, impulsivity, fatigue, and difficulties with fine and gross motor control [10].
Physical Examination
A thorough neurologic examination, including a neuropsychological evaluation, is important to assess the consequences of a brain injury. The neurologic examination evaluates mental status, cranial nerve function, vision, hearing, deep tendon reflexes, and abnormal reflexes. The examination should also evaluate muscle strength, tone, and coordination and assess gait or mobility in a wheelchair. It is important to create a thorough neuropsychological profile with the assistance of a neuropsychologist. This should be done to determine both physical abilities and the cognitive and emotional issues that will affect the patient’s function. Cervical injury can be associated with TBI, especially in patients with a Glasgow Coma Scale score below 8 [11,12]. This must be recognized early to accurately assess injury severity and to determine treatment course.
Functional Limitations
Motor
Patients may have difficulty with mobility and self-care as a result of isolated motor weakness or coordination of either the upper or the lower extremities. Safe mobility may also be impeded by poor cognition, including deficits with planning and poor impulse control.
Behavior
Individuals often experience subtle or dramatic personality changes that alter relationships with others. These may include problems with the initiation of responses, verbal or physical aggression, altered emotional control, social disinhibition, depression, apathy, decreased sense of self-worth, and altered sexual function.
Social
Patients often are unable to return to work at their previous level of function. As a consequence, they may suffer significant economic strain and may have difficulty with their relationships, including their marriage. Studies have failed, however, to consistently show a significantly higher rate of divorce among those married at the time of injury [13]. Family members may be helpful in identifying issues of social isolation, depression, and anger.
Diagnostic Studies
Initial diagnostic studies can provide clues to the severity of the injury and will have prognostic implications. The IMPACT study suggests that in moderate to severe TBI, age, Glasgow Coma Scale motor scores, pupillary response, computed tomography characteristics, and the presence of subarachnoid hemorrhages are the most powerful independent prognostic factors for patient outcome. Other prognostic factors include hypotension, hypoxia, eye and verbal components of the Glasgow Coma Scale, glucose level, platelet number, and hemoglobin concentration [14].
Imaging Studies
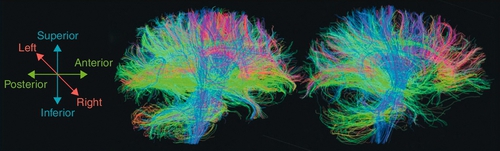
The initial computed tomography scan has been shown to be useful as an outcome predictor with use of the Traumatic Coma Data Bank classification or the Rotterdam computed tomography score [15]. Previous studies and guidelines have recommended computed tomography scans for all TBI patients with a Glasgow Coma Scale score of 14 and of 15 in the presence of risk factors, such as emesis, advanced age, duration of amnesia, injury mechanism, neurologic deficits, or anticoagulation (Table 162.2) [2,15–17]. More sophisticated testing has been introduced, including single-photon emission computed tomography, functional magnetic resonance imaging, and positron emission tomography, but for the most part, these are of little use in assessing the functional limitations caused by the injury. In patients with otherwise normal findings on neuroimaging, diffusion tensor imaging is emerging as a potential diagnostic tool for mild brain injury as it can detect white matter microstructure changes (Fig. 162.1) [18,19]. Despite the potential of new imaging techniques, TBI remains primarily a clinical diagnosis [20,21].
At the time of outpatient follow-up, it may be necessary to remind the patient and his or her caregivers of the extreme limitations of these studies and to focus on that patient’s functional abilities as the more important measure of the extent of the injury. In general, follow-up radiologic examinations are useful tools if the patient has excessively slow progress or has demonstrated a decline in function. These may be helpful in determining new or expanding lesions. Otherwise, these are generally of limited utility.
Biomarkers
The use of biomarkers to assess the magnitude of total brain injury and to localize brain injury is still in the investigatory phase. These markers may prove to be useful in patients with mild TBI with otherwise normal imaging findings as well as in patients whose injury severity cannot be accurately assessed because of confounders mentioned previously.
Biochemical markers of neuronal, glial, and axonal damage, such as neuron-specific enolase, S100B, and myelin basic protein, respectively, are readily detectable in biologic samples such as serum and cerebrospinal fluid and are being studied in patients with ischemic brain injury and TBI. These and others may soon be of use as an adjunct to neuroimaging in the early assessment of primary and evolving damage in traumatic and ischemic brain injury [22].
Functional Assessment Tools
One of the best diagnostic tools is the Glasgow Coma Scale, which is used for the initial evaluation of the severity of the patient’s injury (see Table 162.1). A review of this initial score will help in the determination of the extent of the injury and thus with prognostication. Later, as a review of function in the outpatient setting, progress can be measured by the Disability Rating Scale. Post-traumatic amnesia is important for prognostication as well and can be assessed by the Galveston Orientation and Amnesia Test. For the current level of functional recovery to be characterized, the Rancho Los Amigos Scale is helpful in the assessment of the patient’s awareness and interaction with the environment.
Neuropsychological Testing
This battery of tests, performed by a neuropsychologist, is the best means of determining the full spectrum of cognitive, affective, and emotional function of the individual. This may be completed before discharge from inpatient rehabilitation and should be repeated when a change in function needs to be documented. This testing may provide the clinician with critical information needed to understand the ability of the patient to progress toward more independence or responsibility at home or at work. This also may be a critical assessment tool for the documentation of the injury for insurance purposes.
Treatment
Initial
The initial focus of treating a patient with a TBI is to reduce the magnitude of the secondary injury. If the initial injury is of sufficient severity, computed tomography or magnetic resonance imaging is needed to determine the need for surgical intervention. The scans are reviewed for signs of excessive bleeding, edema, and shifting of the brain. If these signs are absent, medical intervention addresses the possible secondary injury that may result. Although it is still unclear as to how long a window of opportunity exists to affect the extent of secondary injury, it is generally accepted that this opportunity is likely to occur within the time of the initial acute hospitalization [4]. For this reason, there is little opportunity to affect this process in the outpatient setting.
Initially, metabolic issues such as blood pressure, electrolytes, hydration and nutrition, infectious processes, sleep disturbances including sleep apnea, and medications need to be addressed. Any imbalance in these may inhibit the function of the surviving brain tissue. Hydration and nutrition should be well maintained. An individual with a brain injury may be unable or unwilling to take nutrients by mouth, and this may necessitate either intravenous or direct gastrointestinal feedings. This may be a significant issue well into the post-acute phase of recovery. A survey for possible infectious processes includes, at a minimum, the pulmonary and genitourinary systems. Even infections that a clinician may otherwise label subclinical can disrupt the function of a damaged brain. For this reason, such infections should be treated as potentially symptomatic.
Medications can have undesired negative effects among those with a brain injury. These need to be reviewed carefully to eliminate any that may interfere with cognitive function. The list is long, but the most common offenders include antiseizure medications, antihypertensive medications, antispasticity medications, neuroleptics, sedatives, hypnotics, and gastrointestinal medications. Some of these may be unnecessary, whereas others may have less disruptive alternatives.
In addition to neuropsychological testing of the cognitive performance of the patient, psychological services are important in the assessment and treatment of affective disorders, which may include depression, apathy, and post-traumatic stress disorder. It is important to consider psychology services as being useful for the family and support system because the stress on these individuals may be tremendous. Psychologists and behavior specialists may be helpful for the intervention into behavior issues.
Arousal
Arousal will fluctuate throughout the day for a person with brain injury. Fatigue may become a long-standing problem. Frequent rests and naps may be needed, even at more than 1 year after injury. Pharmacologic interventions may be initiated for hypoarousal and excessive fatigue. These include amantadine, bromocriptine, carbidopa/levodopa, methylphenidate, modafinil, atomoxetine, amphetamine, nortriptyline, and protriptyline [23]. In a double-blind clinical trial, amantadine has been shown to accelerate the pace of functional recovery in patients with severe brain injury [24].
Attention
Neuropharmacologic agents for attention are similar to those used for arousal. These include neurostimulants, such as methylphenidate, modafinil, and atomoxetine, and dopaminergic agents, including amantadine, bromocriptine, and carbidopa/levodopa. Antidepressants include a long list of mixed as well as selective serotonin reuptake inhibitors; these will be especially useful if there is an element of depression interfering with cognition.
Agitation
Because agitation is a common and often troubling issue among those recovering from a TBI, a careful selection of pharmacologic agents is important to prevent injury, to allow focus on rehabilitation, and to reduce the stress on caregivers. In general, the agents that are preferred help control behavior while producing the least reduction in cognition. Because benzodiazepines are thought to have the potential of interfering with the recovery of the injured brain, these are often not recommended in the early stages of recovery. Other medications are therefore used as first-line agents. As an anxiolytic, buspirone seems preferable. A clinician may use antiseizure medications as a mood stabilizer (e.g., divalproex sodium, carbamazepine), newer antipsychotic medications (e.g., risperidone, quetiapine), beta blockers (e.g., propranolol), and antidepressants for anxious or agitated patients. Because poor attention to the environment may result in behavioral agitation, medications such as amantadine and methylphenidate should also be considered useful agents.
Memory
Because memory requires both arousal and attention, the medications previously discussed may produce improvements in the ability to learn. In addition, there have been limited reports of positive results through the use of donepezil, memantine, and other similar drugs. Memory can also be enhanced through the use of compensatory strategies and services. Speech pathologists can be useful for the introduction of and training in some of these strategies. There are portable electronic devices that can be preprogrammed with important information, and these memory aids can be frequently updated for individuals whose TBI may interfere with the ability to program the electronic memory aids.
Seizures
There is a reasonable body of literature to suggest that the use of antiseizure medications is not warranted if no seizure occurs within the first week after the brain injury. If the patient experiences a seizure after 1 week, the use of anticonvulsant agents may be needed for an extended time until the patient is seizure free for a period of 2 to 5 years; the patient is then to be reevaluated and managed per standard guidelines for patients with new-onset seizures [25,26]. Recommended agents depend on seizure type and usually include carbamazepine, valproic acid, and gabapentin.
Spasticity
Spasticity is a common problem among patients with brain injury (see Chapter 153). Patients may also have hyperactive muscle stretch reflexes and clonus. If these problems are not addressed, early contracture of joints may result. The modified Ashworth scale can be used to measure the degree of spasticity. As a first step of intervention, the clinician should look to reduce noxious stimuli, including anything that may produce pain. Infectious issues, positioning, and seating should be addressed as potential offenders. Stretching should be initiated and may necessitate serial casting and splinting. If medications are needed, these may include tizanidine, clonidine, dantrolene, diazepam, and baclofen. All of these agents have potential side effects and should be used judiciously. Dantrolene is unique in its lack of central effect but often results in acute liver dysfunction.
Rehabilitation
The rehabilitation of patients with brain injury begins during the acute stage of treatment when the risks of secondary brain injury are the greatest. After the acute phase, it is important that the clinician review the potential pharmacologic management and combine this with an interdisciplinary group of therapies, depending on the specific deficits of the patient. Studies have suggested that early admission to a dedicated inpatient brain rehabilitation unit is associated with reduced overall cost as well as improved outcome [27].
Physical Therapy
Physical therapy is important for the restoration of range of motion of the lower extremities and, if needed, through the use of serial casting. This may be aided by neurolysis or blocks at the neuromuscular junction. Later, issues of wheelchair preparation and propulsion may be important for those with sufficient impairment of mobility. Ambulation training with the appropriate assistive device should be frequently reviewed as the patient progresses with ambulation. Safety must always be considered because the patient with TBI may be endangered by impulsivity or poor planning and judgment.
Occupational Therapy
Occupational therapy addresses the preservation of joints when a lack of strength or an excess in tone or spasticity threatens a joint. As strength and ataxia are often issues in the first year, these should be addressed individually. The issues of self-care, including daily activities such as dressing, bathing, and grooming, must be addressed and emphasize the need for a planning strategy for the patient. Cooking and driving evaluations may be needed to advise the patient before his or her return to the home.
Speech Therapy
Early in the care of the patient, the ability to swallow safely needs to be evaluated. In addition, the speech pathologist, ideally working with the neuropsychologist, can identify focal cognitive needs of the patient and addresses these over a length of time. These often involve memory strategies, such as mnemonic training, and pragmatics, which focus on the contextual and social aspects of communication skills. Published cognitive rehabilitation studies have generally supported the efficacy of interventions that target memory [28–31]. These memory strategies, as a part of a comprehensive cognitive rehabilitation program, may enhance a partial restoration of focal activities in regions of the brain associated with memory, such as the hippocampus [32].
Vocational Rehabilitation
Many patients will have difficulty in returning to their previous level of employment. Vocational rehabilitation counselors can evaluate a patient’s skills and determine the need for training.
Procedures
For spasticity, local injections may be preferable to oral medications. These may include nerve root blocks, nerve blocks, motor unit blocks (all with phenol), and neuromuscular junction blocks (with botulinum toxin). When spasticity is severe and not responsive to these interventions, an intrathecal pump may be considered for continuous infusion of baclofen into the cerebrospinal fluid (refer to Chapter 153).
Surgery
Patients with new-onset hydrocephalus may need a shunt placed to reduce the pressure load at the brain. If medications and other measures fail to control spasticity and contractures result, surgery may be an option. If joint contractures occur, a surgical release may be indicated.
Potential Disease Complications
Seizures can result from a TBI. The risk is highest early after the injury but persists for years. Soon after the injury, patients are at risk for aspiration pneumonia and, if their swallowing is impaired, for malnutrition and dehydration. Sleep apnea is a frequent early issue. If not treated, it may exacerbate the symptoms of the TBI. Continuous positive airway pressure may be an effective treatment. As with all trauma patients, there is a risk for deep venous thrombosis (see Chapter 127). This must be treated with prophylactic heparin or, if hemorrhage is a risk, with pneumatic compression devices or an inferior vena cava filter.
Potential Treatment Complications
Medications that are used to treat attention and arousal may lead to excess arousal and agitation. This may also be manifested as somatic complaints or delirium. Medications for agitation and seizures may slow the patient’s recovery over time and may reduce the patient’s function while the medications are taken. Refer to Table 162.3.
Table 162.3
Medications Frequently Used in Traumatic Brain Injury
| Symptoms | Medication | Initial Dose | End Dose |
| Arousal | Amantadine | 50 mg, 8 AM and 2 PM | 100 mg, 8 AM and 2 PM |
| Bromocriptine* | 1.25 mg, 8 AM and 2 PM | 5.0 mg, 8 AM and 2 PM | |
| Carbidopa/levodopa* | 10 mg/100 mg tid | 25 mg/100 mg tid | |
| Methylphenidate | 2.5 mg, AM and 2 PM | 20 mg, AM and 2 PM | |
| Modafinil | 100 mg qd | 100 mg, 8 AM and 2 PM | |
| Dextroamphetamine (Dexedrine) | 5 mg qd | 30 mg, AM and 2 PM | |
| Attention | Methylphenidate | 2.5 mg, AM and 2 PM | 20 mg, AM and 2 PM |
| Adderall | 5 mg bid | 20 mg bid | |
| Modafinil | 100 mg, AM | 100 mg, AM and PM | |
| Amantadine | 100 mg, AM | 150 mg, AM and 2 PM | |
| Bromocriptine* | 1.25 mg, AM | 50 mg, 8 AM and 2 PM | |
| Carbidopa/levodopa* | 10 mg/100 mg tid | 25 mg/100 mg tid | |
| Sertraline (Zoloft) | 50 mg qd | 200 mg qd | |
| Citalopram (Celexa) | 20 mg qd | 40 mg qd | |
| Donepezil (Aricept) | 2.5 mg qd | 5 mg bid | |
| Memantine (Namenda) | 5 mg qd | 10 mg qd | |
| Atomoxetine (Strattera) | 20 mg qd | 60 mg qd | |
| Agitation | Buspirone | 7.5 mg bid | 30 mg bid |
| Carbamazepine | 200 mg bid | 600 mg bid | |
| Risperidone | 1 mg bid | 6 mg/day | |
| Morphine | 10 mg q4h | 10 mg q4h | |
| Propranolol | 10 mg qd | Limited by heart rate and blood pressure | |
| Quetiapine (Seroquel) | 25 mg qd | 800 mg qd |
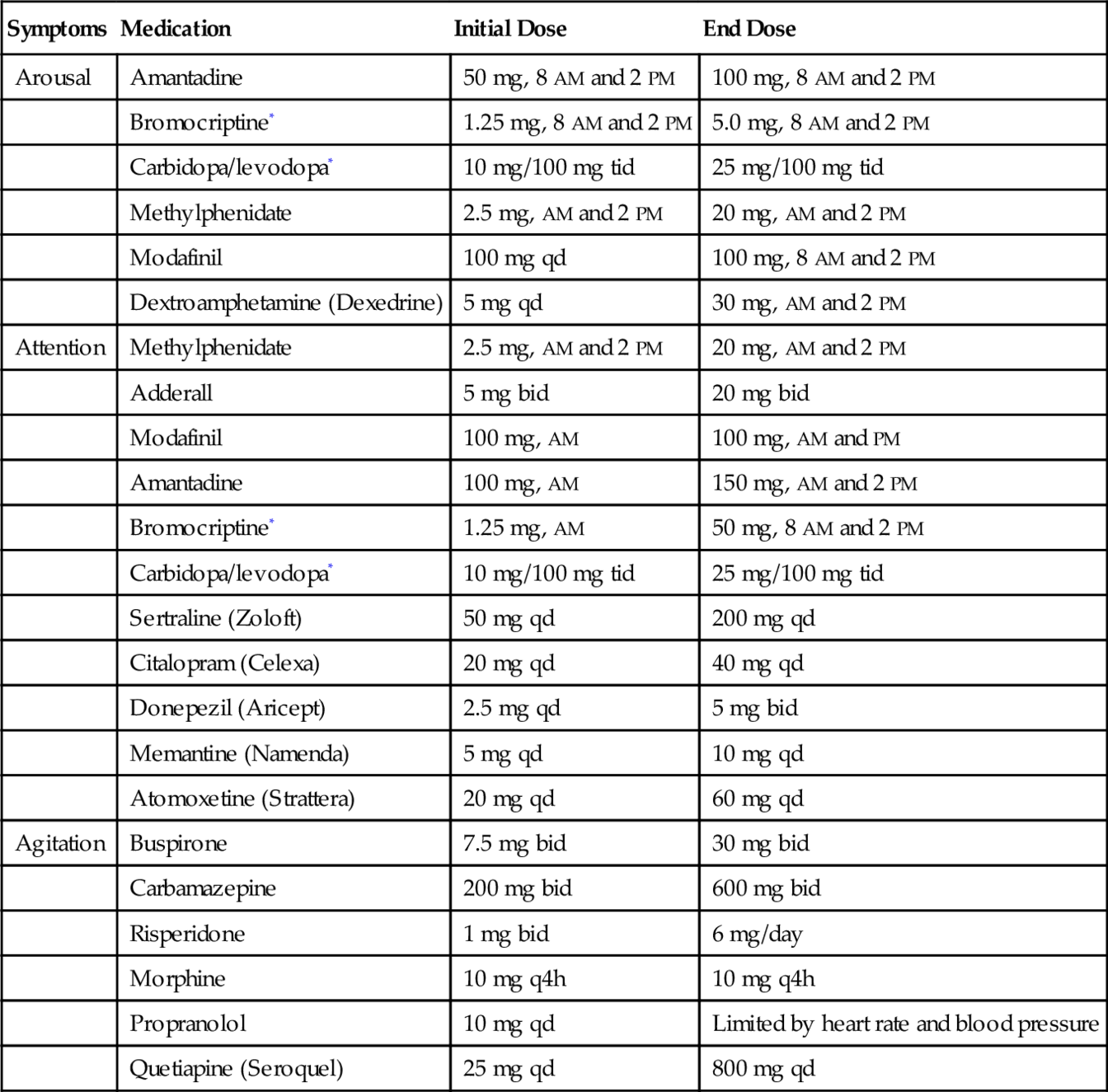
* Limited by hypotension.