CHAPTER 161
Transverse Myelitis
Definition
Transverse myelitis is a focal inflammation across the spinal cord along one or more levels. This inflammation can cause damage to the ensheathing nerve cell fiber myelin, with resultant nervous system dysfunction [1]. The diagnosis may incorporate the terms acute, meaning arising suddenly and intensely, and idiopathic, in which no specific bacterial, viral, or other obvious inflammatory cause can be found. Other descriptors include acute partial, acute complete, and longitudinally extensive. Few population-based studies are available, and comparative or meta-analysis of the literature is difficult because of the different presentations of transverse myelitis being reported. It appears, however, that acute transverse myelitis is rare, with only 1400 new cases annually in the United States, or 1 to 4 cases per million population per year [1,2].
An older study from 1993 in the United States on acute or subacute noncompressive myelopathy showed these cases to be 45% parainfectious, 21% multiple sclerosis, 12% spinal cord ischemia, and 21% idiopathic [3]. With the availability of improved diagnostic tools, possible changes in disease patterns, and longer follow-up, the etiology of transverse myelitis may be clearer. A 2012 study from France on acute partial transverse myelitis with a median follow-up period of 104.8 months reported the etiology of cases as 62% multiple sclerosis, 1% postinfectious myelitis, 1% neuromyelitis optica, 1% Sjögren syndrome, and 34% undetermined (i.e., idiopathic) [4]. However, another French multicenter retrospective study applying the Transverse Myelitis Consortium Working Group criteria [2] for acute transverse myelitis to 288 subjects was more evenly spread. It reported the etiology as 20.5% systemic disease (systemic lupus erythematosus, Sjögren syndrome, antiphospholipid syndrome), 18.8% spinal cord infarct, 10.8% multiple sclerosis, 17.3% infectious or parainfectious, 17% neuromyelitis optica, and 15.6% idiopathic acute transverse myelitis [5].
There is a female predominance of 60% to 75% [4–9] and a bimodal age distribution. Patients having transverse myelitis related to multiple sclerosis, postinfectious transverse myelitis, or idiopathic transverse myelitis are younger, whereas those with transverse myelitis related to spinal cord infarcts or delayed radiation effects are older [4,6,8,10]. Transverse myelitis may recur, with reported rates ranging from 17.5% [9] to 61% [8], and relapse appears to be more common with acute partial transverse myelitis [11].
According to one magnetic resonance imaging (MRI) study, idiopathic acute transverse myelitis most commonly affects the cervical region (60%), followed by the thoracic region (33%) [5]. The onset of transverse myelitis can be acute (within hours or days) or subacute (between 1 and 4 weeks) [1,3]. The period from onset to complete weakness in idiopathic transverse myelitis has been reported to range from 10 hours to 28 days, with a mean of 5 days [10]. Subacute presentations, progressing over days to weeks and ascending, are associated with a good to fair prognosis. Acute and catastrophic presentations with back pain have a poorer outcome [12].
Symptoms
Patients with transverse myelitis may present in the ambulatory clinic or hospital setting with complaints of weakness of the arms and legs, pain, sensory impairments, or difficulties with the bowel and bladder. Weakness may affect the lower limbs or all four limbs with varying severity. Sensory complaints may include hypersensitivity, numbness, tingling, coldness, or burning. Pain is a common symptom in a third to one half of patients and may be localized or shooting in character. Bowel frequency or constipation may occur, and bladder symptoms include increased frequency, retention, and incontinence [1,10].
The history may reveal symptoms of recent infection, immunocompromised or autoimmune condition, space-occupying lesion, demyelinating disease, travel, vaccination, trauma, sexual exposure, animal bites, and insect or tick bites. A careful review may yield systemic symptoms, including the upper respiratory tract with cough and difficulty in breathing, chest pain, rashes, joint aches, muscle pain, vision changes, nausea, diarrhea, constipation, and problems with urinary function. Particular attention should be paid to details pointing toward potentially treatable or reversible conditions responsive to antimicrobials or surgical decompression.
Any history of invasive spinal intervention for pain management should be explored. Cases of acute paraplegia with sensory, bowel, and bladder dysfunction have been reported after epidural steroid injections and lumbosacral nerve root blocks. Inadvertent direct cord injury may occur, or a vascular injury resulting in cord infarction may be the cause [13–15]. Damage to an abnormally low artery of Adamkiewicz as it travels with the nerve root through the neural foramen has been postulated. This dominant radiculomedullary artery arises between T9 and L2 levels in 85% of people, but it may arise from the lower lumbar region to as low as S1 [15]. There has also been a report of transverse myelitis resulting from the infected catheter tip of an intrathecal morphine pump for chronic pain [12].
Physical Examination
The physical examination follows a thorough history taking and will focus on the manifestations and findings relating to myelopathy, such as motor weakness, changes in sensation (pinprick, light touch, vibration, position sense, or temperature), tone, muscle stretch reflexes, coordination, and bowel and bladder functioning. Changes affecting the brain, such as cognitive dysfunction and cranial nerve and visual abnormalities, are generally not seen with idiopathic transverse myelitis and suggest other diagnoses.
Temperature elevation, tachycardia, and tachypnea may indicate an infectious etiology. Infections and autoimmune conditions that cause acute inflammation of the spinal cord may also be manifested in the other body systems. The respiratory, cardiovascular, gastrointestinal, and genitourinary tracts as well as the musculoskeletal and integumentary systems should be assessed accordingly. The findings may assist in determining the level of spinal involvement, guide diagnostic testing for the myelitis, and help rule out other diagnoses.
Functional Limitations
The physiatrist is likely to encounter the patient as a consultation or referral for rehabilitation assessment and management or for specific problems, such as spasticity and pain intervention. As with other spinal cord injuries, the functional limitations in transverse myelitis usually depend on the level or levels of injury and the muscles that are affected or continue to be innervated normally. Debilitation and deconditioning from associated illnesses and prolonged recumbence will also affect function secondarily.
Recovery is often related to the clinical presentation and may or may not be complete. In general, one third of patients with acute transverse myelitis make a good recovery, another third have fair recovery, and the rest either fail to improve or die [5,7,10,16]. In idiopathic transverse myelitis treated with methylprednisolone, by use of the Medical Research Council (MRC) scale for muscle strength (5, normal; 0, no movement is observed), 37.5% were reported to have complete recovery or minimal residual deficit (MRC 5-4), 43% had partial recovery (MRC 3), and 19.4% had severe disability or absent recovery (MRC 0-2). Factors associated with poor outcomes include severe initial symptoms with spinal shock, delayed presentation to the hospital after maximum deficits have already occurred, development of syringomyelia, and extensive MRI lesions [5,10]. If no recovery has occurred by 1 to 3 months, complete recovery is less likely [3,12].
The following functional capability review according to spinal level may be influenced by whether the cord injury is unilateral or bilateral and the degree of completeness. A patient with only C4 innervation preserved and loss of function distally may or may not have respiratory difficulties but will be dependent for most self-care activities. Using appropriate technology and devices, whether they are customized or commercially available, the patient may be able to control the immediate home environment, summon assistance, and mobilize in an electric wheelchair with a chin control or a sip-and-puff interface. Devices that can be controlled by moving and positioning the head, cheek, or tongue and by infrared-sensitive or voice-activated mechanisms include door openers and various electronic devices such as the television and personal computer.
A patient with C5 level may be able to self-feed and groom with equipment such as a glove with universal cuff allowing attachment of tools (e.g., fork, spoon, or comb). The patient can independently use a powered wheelchair and propel a lightweight manual wheelchair with rim projections (“quad knobs”) for limited distances over level ground. C6 innervation allows independence with upper extremity dressing, bathing with equipment, and functional use of a manual wheelchair indoors. The patient with superior balance and motor control could potentially perform independent or supervised transfers with a sliding board, self-catheterize with appropriate aids, and drive a specially adapted automatic transmission vehicle with powered steering, hand-controlled accelerator and brake. A C7 level allows independence in all self-care activities with equipment and independent transfers because of preserved ability to push off with the elbow extensors, and the patient may be able to live alone. A patient with C8 and T1 innervation will have improved manual dexterity and strength for self-care, is independent with a manual wheelchair, and should be able to self-catheterize.
The patient with preservation of upper thoracic innervation has a degree of trunk control that increases stability during use and propulsion of a manual wheelchair. It also adds to ease and independence with bladder and bowel self-management. With bracing of the hips, knees, and ankles (knee-ankle-foot orthoses), minimal ambulation can be attempted, although this would be more for encouragement and exercise purposes than truly functional. Independent ambulation even with bracing and bilateral axillary or forearm crutches is usually not realistic unless the patient has preservation of some upper lumbar innervation. Further preservation of lumbar and sacral innervation will increase ease of ambulation with better trunk and pelvic control. There have been developments in exoskeleton systems to assist standing and ambulation, such as the ReWalk (Argo Medical Technologies Inc., Marlborough, Mass) and Ekso (Ekso Bionics, Richmond, Calif), although these are currently still limited by the individual patient’s abilities, terrain, and need for safety supervision. The patient with incomplete spinal injury is less predictable, and functional abilities will largely depend on the degree and nature of neurologic preservation.
Diagnostic Studies
With increasingly greater resolution, T1 versus T2 weighting, and other techniques to enhance and to suppress the appearance of tissues of different densities, the best tool when transverse myelitis is suspected is MRI. MRI not only allows visualization of the lesion but also helps rule out potentially treatable causes, such as tumor, abscess, and other lesions causing compressive myelopathy. Contrast material can be given to highlight lesions [17], and myelography may be considered if MRI is not available.
Although it is not definitive, there are MRI features that help differentiate transverse myelitis from other disorders, such as multiple sclerosis (Figs. 161.1 and 161.2). Transverse myelitis is more likely to have high signal intensity on T2-weighted images extending longitudinally over more segments [17,18]. The number of segments involved may be 1 or 2, to as many as 11. The entire cord or sometimes only the medulla may be affected [17–20]. In transverse myelitis, the lesion appears more likely to affect the central region of the cord and to involve more than two thirds of the cord diameter.
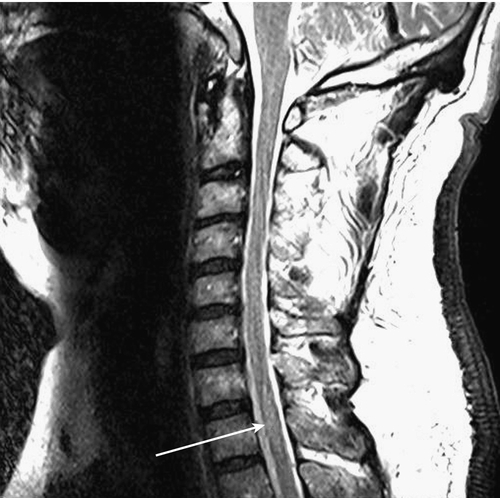
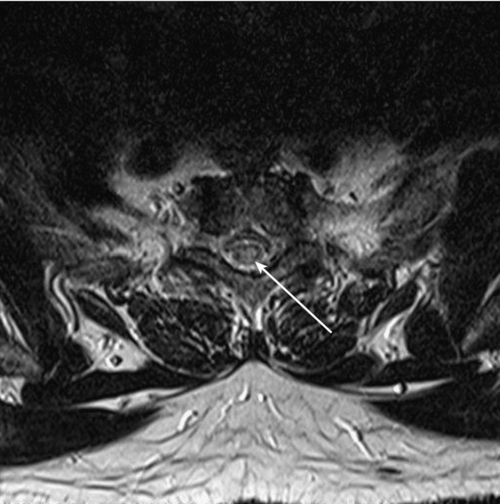
In multiple sclerosis, the lesion appears more peripheral and generally involves less than half of the diameter of the cord [17]. The lesion in transverse myelitis is more likely to resemble a spinal cord tumor, and biopsy may even be mistakenly performed [17,18]. MRI of the brain with contrast enhancement is often performed to help determine whether the patient’s condition is a prelude to multiple sclerosis rather than “idiopathic” transverse myelitis. In idiopathic partial transverse myelitis, a study that does not show brain lesions translates to the likelihood of evolving multiple sclerosis at 15% to 44%. When brain lesions such as white matter plaques (especially periventricular) are seen, the chance for development of multiple sclerosis increases to 44% to 93% [21]. Asymmetric motor or sensory symptoms and absence of peripheral nervous system involvement at presentation suggest acute myelopathic multiple sclerosis, whereas symmetric symptoms and neurophysiologic evidence of peripheral nervous system involvement suggest acute transverse myelitis [22,23].
Immunoglobulin G antibodies may be useful for determining neuromyelitis optica as the etiology in patients with acute complete transverse myelitis. Longitudinally extensive transverse myelitis spanning three or more vertebral segments is an important feature of neuromyelitis optica, and detection of anti–aquaporin 4–specific antibodies (anti-AQP4) is useful to determine both increased risk for recurrence and conversion to neuromyelitis optica [11,24].
Other tests include blood counts and chemistry; tests for autoimmune conditions, such as antinuclear antibodies, anti–double-stranded DNA antibodies, anti-Sm antibodies, and erythrocyte sedimentation rate; SS-A antibody for Sjögren disease; immunoglobulin levels; and VDRL test. Vitamin B12 levels may be tested, and Mycoplasma pneumoniae or Mycobacterium cultures may be performed. Lyme titers and titers for various viruses including human immunodeficiency virus, West Nile virus, poliovirus, hepatitis virus, Epstein-Barr virus, cytomegalovirus, and enteric cytopathic human orphan virus may be elevated.
A lumbar puncture allows the assessment of central nervous system pressure as well as obtains cerebrospinal fluid for cell count, determination of protein and glucose concentrations, measurement of immunoglobulins, and protein electrophoresis. Oligoclonal bands detected in the cerebrospinal fluid are useful in making a diagnosis. In one report, they were present in three of five patients with multiple sclerosis–associated transverse myelitis but in none of four patients with parainfectious transverse myelitis [3]. Vascular flow studies or clotting parameters may be needed if spinal hematoma, thrombosis, or vasculitis is suspected. Electrodiagnostic studies, including somatosensory and motor evoked potentials, may be useful for both diagnostic purposes and monitoring of treatment progress [25]. A urinary evaluation may include cystography, voiding cystourethrography, and cystoscopy. Baseline renal ultrasound and urodynamic evaluation have been recommended because of the very high rates of persistent long-term bladder dysfunction [26,27]. Bowel evaluation may require radiography, computed tomography, MRI, or colonoscopy to rule out obstruction.
In 2002, the Transverse Myelitis Consortium Working Group proposed the criteria in Table 161.1 for the diagnosis of idiopathic acute transverse myelitis [2].
Table 161.1
Criteria for the Diagnosis of Idiopathic Acute Transverse Myelitis
| Inclusion Criteria | Exclusion Criteria |
|
• Development of sensory, motor, or autonomic dysfunction attributable to the spinal cord • Bilateral signs or symptoms (although not necessarily symmetric) • Clearly defined sensory level • Exclusion of extra-axial compressive etiology by neuroimaging (MRI or myelography; CT of spine not adequate) • Inflammation within the spinal cord demonstrated by CSF pleocytosis or elevated IgG index or gadolinium enhancement. If no inflammatory criterion is met at symptom onset, repeated MRI and lumbar puncture evaluation between 2 and 7 days after symptom onset meet criteria. • Progression to nadir between 4 hours and 21 days after the onset of symptoms (if patient awakens with symptoms, symptoms must become more pronounced from point of awakening) |
• History of previous radiation to the spine within the last 10 years
• Clear arterial distribution clinical deficit consistent with thrombosis of the anterior spinal artery
• Abnormal flow voids on the surface of the spinal cord consistent with AVM
• Serologic or clinical evidence of connective tissue disease (e.g., sarcoidosis, Behçet disease, Sjögren syndrome, SLE, mixed connective tissue disorder)*
• CNS manifestations of syphilis, Lyme disease, HIV, HTLV-1, mycoplasma, other viral infection (e.g., HSV-1, HSV-2, VZV, EBV, CMV, HHV-6, enteroviruses)*
• Brain MRI abnormalities suggestive of multiple sclerosis*
• History of clinically apparent optic neuritis*
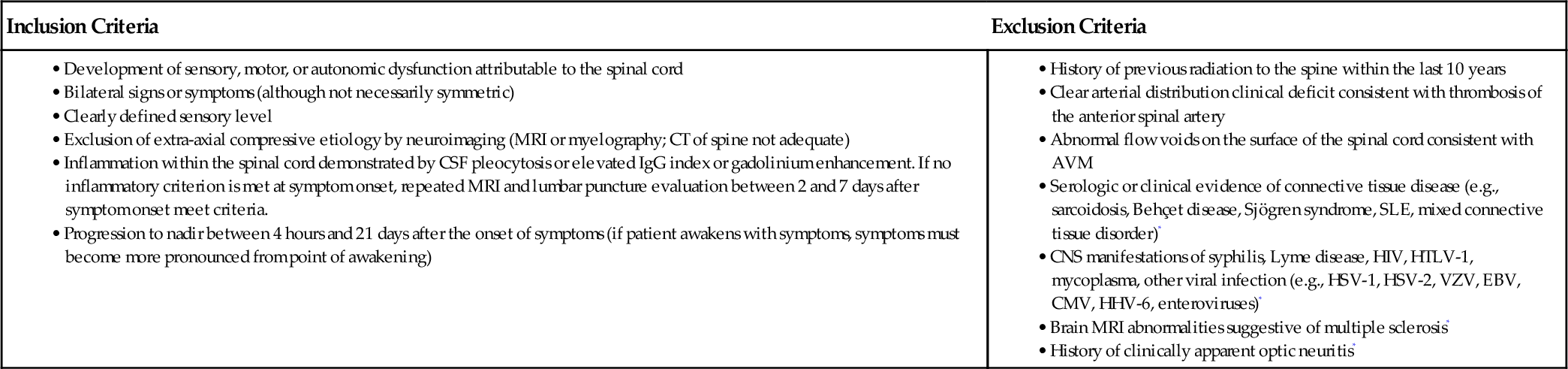
Modified from Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;59:499-505.
AVM, arteriovenous malformation; CMV, cytomegalovirus; CNS, central nervous system; CSF, cerebrospinal fluid; CT, computed tomography; EBV, Epstein-Barr virus; HSV, herpes simplex virus; IgG, immunoglobulin G; HHV, human herpes virus; HIV, human immunodeficiency virus; HTLV-1, human T-lymphotropic virus 1; MRI, magnetic resonance imaging; SLE, systemic lupus erythematosus; VZV, varicella-zoster virus.
* Do not exclude disease-associated acute transverse myelitis.
A comparison by de Seze [6] of the clinical findings, MRI results, laboratory profiles, and outcomes of patients with acute myelopathy according to etiology is presented in Table 161.2.
Table 161.2
Comparison of Findings Based on Etiology [6]
| Etiology | Findings | Prognosis |
| Multiple sclerosis | MRI: lesions small, localized in lateral or posterior cord, more cervical CSF: oligoclonal bands |
Clinical outcome good but relapse in 47% at mean of 21 months |
| Systemic disease (SLE, Sjögren syndrome) | Severe motor and sphincter problems MRI in SLE: large and centromedullary lesions CSF: > 30 cells |
Clinical outcome poor |
| Spinal cord infarct | No clear diagnostic criteria acutely; > 50 years old; severe motor and sphincter problems MRI: isolated centromedullary lesions CSF: absent or low cells, no oligoclonal bands |
Outcome poor or fair in 91% of cases |
| Parainfectious myelopathy | Severe motor and sphincter problems MRI: large centromedullary lesions, cervicodorsal frequently CSF: > 30 cells, no oligoclonal bands Serologic confirmation rarely obtained |
Clinical outcome good |
| Delayed radiation myelopathy | History of irradiation; delay can exceed 10 years MRI: high-intensity cord signals with focal swelling, follow-up cord atrophy CSF: normal |
Clinical outcome good in early (10-16 weeks after) radiation myelopathy, poor in delayed |
| Unknown etiology | Long-term follow-up produces diagnosis in 50% of cases |
CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; SLE, systemic lupus erythematosus.
Treatment
Initial
Although the physiatrist may manage stable long-standing transverse myelitis on an outpatient basis, hospitalization may be necessary to monitor vital signs, to manage respiratory status and bowel or bladder complications, and to carry out diagnostic investigations, particularly during the acute presentation [26,31]. Abnormalities of the vital signs, such as tachypnea or tachycardia, may suggest impaired oxygenation or blood flow that may need to be managed urgently. The ability to provide antiviral agents, antibacterial agents, and surgical decompression may also be critical, depending on whether a specific cause has been identified.
Various medications have been tried for idiopathic transverse myelitis without clear success in changing the course. Intravenous methylprednisolone has been advocated to prevent further damage to the spinal cord as a result of swelling [19,20]. During the acute phase, it may lead to faster recovery and less disability, and it is well tolerated according to several small observational studies [21]. Cyclophosphamide exerts an immunosuppressive and immunomodulatory effect through suppression of cell-mediated and humoral immunity (i.e., on the T cells and B cells) [21]. Cyclophosphamide in combination with methylprednisolone has some success on lupus-related lesions [20,29]. However, there appears to be an absence of any beneficial effect of immunosuppressive drugs (cyclophosphamide, azathioprine, intravenous immune globulin) in patients with idiopathic acute transverse myelitis [5]. Plasma exchange to remove autoreactive antibodies and other toxic molecules from plasma can be effective, especially within 20 days of onset, to increase chances of a good clinical response [21].
Rehabilitation
Rehabilitation is a crucial component of the treatment for any spinal cord injury, and the more affected or severe cases of transverse myelitis will require a comprehensive multidisciplinary rehabilitation program led by a physiatrist. Physical and occupational therapists on the team can work with patients on strengthening, endurance, balance, coordination, joint range of motion, reconditioning, mobility, and independence with activities of daily living. If pain is present, appropriate medications and heat, cold, and electrical modalities including transcutaneous electrical stimulation may be helpful.
An orthotist can improve mobility with bracing devices such as an ankle-foot orthosis or knee-ankle-foot orthosis. An assessment for appropriate equipment, such as wheelchair, and other assistive and walking devices, is needed. Education of the patient and family about the disease, resultant impairments, potential complications, and plans and prognosis for rehabilitation is important. The psychological state of the patient should not be neglected, and there should be monitoring for depression. Discharge planning needs and issues potentially affecting the patient’s community reintegration should be assessed.
Transverse myelitis may or may not be a transient condition. Recovery may occur, and it is important to minimize the effects of even temporary denervation. All muscles and joints should be kept as active as possible, and having the joints go through a full range of motion daily will help prevent contractures. Passive and active exercises at all times and electrical stimulation are methods to keep muscles as flexible and strong as possible. If respiration is compromised, exercises for muscles of inspiration may be started, glossopharyngeal breathing may need to be taught, and electrical stimulation of the diaphragm may need to be considered [31].
Spasticity is a possible complication as with other upper motor neuron lesions. Regular stretching and use of antispasticity medications, such as baclofen, diazepam, and tizanidine, can minimize and decrease development of joint contractures. An antiepileptic drug (e.g., gabapentin) can also have a degree of antispasticity effect. Checking the skin thoroughly on a daily basis can potentially avoid skin breakdown and associated infections. Insensate areas of high pressure should be relieved with special cushions and mattresses, such as egg crate foam and alternating pressure overlays, and pressure-relieving ankle-foot orthoses may be helpful.
Bladder and bowel programs should be started immediately because a neglected neurogenic bowel or bladder may lead to stool obstruction or kidney damage. An indwelling catheter can initially be used for bladder drainage, but intermittent catheterization, independently or otherwise, is commonly instituted whenever possible. Long-term follow-up of 2 to 10 years in pediatric patients with transverse myelitis has shown that residual bladder dysfunction is common even with improvement of paraparesis and lack of urologic symptoms. In one study, 86% had persistent bladder dysfunction and 77% had persistent bowel dysfunction [27].
A bowel program includes adequate fluids, proper diet, activity, and scheduled bowel movements. Upper motor neuron bowels may need a stool softener (e.g., docusate), osmotic laxative (lactulose), or stimulant laxative (senna or bisacodyl) for evacuation. Digital stimulation of the rectum is often effective and needs to be taught. With areflexic lower motor neuron bowels, use of bulk laxatives like psyllium or methylcellulose to obtain formed stools may help during digital manual evacuation. Bowel training is often started on a daily basis in the hospital, but the frequency can be extended to every 2 or 3 days once an individual returns home.
Individuals who require assistive devices for mobility must be trained in use of a wheelchair, walker, crutches, or cane, including maneuvering over steps and curbs. If transfers and ambulation require assistance, training of family members or assistants becomes crucial.
For patients with transverse myelitis at the cervical level, various types of equipment and temporary or permanent orthoses can be provided to help with self-care activities. Proper bathroom equipment and modifications, such as a tub bench, commode, hand-held shower, raised toilet seat, and grab bars, may make the difference between dependence and independence. Selection of appropriate aids is essential to maximize function, and many are expensive. Timing of these purchases may need to be carefully considered in this possibly transient condition. Despite a reasonable prognosis for eventual recovery, inaction may result in secondary complications, and it is important to keep an individual as functionally independent and active as possible throughout the entire recovery period.
Procedures
Procedures in transverse myelitis are determined by which systems are affected by the spinal cord injury. Renal ultrasound and urodynamic evaluations are relatively routine procedures for these patients to assess and monitor bladder dysfunction. Intramuscular botulinum toxin injections or alcohol or phenol nerve and motor point blocks may be needed for spastic limb muscles. An intrathecal baclofen pump may be effective in intractable cases and allows much smaller doses and concomitantly fewer side effects. Other procedures include implantation of diaphragmatic electrodes (phrenic nerve stimulation) when respiratory muscles have been affected. Some patients receive functional electrical stimulation systems to help maintain fitness or to increase hand and ambulatory function. Although not routinely done presently, anterior sacral root stimulation may be effective for bladder management.
Surgery
There is no specific curative surgical procedure for idiopathic transverse myelitis. However, when there are compressive abnormalities such as abscess, herniated nucleus pulposus, spinal stenosis, and tumor, surgery may be needed as soon as possible to relieve pressure on the spinal cord. Timely management of compressive lesions may reverse neurologic injury to the cord or at the least stop further injury.
Secondary complications from spinal cord dysfunction may require surgical intervention. These include skin breakdown, accidental injury including fractures from lack of sensation in muscles and joints, development of kidney stones, and infections. Tendon transfers may be considered at a later stage to increase an individual’s functioning. Nerve transfer in patients with permanent upper limb deficits may be considered to restore or to improve ability to voluntarily activate a muscle. In a recent case report, a child who underwent multiple fascicle transfers from median and ulnar nerves to the musculocutaneous nerve, spinal accessory to suprascapular nerve, and medial cord to axillary nerve had excellent recovery of elbow flexion. However, shoulder abduction had improved only minimally after 22 months [32].
Potential Disease Complications
Potential disease complications resulting from spinal cord injury with its attendant sequelae are generally similar irrespective of the etiology. A common complication is pressure ulcer of the skin if pressure relief is not done regularly. Awareness of and monitoring for deep venous thrombosis and pulmonary embolism should be routine. There may be varying degrees of respiratory muscle weakness, and when it is severe, mechanical ventilation assistance may be required. Patients are at increased risk for pneumonia or sleep apnea from the illness, compounded by any sedating medications or respiration-depressing narcotics given.
Spasticity and joint contractures may result over time. Heterotopic ossification may surround a joint, further promoting contractures. Gastrointestinal complications may begin with an acute ileus followed by chronic constipation. Urinary tract infections are common because retained urine and instrumentation both increase the likelihood of infection. Autonomic dysreflexia may occur, especially for lesions above T6. Pain, a frequent complaint after spinal cord injury, is often attributed to either musculoskeletal sources or a “central” or neurogenic pain mechanism and in some studies affects more than 90% of individuals. Treatment initiated for this pain includes tricyclic antidepressants, anticonvulsants, analgesics, nonsteroidal anti-inflammatory drugs, or short courses of cyclooxygenase-2 inhibitors. Depression and anxiety may occur and usually respond to supportive counseling but may need antidepressants such as the selective serotonin reuptake inhibitor or the serotonin-norepinephrine reuptake inhibitor drugs.
Overuse syndromes can result because muscles and joints are commonly stressed in trying to maintain or to learn new functions. Shoulder pain is a prominent issue, and the problems of tendinitis, arthritis, rotator cuff tear, impingement, and contracture must be properly identified and managed or rehabilitated. Steroid and local anesthetic injections in the joint may sometimes be needed, but use of proper transfer techniques or specific adaptive equipment is often helpful. Pressure from prolonged resting on superficial nerves can also cause pain or weakness. There may be difficulty with reproduction and fertility problems in the younger patients, and sexuality issues and solutions should be addressed or referred to a specialist.
Potential Treatment Complications
Treatment complications may result from side effects of medications and equipment required to treat the disease manifestations. Skin complications may result from ill-fitting devices or poorly applied dressings. Strictures or tracheal inflammation can result from tracheostomy tubes; if mechanical ventilation is required, failure of equipment can result in hypoxia or worse. Respiratory infections occur frequently with prolonged mechanical ventilation in high tetraplegia. High-dose steroids used to treat initial inflammation may cause gastritis, ulceration, or hemorrhage in the gastrointestinal tract. Deep venous thrombosis prophylaxis and anticoagulant treatment may result in or exacerbate bleeding tendencies. Catheterization may cause urinary tract infections or false passages in the urethra, making further catheterization difficult, with possible development of strictures. If bowel programs are not well managed, anal irritation may go on to skin maceration and breakdown around the sacral region.
Acknowledgment
Deborah Reiss Schneider, MD, for the chapter in the first edition on which this is based.