CASE 16 Coronary Perforation
Cardiac catheterization
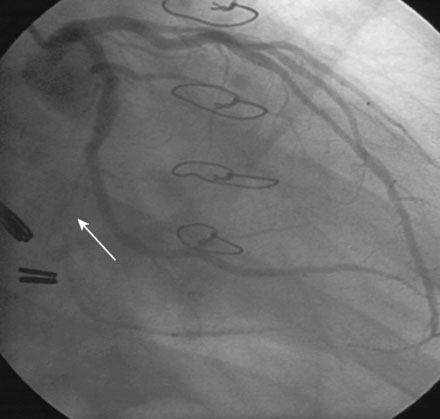
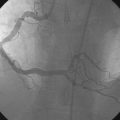
Left ventricular systolic function was preserved, with an estimated ejection fraction of 60% and only mild inferior wall hypokinesis. A widely patent right internal mammary artery graft provided an excellent distal blood supply to a severely diseased right coronary artery (Figure 16-1). Stents placed in the diagonal and obtuse marginal arteries were widely patent and the previously-noted long occluded segment of the distal circumflex remained unchanged (Figure 16-2). A long, tubular stenosis of moderate severity was apparent in the midportion of the left anterior descending artery (Figure 16-3 and Videos 16-1, 16-2). Given the uncertainty of the stenosis severity, the operator measured fractional flow reserve using a pressure wire and intracoronary adenosine to achieve maximal hyperemia. With classic anginal symptoms despite maximal medical therapy and a fractional flow reserve measuring 0.78, the physician decided to revascularize the left anterior descending artery percutaneously. Following administration of intravenous heparin and eptifibatide, the operator directly stented the diseased segment using a 6 French left coronary guide catheter with a 3.0 mm diameter by 32 mm long sirolimus-eluting stent inflated to 12 atmospheres (Figure 16-4).
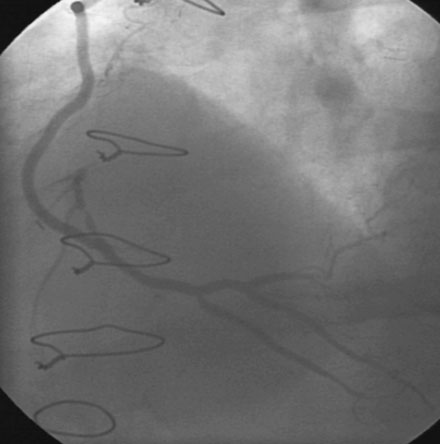
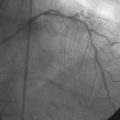
FIGURE 16-1 Patent right internal mammary artery graft to the severely diseased right coronary artery.
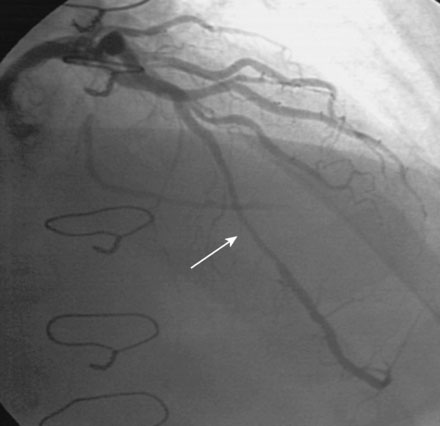
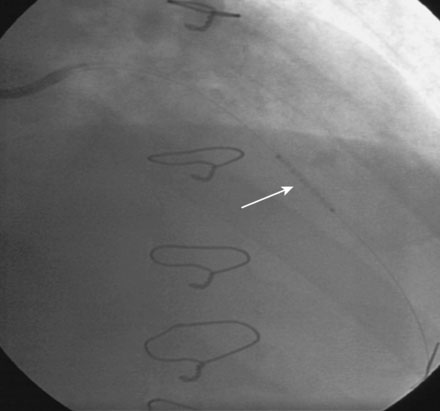
FIGURE 16-3 Long, tubular stenosis in the midportion of the left anterior descending artery (arrow).
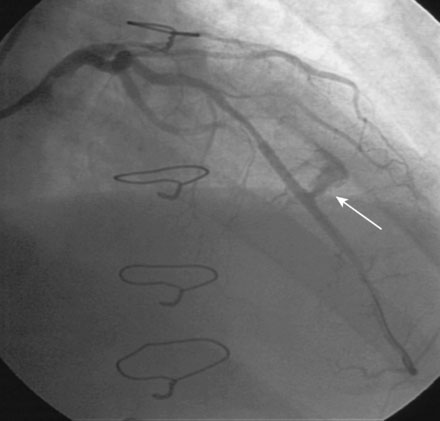
Immediately following stent deployment, the angiogram demonstrated free extravasation of contrast emanating from the midportion of the stented segment consistent with a coronary artery perforation (Figure 16-5 and Video 16-3). The patient reported substernal chest and left shoulder pain; ST-segment elevation was apparent on the monitored leads. Central aortic pressure fell to a systolic pressure of 70 mmHg and the heart rate increased to 110 beats per minute. A 3.0 mm diameter by 20 mm long compliant balloon was immediately advanced over the 0.014 inch guidewire and inflated at the site of contrast extravasation (Figure 16-6). This maneuver, along with simultaneous infusion of saline and initiation of intravenous dopamine, led to immediate stabilization of blood pressure.
Working quickly, the operator deflated the balloon, removed the guidewire, and replaced the 6 French sheath and guide catheter with an 8 French system. After repositioning an exchange-length, 0.014 inch guidewire in the left anterior descending artery, the physician centered a 3.0 mm diameter by 19 mm long polytetrafluoroethylene-covered (PTFE) stent (Jostent Graftmaster) centered on the site of contrast extravasation (Figure 16-7), successfully sealing the perforation (Video 16-5). The dopamine infusion was stopped with no further hypotension and he was observed in the cardiac catheterization laboratory for an additional 15 minutes; a final angiogram revealed no further evidence of contrast extravasation.
Discussion
Coronary artery perforation complicates less than 0.5% of coronary interventions1 but is potentially lethal, particularly in the event of free extravasation into the pericardial space as exemplified in this case. More commonly seen with ablative techniques such as rotational or directional atherectomy, coronary perforation may be caused by vessel disruption from barotrauma (the likely cause in this case) or from deep dissection extending through the adventitia. Guidewire perforations may occur at the site of the lesion during attempts at crossing a stenosis or may occur distally from migration of the wire tip into a small vessel, with subsequent perforation.
Small perforations may seal with reversal of anticoagulation and prolonged balloon inflation at the perforation site. Large, free-flowing rents in the artery, like the one shown in this case, usually require sealing with a PTFE-covered stent.2,3 These devices consist of a thin layer of PTFE sandwiched between two stents. They are fairly bulky and may be difficult to position and are not available in sizes smaller than 2.5 mm diameter. Importantly, they require large-bore guides, usually necessitating a change in the sheath and guide during an emergency. If the patient cannot tolerate release of the inflated balloon used to temporarily staunch the bleeding, then access should be obtained in the contralateral femoral artery and the appropriately sized sheath and guide catheter placed while the balloon remains inflated.
In the event of an obvious coronary perforation:
1 Fasseas P., Orford J.L., Panetta C.J., et al. Incidence, correlates, management and clinical outcome of coronary perforation: analysis of 16,298 procedures. Am Heart J. 2004;147:140-145.
2 Briguori C., Nishida T., Anzuini A., DiMario C., Grube E., Colombo A. Emergency polytetrafluoroethylene-covered stent implantation to treat coronary ruptures. Circulation. 2000;102:3028-3031.
3 Lansky A.J., Yank Y., Khan Y., Costa R.A., Pietras C., Tsuchiya Y., Cristea E., Collins M., Mehran R., Dangas G.D., Moses J.W., Leon M.B., Stone G.W. Treatment of coronary artery perforations complicating percutaneous coronary intervention with a polytetrafluoroethylene-covered stent graft. Am J Cardiol. 2006;98:370-374.