CHAPTER 156
Spinal Cord Injury (Thoracic)
Jesse D. Ennis, MD, FRCP(C); Jane Wierbicky, RN, BSN; Shanker Nesathurai, MD, MPH, FRCP(C)
Definition
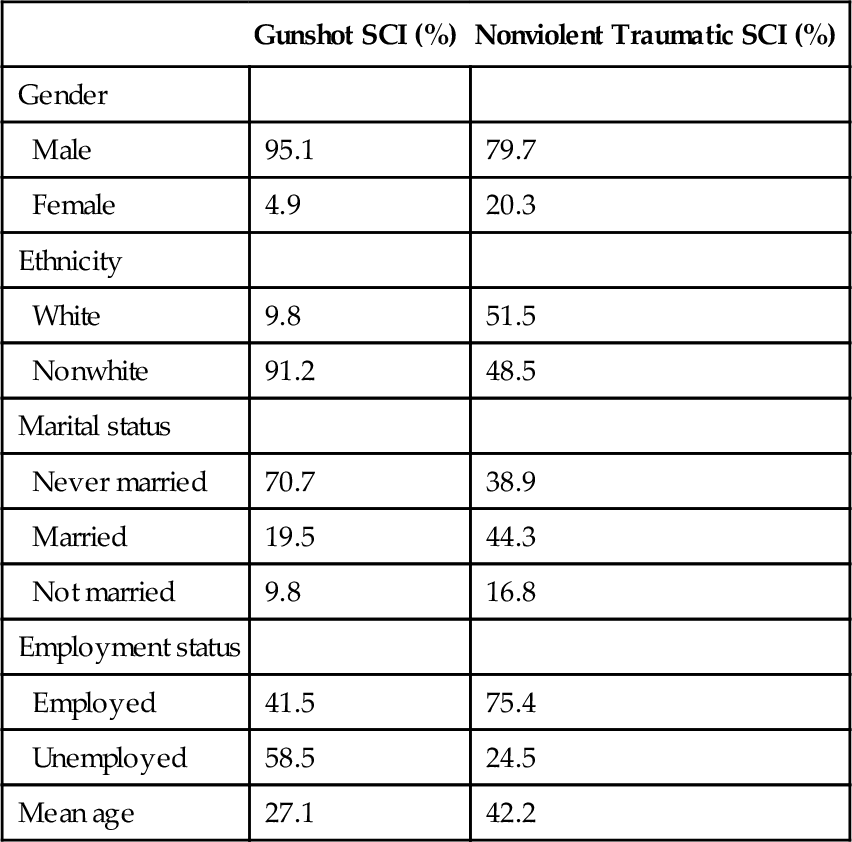
Spinal cord injury (SCI) is a common cause of paralysis, particularly in young men (Table 156.1). Just more than one third of all injuries to the spinal cord occur at the thoracic level, most commonly at T12 [1]. With thoracic SCI, 68% of patients will have complete injury, 8% will have a sensory incomplete injury, and 23% will have a motor incomplete injury. Mean age at the time of injury has increased over time: from 2005 to 2011, the mean age was 41 years, compared with 28.7 years from 1973 to 1979. Overall, there is still higher representation of males with SCI, with 80.6% of patients being male and 19.6% being female [1]. Compromise to the thoracic spinal cord typically results in paraplegia. Unlike paraplegia that results from compromise of the cauda equina associated with lumbar spine injuries, the clinical findings are consistent with upper motor neuron injury. However, lower limb paralysis is not the only impairment. The thoracic spinal cord also segmentally innervates the intercostal muscles as well as the upper and lower abdominal muscles. The intercostal muscles are innervated by the T1 to T12 spinal segments. The upper abdominal muscles are innervated by the T8 to T10 spinal segments; the T11 to T12 spinal segments innervate the lower abdominal muscles [2].
A quantitative three-dimensional anatomy of the thoracic spine reveals three distinct zones: the cervical-thoracic transition zone, the middle region, and the thoracic-lumbar transition zone [3,4]. The T1-T4 region is characterized by a narrowing of the vertebral end plate and spinal canal widths [3]. The middle thoracic region (T4-T9) is notable for its relatively narrow end plate and small spinal canal. The rib articulations provide an increased degree of protection at this level. An enlargement of the spinal canal characterizes the lower thoracic region (T10-T12) [3]. There is also less rigidity of the spine at the T11 and T12 segments because of the lack of ventral attachment of the ribs [3]. Therefore there is an increased vulnerability to SCI at the lower thoracic levels. Compared with the cervical and lumbar spinal levels, the blood supply is more tenuous in the thoracic spinal cord, and therefore ischemia poses a greater threat to neurologic function in this area [4].
Symptoms
The presenting symptoms of thoracic SCI are consistent with the alteration to the motor, sensory, and autonomic pathways. The chief symptoms are weakness or paralysis of the abdominal and lower extremity musculature and loss of sensation in the lower limbs, thorax, and perineum. Patients may also experience altered bowel or bladder function in addition to spasticity and sexual dysfunction.
With lesions above the T6 level, patients may experience the symptoms of autonomic dysreflexia. Autonomic dysreflexia is characterized by pounding headaches, nasal congestion, anxiety, visual disturbances, pallor below the level of injury, and sweating and flushing above the level of injury. In patients with an old, stable injury who are experiencing new or progressive symptoms (e.g., increasing weakness, loss of sensation), the clinician should consider the possibility of a syrinx.
Patients with SCI are often insensate to the pain that accompanies deep venous thrombosis, and therefore both the clinician and the patient should be attentive to clinical signs such as edema, erythema, and increased tone. Heterotopic ossification may mimic deep venous thrombosis because the symptoms include swelling, decreased range of motion, erythema, increased spasticity, pain, and low-grade fever.
Pain originating from either musculoskeletal or neurologic sources is common. Neuropathic pain resulting from central or peripheral nervous disruption may be described as burning or shooting. An analysis of several studies addressing prevalence of pain after SCI showed a variable overall prevalence of pain ranging from as low as 26% to as high as 96% [5].
Physical Examination
The diagnosis of a thoracic-level SCI necessitates a thorough physical examination, including a comprehensive neurologic assessment. Findings on physical examination include a motor and sensory level. Depending on whether the injury is partial or complete, there may be sparing of sacral sensation or anal sphincter motor function below the neurologic level of injury. In the acute period, the motor examination is characterized by loss of muscle tone and deep tendon reflexes. During subsequent days and weeks, there is emergence of increased muscle tone, reflexes, and pathologic reflexes. Cutaneous reflexes including the plantar response, cremasteric reflex, and bulbocavernosus reflex are initially depressed and follow a variable course to gradual return. The initial evaluation of the patient also includes the assessment of vital signs and the cardiovascular, respiratory, musculoskeletal, gastrointestinal, and genitourinary systems. A thorough examination of the skin is necessary. In thoracic SCI, pressure ulcers are more common over bone prominences such as the sacrum, calcaneus, and greater trochanter. In addition, it is important to evaluate the patient for spasticity and contractures.
New neurologic abnormalities on physical examination should alert the clinician to consider imaging studies to exclude syringomyelia. In this case, typical physical examination findings include change in sensory level, change in motor level, and reflex abnormality as well as spasticity.
Functional Limitations
Persons who suffer from thoracic SCI can have significantly different levels of disability, depending on their degree of paralysis and associated potential complications (e.g., contractures, spasticity). A patient with high thoracic paraplegia (i.e., T2 level) typically has some component of truncal instability; as a result, the patient’s wheelchair requires a high back. In contrast, a person with low thoracic paraplegia generally has preservation of most of the intercostal and abdominal muscles and could opt for a chair with a low back. Intercostal muscle impairment in patients with SCI in the upper thoracic region may cause an impaired cough and a decreased ability to mobilize secretions.
Functional goals for individuals with thoracic SCI include the ability to complete activities of daily living and instrumental activities of daily living with or without the use of assistive equipment. Tasiemski and colleagues [6] have described a positive association of involvement in sports and recreational activities with increased life satisfaction in a community sample of people with SCI. Numerous sports and recreational organizations offer adaptive sports programs for people with disabilities.
Bowel and bladder function may cause social embarrassment, leading to self-imposed social isolation. Sexual dysfunction may result in a loss of intimacy. The availability of partners is a concern for many patients because their disability as well as environmental and social barriers may preclude their involvement in some of the more typical dating activities.
Depression is common in patients with SCI; reported rates of depression in the newly injured range between 20% and 44% [7]. The most recent large-scale retrospective study of veterans with SCI showed a depression rate of 22% [8]. Depression has been associated with an increase in secondary complications and poor compliance with self-care activities [7]. Referral to mental health professionals is encouraged for patients at risk.
Diagnostic Studies
The diagnosis of thoracic SCI is often corroborated with magnetic resonance imaging. The stability of the injury is assessed by evaluation of the anterior, middle, and posterior columns of the spine. Magnetic resonance imaging is also the study of choice when syringomyelia is suspected.
Urodynamic testing is commonly used to evaluate bladder function in the individual with SCI. Urodynamic studies involve filling of the bladder with fluid or gas and use of electromyographic and fluoroscopic techniques to evaluate voiding function. Annual evaluations often include an ultrasound examination to further assess the integrity of the renal system.
Patients with grade IV pressure ulcers may require a bone scan or magnetic resonance imaging study to detect osteomyelitis. The triple-phase bone scan is also used in the diagnosis of heterotopic ossification (see Chapter 130). Doppler surveillance studies are sometimes performed to detect deep venous thrombosis in this highly susceptible population (see Chapter 127). Routine colonoscopy and fecal occult blood testing may be appropriate for patients 50 years and older [9]. In patients susceptible to autonomic dysreflexia, appropriate precautions must be used during colonoscopy.
Treatment
Initial
Skin Management
In an analysis of long-term medical complications among subjects enrolled in the National Spinal Cord Injury Statistical Center database, pressure ulcers were the most commonly reported postinjury complication. McKinley and colleagues [10] reported a 15.2% incidence of pressure ulcers in the first annual follow-up postinjury year. The rates increased steadily during all follow-up years in both complete and incomplete SCIs. Common sites for pressure ulcers are the sacrum, greater trochanter, and heels. Excessive pressure, shearing, friction, and maceration can increase the risk for pressure ulcers. Other risk factors include spasticity, impaired sensation, immobility, poor nutrition, weight gain, and incontinence [11].
The maintenance of skin integrity is an ever-present goal in patients with SCI. Pressure ulcer formation will lead to the development of scar tissue and an even greater likelihood of ulcer recurrence. Seating surfaces should be reevaluated on a regular basis, ensuring that they have not worn out and still fit the patient’s weight and size. Patients are encouraged to perform daily skin examinations, and most paraplegic patients are able to independently perform pressure-relieving strategies, such as wheelchair pushups. These techniques should be performed every 15 minutes to minimize excessive pressure.
Patients who have a pressure ulcer must minimize pressure to that area until the wound is healed. A variety of débridement methods are available for removal of necrotic debris from pressure ulcers (see Chapter 148).
Pain
The presentation of pain among the SCI population can be varied in nature; both neuropathic pain and pain resulting from abnormal mechanical stresses (e.g., tendinitis) are common. Many times, patients with musculoskeletal pain have a well-defined disorder that is amenable to standard physiatric treatment (e.g., rotator cuff tendinitis, lateral epicondylitis). Non-narcotic analgesics and nonsteroidal anti-inflammatory drugs can be used to treat musculoskeletal causes of pain. Neuropathic pain generally is not responsive to these medications; however, tricyclic antidepressants, antiseizure medications, and pregabalin have been effective in its treatment but should be prescribed with caution [12].
Bladder Management
Most patients with thoracic-level SCI will have upper motor neuron bladder dysfunction, characterized by low urinary volumes, high bladder pressures, bladder trabeculation, and diminished bladder compliance. Detrusor-sphincter dyssynergia (co-contraction of the bladder and sphincter) is common. This can contribute to vesicoureteral reflux, which may result in hydronephrosis and subsequent chronic renal failure. Detrusor-sphincter dyssynergia can be treated with medical interventions that decrease bladder tone (e.g., oxybutynin) or, alternatively, decrease sphincter tone (e.g., terazosin, tamsulosin, or sphincter chemodenervation) [13,14] (see Chapter 137).
Bladder management strategies should be individualized, but intermittent catheterization is the preferred treatment option. A typical intermittent catheterization program requires bladder emptying four to six times per day, with volumes remaining less than 500 mL. Most paraplegic patients have the manual dexterity to perform self-catheterization; some individuals, because of biologic or sociomedical factors, must use indwelling catheters (with suprapubic preferred to urethral). Indwelling catheters are associated with a higher incidence of bladder stones and bladder carcinoma [15]. In addition, in men, urethral catheters are associated with prostatitis, epididymitis, and urethral strictures. Long-term use of urethral catheters in women may result in urethral dilation.
Bowel Management
The patient with thoracic-level injuries will most likely have constipation; therefore a bowel program is necessary. A reasonable goal for a bowel regimen is to achieve socially acceptable fecal continence, with bowel evacuations at least three times per week. A bowel regimen may include medications (Table 156.2). In addition, bowel evacuation is scheduled after a meal to capitalize on the intrinsic increase in peristalsis after meals (i.e., the gastrocolic reflex). Bowel care programs done on a raised toilet seat use the benefits of gravity. Digital stimulation (gentle insertion of the finger) of the rectum or insertion of a suppository can activate the rectocolic reflex by stimulating peristalsis and promoting regular bowel movements. Enemas (Fleet, soapsuds) should not be part of a regular bowel program. However, these agents are useful in emptying the colon before initiation of a bowel program or treatment of fecal obstipation [9]. The administration of an enema can precipitate autonomic dysreflexia in susceptible patients. If administration of an oral osmotic laxative is required, consideration should be given to polyethylene glycol 3350 (PEG 3350) over the traditional use of lactulose. This is supported by a recent Cochrane review that demonstrated greater efficacy and less abdominal pain compared with lactulose for all-cause chronic constipation [16] (see Chapter 138).
Table 156.2
Oral Adjunctive Bowel Medications [9,16]
| Medication | Brand | Mechanism of Action | Strength | Dose |
| Docusate sodium | Colace | Stool softener | 100 mg (capsules) | 1 tablet bid |
| Senna | Senokot | Colonic stimulant | 8.6 mg (tablets) | 1-2 tablets qhs |
| Bisacodyl | Dulcolax | Colonic irritant | 5 mg (tablets) | 2 tablets qd |
| Polyethylene glycol (PEG 3350) | Lax-A-Day, MiraLax | Osmotic laxative | 17 g (powder) | 17 g daily (dissolved in liquid) |
| Psyllium powder | Smooth Texture Sugar-Free Unflavored Metamucil | Bulk-forming agent | 5.4 g per tsp | 1 tsp qd-tid |
| Metoclopramide | Reglan | Prokinetic agent | 10 mg (tablets) | 1 tablet qid |
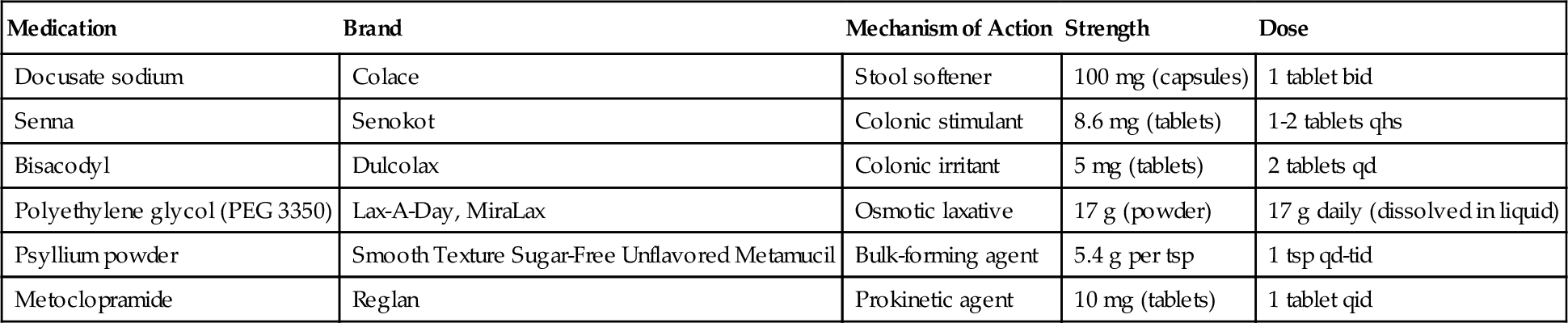
Modified from Bergman S. Bowel Management. In Nesathurai S, ed. The Rehabilitation of People with Spinal Cord Injury, 3rd ed. Arbuckle Academic Publishers, 2013.
Mental Health
Psychosocial adaptation subsequent to SCI is a lifelong process. There is no single “classic” presentation of this phenomenon. Anger, hostility, anxiety, and depression often result from overwhelming losses confronting this population. Suicide is among the leading causes of death in these patients. In individuals with depression or other psychological sequelae, consultation with an appropriate mental health care professional is recommended. Continued follow-up is encouraged, when appropriate.
Sexual and Reproductive Function
Sexual desire is not necessarily affected by SCI. However, associated depression, fears of inadequacy, and poor body image may consequently alter sexual desire. Sexual function (e.g., erection and ejaculation in men and lubrication in women) in patients with thoracic-level injuries may be altered. In general, patients with more complete lesions have more impairment. Men with thoracic-level lesions (with intact sacral reflexes) generally can achieve reflex erections with direct genital stimulation. However, many times, these reflex erections are of insufficient rigidity and duration for satisfactory vaginal penetration [17].
Many patients with SCI have numerous questions and fears about sexuality and sexual function. Treatment should address concerns related to body image, dating, and initiation and maintenance of intimate relationships. Peer counselors can share their experiences, and their advice can be beneficial. Peer counselors may be located through local independent living centers or through advocacy organizations such as the National Spinal Cord Injury Association or Canadian Paraplegia Association. In addition, mental health professionals (e.g., psychologists, psychiatrists, social workers) can be a valuable resource to the patient and rehabilitation team.
Several options available to men with erectile dysfunction include oral medications (e.g., sildenafil [18–24], tadalafil [18,24–26], vardenafil [24]), vacuum devices, penile injection programs (papaverine) [27], and surgically implanted prostheses. Ejaculatory dysfunction is also common, including retrograde ejaculation into the bladder [28]. Chronic SCI is also associated with poor semen quality and decreased spermatogenesis [29]. Elevated scrotal temperatures (from chronic sitting) and frequent urinary tract infections may negatively affect semen quality. Although studies have indicated that approximately 12% to 15% of men with SCI report ejaculation, fatherhood has become a possibility with use of semen retrieval methods such as penile vibrostimulation and electroejaculation along with improved assisted reproductive technology [30]. The risk of autonomic dysreflexia exists among susceptible patients with an injury level of T6 and above; therefore, assisted semen retrieval should be initiated by medical teams well trained in semen retrieval methods and the treatment of autonomic dysreflexia [31].
Women with thoracic-level lesions may note changes in vaginal lubrication. However, at this level, women may achieve reflex lubrication [32]. Direct stimulation of the genital region may result in sufficient lubrication. A water-soluble lubricant is recommended for patients with complaints of decreased vaginal lubrication. One randomized placebo-controlled study on the use of sildenafil to enhance sexual arousal in women with SCI failed to show a statistically significant benefit [33], despite an earlier pilot study that appeared to show benefit [34].
Orgasm for both men and women after injury may be nonexistent or described as a primarily emotional event or a pleasurable sensation in the pelvic region or sensory level with generalized muscle relaxation [32].
Women with thoracic-level SCI remain fertile. Contraceptive options include barrier methods (condoms, diaphragm) and oral contraceptives. Intrauterine contraceptive devices are contraindicated because of the lack of sensation and the risk for development of pelvic inflammatory disease. Patients with SCI are at increased risk for the development of thromboembolism, and the administration of oral contraceptives further increases this risk and should be discussed.
The care of pregnant women with SCI has special challenges. Potential complications include premature labor, increased risk of urinary tract infection, increased spasticity, autonomic dysreflexia, and constipation. In pregnancy, autonomic dysreflexia is manifested most frequently during labor; therefore hemodynamic monitoring is considered for all at-risk patients [35].
Pregnant women with thoracic SCI levels above T10 may be unable to sense fetal movements and contractions. Therefore, uterine palpation, serial cervical examinations, and fetal monitoring may be recommended. Regional anesthesia is preferred [36].
Deep Venous Thrombosis
SCI predisposes individuals to both deep venous thrombosis and pulmonary embolism. A systematic literature review revealed the prevalence of deep venous thrombosis in SCI to vary from 9% to 100% [37]. Of these, it is estimated that only 20% will extend into proximal veins, with associated elevated risk of pulmonary embolism. The rate of pulmonary embolism has been found to be 8% to 14% in acute SCI, with rates of fatal pulmonary embolism as high as 5% [37]. Putative mechanisms include immobility as a result of paralysis and failure of the venous-muscle pump as well as the possible contribution of a generalized hypercoagulable state.
Patients are administered fractionated or unfractionated heparin during the initial weeks after injury and may use thigh-high compression stockings and pneumatic compression. Current literature suggests continuation for 8 to 12 weeks after injury. For more details on the prevention and treatment of deep venous thrombosis, see Chapter 127.
Spasticity Management
Spasticity should be treated when it results in significant pain, contributes to contractures, impairs hygiene, interferes with functional tasks, or impedes nursing care. In the first instance, clinically significant spasticity should be treated by removal of noxious stimuli that may be contributing to the condition, such as urinary tract infection, ingrown toenails, and tight clothing. Second, physical interventions such as daily stretching of muscles with terminal sustained stretch can be considered. If these are unsuccessful, medications such as tizanidine and baclofen as well as interventional procedures (e.g., chemodenervation) can be considered (see Chapter 153 for details).
Heterotopic Ossification
Heterotopic ossification is most often seen in the first 6 months after injury in the spinal cord–injured population, especially in the first 2 months [38,39]. The incidence of heterotopic ossification among individuals with SCI ranges from 10% to 53% [39]. It is estimated that 20% to 30% of those with heterotopic ossification will experience a significant loss of joint mobility [38]. Treatment may include the administration of etidronate, which limits ossification, and physical therapy to maintain range of motion (see Chapter 130).
Osteoporosis
Osteoporosis among patients with SCI is common. Immobilization and the lack of weight-loading activities are among the chief causes of osteoporosis. Other factors may include alterations in blood circulation, lack of muscle traction on bone, and hormonal changes [40]. The loss of bone density develops in the acute stage of injury, with demineralization occurring below the level of injury [41]. Patients with SCI are at significant risk for long bone fracture, and care must be taken to prevent fractures resulting from range of motion exercises and falls. There is evidence from several studies that bisphosphonates may slow or stop the loss of bone density after SCI, but guidelines as to their use have not yet been developed [40]. In the absence of clear guidelines, investigation, treatment, and monitoring for osteoporosis in SCI patients remains somewhat empirical. Supplementation with vitamin D is often recommended, although calcium supplementation remains controversial because of the associated risk of urinary calculi [12] (see Chapter 140 for further details).
Autonomic Dysreflexia
For the treatment of autonomic dysreflexia, it is necessary to remove the precipitating noxious stimulus. Patients should be placed in an upright position, if possible, to decrease blood pressure, and a search for a causative agent is initiated. Most cases of autonomic dysreflexia are related to bladder distention or bowel distention [42]. However, noxious stimuli such as ingrown toenails, pressure ulcer, and renal calculi are not uncommon. Vasodilating medications such as nitropaste may be required to decrease the blood pressure while the clinician seeks the causative factor [43]. Nitrates are contraindicated in individuals who have ingested cyclic guanosine monophosphate–specific phosphodiesterase type 5 inhibitors, such as sildenafil, as the nitrates can potentiate the hypotensive effects of sildenafil and cause severe hypotension [37].
Rehabilitation
A comprehensive rehabilitation program is essential to optimize functional independence. The program should address functional goals related to mobility, transfers, and self-care as well as issues related to health maintenance and self-advocacy. Depending on the outstanding treatment goals, the treatment team may include a physical therapist, occupational therapist, orthotist, nurse, and mental health provider.
Mobility is a major issue that needs to be addressed both initially and then periodically as the patient’s condition changes (e.g., women who become pregnant may require assistance with functional activities as the pregnancy progresses). Typically, patients with thoracic SCI are able to achieve mobility with a manual wheelchair. Higher levels of thoracic SCI are associated with greater truncal instability. This may affect stability in a wheelchair and should be addressed with the seating prescription. Most patients with thoracic injuries, with training, are able to transfer independently.
For assistance with independence in self-care, adaptive equipment, such as long-handled shoehorns and reachers, can be recommended. Home environmental modifications may also be necessary (i.e., ramp to enter home). Patients with thoracic SCI should be able to drive a modified car or van.
Passive interventions, such as therapeutic heat and cold as well as transcutaneous electrical nerve stimulation, may be beneficial in the management of pain. However, particular caution must be used with therapeutic heat or cold modalities over insensate areas.
As well, with higher levels of injury, there is increasing loss of intercostal muscle innervation and increased level of respiratory impairment. Techniques including incentive spirometry, manual assisted cough, and insufflation-exsufflation can be used to reduce pulmonary complications of thoracic SCI.
Physical interventions, such as daily stretching of muscles with terminal sustained stretch, can be considered a first-line rehabilitative treatment for spasticity. Positioning as well as serial casting and splinting of the affected limbs can minimize spasticity.
Other rehabilitation treatments may also be considered. Functional electrical stimulation may improve muscle strength, decrease muscle atrophy, and improve lower limb endurance [44]. Body weight–supported treadmill training is prescribed in many centers. This intervention has been shown to be as effective as traditional gait training practices in the first year after injury with regard to functional outcomes [44]. The use of lower limb bracing may be an option in individuals with thoracic SCI, predominantly in those with lower thoracic level.
Procedures
A number of procedures can be used to address issues such as spasticity and pain. Interventional approaches for the treatment of spasticity include botulinum toxin injection, motor branch blocks, and peripheral nerve blocks (see Chapter 153). To decrease sphincter tone in men, botulinum toxin can be injected into the sphincter. This treatment in women is associated with an unacceptably high incidence of urinary incontinence. A patient with dysreflexia caused by a bladder stone may require a urologic procedure for stone removal. For men with ejaculatory dysfunction, retrieval of sperm for insemination has been successfully accomplished by electroejaculation and vibrostimulation methods. These procedures may result in dysreflexia and are performed under medical supervision.
Surgery
Pressure ulcers that do not heal with conservative methods may require surgical closure. Direct closure, skin grafts, musculocutaneous flaps, and skin flaps are among the surgical treatments available for wound closure. Mobilization after surgical closure must be done under close supervision, with careful monitoring of the surgical wound.
A variety of surgical procedures are used in patients who cannot be satisfactorily maintained with an intermittent catheterization program. Sphincter tone can be reduced with a sphincterotomy for men. Men must wear external collection devices after this procedure because it results in continuous incontinence. Sphincterotomies may occasionally require revision because of the development of fibrosis that obstructs outflow. Sphincterotomies may result in erectile dysfunction in some men. In contemporary practice, sphincterotomies are performed less frequently.
Bladder augmentation is occasionally performed to increase bladder capacity. A piece of small bowel is interposed with the bladder tissue to increase vesical volume. Patients with chronic dysreflexia resulting from persistent bowel management difficulties, such as frequent impaction, may be candidates for ileostomy or colostomy procedures. Patients with hemorrhoids aggravated by digital stimulation occasionally require surgical consultation if the hemorrhoids are not relieved by more conservative methods (e.g., medicated suppositories or topical steroid creams).
On occasion, men with erectile dysfunction that is not amenable to less invasive therapies may opt for an implantable penile prosthesis. Again, with the introduction of medications to treat erectile dysfunction, these surgeries are less frequently performed. Surgical placement of an intrathecal morphine or baclofen pump may be beneficial for patients with severe pain or spasticity that is not responsive to noninvasive treatments. Surgical interventions are indicated in some cases of heterotopic ossification. Patients with functionally limited joint mobility or severe and chronic spasticity may benefit from surgical resection of the lesion.
Potential Disease Complications
Individuals with thoracic-level SCI are more likely to have associated injuries that are serious than are those with cervical or lumbosacral spinal cord lesions. In one study, patients with traumatic thoracic spine fractures had an 86.2% occurrence of additional traumatic injury, such as rib fractures (42.1%), pulmonary contusions (37.5%), pneumothorax or hemothorax (34.9%), cervical spine injury (31%), lumbar spine injuries (28.4%), clavicular fractures (11.9%), sternal fractures (10.7%), and scapular fractures (10.3%) [45]. Thoracic SCI may be associated with a lower life expectancy. The leading causes of death among people with SCI in the national Spinal Cord Injury Model Systems database are respiratory, infectious, and cardiovascular diseases [1]. Complications arising from a thoracic-level injury result from immobility, changing sensory patterns, and alterations in autonomic nervous system function.
Potential Treatment Complications
The anticholinergic side effects of tricyclic antidepressants, including dry mouth, blurred vision, and urinary retention, can pose additional difficulties for the patient with SCI. Sphincterotomies, performed to alleviate detrusor-sphincter dyssynergia, may result in urinary incontinence and occasionally sexual dysfunction in men. Voiding by the Credé maneuver may lead to vesicoureteral reflux. The long-term use of indwelling catheters is associated with prostatitis, epididymitis, strictures, bladder stones, and bladder carcinoma.
Digital stimulation of the bowel can result in autonomic dysreflexia and hemorrhoids.
Medications used to treat autonomic dysreflexia can result in hypotension. The blood pressure must be closely monitored.
When the less invasive methods of treating spasticity are ineffective, more interventional approaches to spasticity, including chemodenervation with botulinum toxin or neurolysis with phenol injections, motor branch blocks, peripheral nerve blocks, and intrathecal baclofen pump insertion, may be considered. Injections may result in bleeding or infection. Nerve blocks may result in dysesthesias and weakness. Patients with intrathecal baclofen pumps may experience drowsiness, weakness, catheter breakage, or infection.