CHAPTER 150
Pulmonary Rehabilitation (Neuromuscular)
Definition
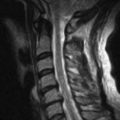
Neuromuscular disorders such as amyotrophic lateral sclerosis and myopathic disorders most often result in respiratory morbidity and mortality caused by weakness of the respiratory muscles. The three respiratory muscle groups are the inspiratory muscles, the expiratory (predominantly abdominal and upper chest wall) muscles for coughing, and the bulbar-innervated muscles. Whereas the inspiratory and expiratory muscles can be completely supported by physical aids such as continuous noninvasive ventilation (NIV), as presented here, and some patients have used this for more than 50 years without resort to tracheostomy (Fig. 150.1), there are no effective noninvasive measures to assist bulbar-innervated muscle function [1,2].

Symptoms
Patients with diminished ventilatory reserve who are able to walk complain of exertional dyspnea; but wheelchair users’ symptoms may be minimal except during intercurrent respiratory infections, when they complain of anxiety, inability to fall asleep, and possibly dyspnea. Morning headaches, fatigue, sleep disturbances, and hypersomnolence result from nocturnal hypoventilation or the decreased ability to recruit accessory respiratory muscles during sleep [3].
Physical Examination
Signs of inspiratory muscle impairment and hypoventilation can include tachypnea, paradoxical breathing, hypophonia, nasal flaring, accessory respiratory muscle use, cyanosis, flushing or pallor, anxiety, and airway secretion congestion. Lethargy, obtundation, and confusion signal carbon dioxide (CO2) narcosis. Often there are no signs at all despite symptoms of hypoventilation. The rest of the physical examination is usually typical for neuromuscular disorders (see, for example, Chapters 132 and 135).
Functional Limitations
With diminished respiratory reserve, walking becomes limited by dyspnea in going up stairs or walking long distances. Once a patient is wheelchair dependent and incapable of independently performing few activities of daily living, there are no additional functional limitations associated with chronic hypoventilation. However, symptoms of hypoventilation and CO2 levels may increase until patients become obtunded or develop CO2 narcosis.
Diagnostic Studies
Respiratory muscle dysfunction and hypoventilation are diagnosed by end-tidal CO2 monitoring (capnography), oximetry, spirometry, and assessment of cough peak flows. The end-tidal CO2 is 2 to 6 mm Hg less than arterial PCO2. The vital capacity (VC) is measured in sitting and supine positions and should not be significantly different in either position. Because hypoventilation is worse during sleep, the supine VC is more important. Orthopnea is common when the VC is less than 25% of normal or the VC in the supine position is at least 20% less than in the sitting position. The normal difference in VC between sitting and the supine position is less than 7%. Patients wearing thoracolumbar bracing should have the VC measured both with the brace on and with it off because a brace that fits well can increase VC, whereas one that restricts respiratory muscle movement can decrease it.
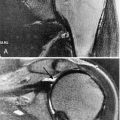
Patients are taught glossopharyngeal breathing (GPB), and its progress is measured spirometrically, as is air stacking. Air stacking and GPB are used for lung volume recruitment. Patients air stack by receiving consecutively delivered volumes of air through a manual resuscitator or volume-cycled ventilator that are held by the glottis to the greatest volume possible. The maximum volume that can be held by the glottis, the maximum insufflation capacity (MIC), is determined spirometrically. Likewise, GPB can often provide volumes of air to or beyond those achieved by air stacking and is also measured spirometrically [4]. A nasal interface or oral-nasal interface can be used for air stacking when the lips are too weak for effective air stacking through the mouth (Fig. 150.2).
Cough peak flows are measured with a peak flow meter (Access Peak Flow Meter; Healthscan Products Inc, Cedar Grove, NJ). Cough peak flows of 160 L/min are minimal and often ineffective [4]. The attainment of (unassisted) cough peak flows above 160 L/min is the best indicator for successful tracheostomy tube removal irrespective of remaining pulmonary function [4]. Patients with VCs of less than 1500 mL have assisted cough peak flows measured from a maximally air stacked volume of air with an abdominal thrust delivered simultaneously with glottic opening [5]. A cough produced from a deep air stacked volume and with an abdominal thrust applied concomitantly with glottis opening is a “manually assisted cough”; its efficacy is also determined by peak flow meter.
For the stable patient without intrinsic pulmonary disease, arterial blood gas sampling is unnecessary. Besides the discomfort, 25% of patients hyperventilate as a result of anxiety or pain during the procedure [6].
For symptomatic patients with normal VC, an unclear pattern of oxyhemoglobin desaturation, and no apparent hypercapnia, polysomnography is warranted [7]. Polysomnography is unnecessary for symptomatic patients with decreased VC because it is programmed to interpret every apnea and hypopnea as resulting from central or obstructive events rather than from inspiratory muscle weakness.
Whereas all clearly symptomatic patients with diminished lung volumes require a trial of NIV to ease symptoms, if symptoms are questionable, nocturnal continuous capnography and oximetry are useful and most practically done in the home. A questionably symptomatic patient with decreased VC, multiple nocturnal oxyhemoglobin desaturations below 95%, and elevated nocturnal CO2 should be encouraged to undergo a trial of nocturnal NIV.
Treatment
Initial and Habilitation
Lung Volume Recruitment
The initial intervention goals are to promote normal lung and chest wall growth for children; to maintain lung and chest wall compliance, lung growth, and chest wall mobility; and to increase cough peak flows to prevent intercurrent respiratory tract infections from developing into pneumonias and respiratory failure.
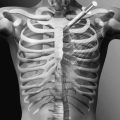
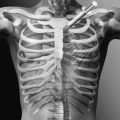
Pulmonary compliance is diminished and chest wall contractures and lung restriction occur when the lungs cannot be expanded to predicted inspiratory capacity. As the VC decreases, the largest breath one can take expands only a fraction of lung volume. Like limb articulations, regular mobilization is required. This can be achieved only by providing deep insufflations, air stacking, or nocturnal NIV [8]. The primary objectives of lung expansion therapy are to increase VC and voice volume, to maximize cough peak flows (Fig. 150.3), to maintain pulmonary compliance, to diminish atelectasis, and to master NIV because anyone who can air stack through a mouthpiece can use mouthpiece NIV and therefore be successfully extubated and decannulated even if not ventilator weaned (Table 150.1).
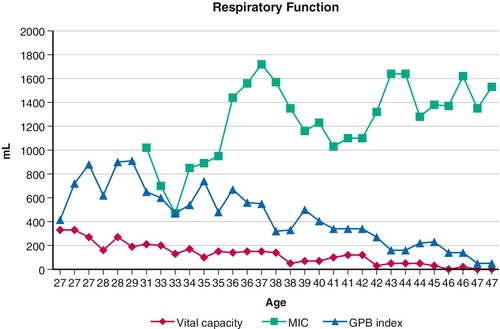
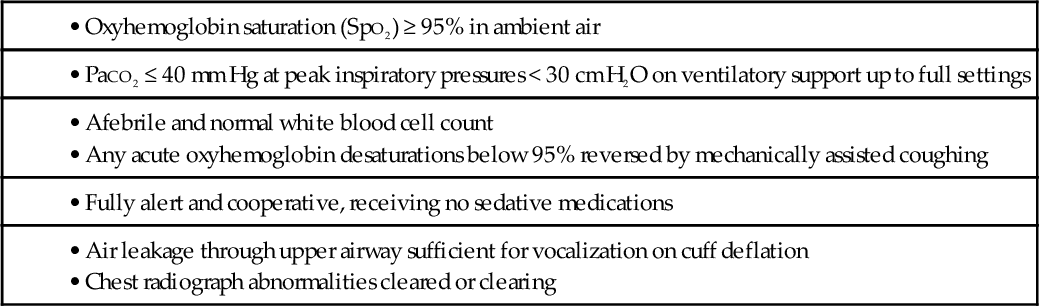
Table 150.1
Extubation Criteria for “Unweanable” Ventilator Users
• Oxyhemoglobin saturation (SpO2) ≥ 95% in ambient air
• Afebrile and normal white blood cell count
• Any acute oxyhemoglobin desaturations below 95% reversed by mechanically assisted coughing
• Fully alert and cooperative, receiving no sedative medications
• Air leakage through upper airway sufficient for vocalization on cuff deflation
• Chest radiograph abnormalities cleared or clearing
Bulbar muscle function and glottis function are objectively quantitated by the extent to which the MIC exceeds VC (MIC − VC). Thus, one who cannot air stack cannot close the glottis and must be passively insufflated by a pressure-cycled ventilator or CoughAssist (Respironics International Inc, Murrysville, Pa) at pressures of 40 to 70 cm H2O or by a manual resuscitator with the exhalation valve blocked. The maximum passive insufflation volume has been termed the lung insufflation capacity [8]. In 282 evaluations of VC, MIC, and lung insufflation capacity, the mean values were 1131 ± 744 mL, 1712 ± 926 mL, and 2069 ± 867 mL, respectively [9].
Cough Flow Augmentation
Manually assisted coughing involves air stacking to a deep lung volume for any patient with less than 1500 mL of VC, then applying an abdominal thrust timed to glottic opening. For 364 patients able to air stack, whereas the mean VC was 997 mL, the mean MIC was 1648 mL, and although cough peak flows were 135 L/min, mean assisted cough peak flows were 235 L/min. This can be the difference between developing pneumonia and coughing effectively to prevent it [10]. The inability to generate 160 L/min of assisted cough peak flows despite having a VC or MIC greater than 1 L indicates upper airway obstruction, which can be due to severe bulbar muscle dysfunction or other lesion that should be identified by laryngoscopy so that reversible lesions can be corrected.
Mechanically assisted coughing (MAC) is the combination of the use of mechanical insufflation-exsufflation (CoughAssist) with an exsufflation-timed abdominal thrust. Deep insufflations followed immediately by deep exsufflations at pressures of 50 to − 50 or greater cm H2O are usually the most effective and preferred. MAC can be provided by an oral-nasal mask, a simple mouthpiece, or a translaryngeal or tracheostomy tube. When it is delivered by a translaryngeal or tracheostomy tube, the cuff, when present, should be inflated. The CoughAssist can be manually or automatically cycled. Manual cycling facilitates caregiver-patient coordination of inspiration and expiration with insufflation and exsufflation, but it requires hands to deliver an abdominal thrust, to hold the mask on the patient, and to operate machine cycling.
Procedures
Lung Volume Recruitment
Before patients’ VCs decrease to 70% of predicted normal, they are instructed to air stack 10 to 15 times two or three times daily, usually with a manual resuscitator. Because of the importance of air stacking, NIV is provided through ventilators using volume rather than pressure cycling.
Infants cannot air stack or cooperate with passive insufflation therapy. All small children with paradoxical breathing require nocturnal NIV to prevent pectus excavatum and to promote lung growth as well as for inspiratory muscle assistance [11]. Deep insufflations can also be timed to the child’s breathing and delivered through an oral-nasal interface. Children can become cooperative with deep insufflation therapy by 14 to 30 months of age.
Cough Flow Augmentation
Cough flows are augmented and sputum expulsed by delivery of about five cycles of MAC followed by a short period of normal breathing or ventilator use to avoid hyperventilation until offending sputum is expulsed and oxyhemoglobin saturation levels, if diminished, return to 95% or greater. Insufflation and exsufflation times are adjusted to provide maximum observable chest expansion and rapid full lung emptying. In general, 2 to 4 seconds are required. Treatment continues until no more secretions are expulsed and secretion-related oxyhemoglobin desaturations are reversed. Use can be required as often as every 30 minutes around-the-clock during chest infections.
The use of mechanical insufflation-exsufflation through the upper airway can be effective for children as young as 11 months, who can occasionally facilitate its efficacy by not crying or closing the glottis. Between 2.5 and 5 years of age, most children cooperate with it. Before that, the insufflations and exsufflations and exsufflation-timed abdominal thrusts are timed to the child’s own breathing cycle.
Conventional airway suctioning misses the left main stem bronchus about 90% of the time [12]. However, MAC can provide effective flows in both left and right airways without the discomfort or airway trauma. Patients prefer MAC to suctioning [13]. Deep suctioning, whether by tube or upper airway, can be discontinued for most patients.
VC, pulmonary flow rates, and SpO2, when abnormal, can improve immediately with clearing of airway secretions by MAC [14]. An increase in VC of 15% to 42% was noted immediately after treatment in 67 patients with “obstructive dyspnea,” and a 55% increase in VC was noted after MAC for patients with neuromuscular disorders [15]. We have observed 15% to 400% (200 to 800 mL) improvements in VC and normalization of SpO2 for patients during chest infections [16].
Of the three muscle groups required for effective coughing, MAC can take the place of only the inspiratory and expiratory muscles. Thus, ventilator users with intact bulbar muscles can usually air stack to volumes of 3 L or more and, unless very scoliotic or obese, can achieve effective assisted cough peak flows of 6 to 9 L/s and do not need MAC. The patients who need MAC the most have moderately impaired bulbar muscle function that limits assisted cough peak flows to less than 300 L/min. This is typical of Duchenne muscular dystrophy and other myopathies [10]. Patients with respiratory muscle weakness complicated by scoliosis and inability to capture the asymmetric diaphragm by abdominal thrusting can also greatly benefit from MAC (Table 150.2).
Table 150.2
Management of Patients with Spinal Cord Injury
| Level* | Vital Capacity | Bulbar Function/Neck Function† | Daytime | Nocturnal |
| Above C1 | 0 | Inadequate/inadequate | TIPPV | TIPPV |
| C2-C3 | < 200 mL | Adequate/inadequate | EPP/DP | NIPPV/MIPPV |
| Below C2 | > 200 mL | Adequate/adequate | MIPPV/IAPV | NIPPV/MIPPV |
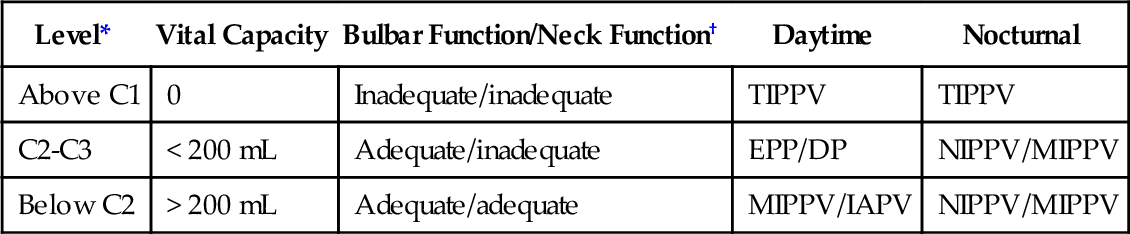
DP, diaphragm pacing; EPP, electrophrenic pacing; IAPV, intermittent abdominal pressure ventilation; MIPPV, mouthpiece intermittent positive pressure ventilation; NIPPV, nasal intermittent positive pressure ventilation; TIPPV, tracheostomy intermittent positive pressure ventilation.
* Motor levels.
† Adequate neck function involves sufficient oral and neck muscle control to rotate, flex, and extend the neck to grab and use a mouthpiece for intermittent positive pressure ventilation. Adequate bulbar function prevents aspiration of saliva to the degree that the SpO2 baseline decreases below 95%.
Surgery
No surgery is indicated in these patients.
Potential Disease Complications
The great majority of morbidity and mortality for patients with neuromuscular disease is due to complications of respiratory muscle weakness. In particular, these patients develop life-threatening chronic alveolar hypoventilation. Life can be prolonged by ventilatory assistance or support, which is usually administered invasively but can be administered noninvasively by use of physical medicine respiratory muscle aids. Respiratory orthopnea, symptoms of hypoventilation noted before, and paradoxical breathing in children indicate the need for nocturnal noninvasive intermittent positive pressure ventilation (NIV) [11]. Because, in general, only patients improperly treated with supplemental oxygen develop CO2 narcosis and respiratory failure is generally caused by ineffective cough and airway secretion management, any patient finding that NIV use is more burdensome than his or her symptoms is told that it is all right to discontinue it for now but to return regularly for reevaluation.
Although the inspiratory muscles can be assisted by applying pressures to the body, negative pressure body ventilators cause obstructive apneas, are less effective than NIV, and become even less effective with age and decreasing pulmonary compliance [17]. A useful body ventilator, the intermittent abdominal pressure ventilator (IAPV) or Exsufflation Belt (Respironics International Inc, Murrysville, PA), involves the intermittent inflation of an elastic air sac that is contained in a corset or belt worn beneath the patient’s outer clothing. The sac is cyclically inflated by a positive pressure ventilator. This moves the diaphragm upward to assist in expiration. With bladder deflation, gravity causes the abdominal contents and diaphragm to return to the resting position, and inspiration occurs. A trunk angle of 30 degrees or more from the horizontal is required for effectiveness. A patient can add to the IAPV-delivered volume by breathing or GPB along with it. The IAPV augments tidal volumes by 300 mL to as high as 1200 mL and is often preferred to mouthpiece NIV by patients with little autonomous breathing ability during daytime hours [18].
Noninvasive Intermittent Positive Pressure Ventilation
NIV can be delivered by lip seal, nasal, and oral-nasal interfaces during sleep or by a mouthpiece or nasal interface during daytime hours. Mouthpiece and nasal NIV are open systems that require the user to rely on central nervous system reflexes to prevent excessive insufflation leakage during sleep [3,19]; thus, supplemental oxygen and sedatives can render it ineffective. NIV can be introduced in the clinic or home setting.
There are numerous commercially available nasal interfaces (continuous positive airway pressure masks). Several should be tried and their use alternated to avoid prolonged skin pressure. Excessive insufflation leakage can be avoided, if necessary, by switching from an open to a closed system with use of a lip seal–nasal prongs system. Such interfaces deliver air through the mouth and nose during sleep and require minimal strap pressure. This optimizes skin comfort and minimizes air (insufflation) leakage.
The most useful method for daytime ventilatory support is NIV through a 15-mm angled mouthpiece. Some keep the mouthpiece in the mouth all day, but most have it fixed near the mouth by a metal clamp attached to a wheelchair for this purpose [20–22]. The mouthpiece can also be fixed onto motorized wheelchair controls (e.g., sip and puff, chin, and tongue controls). Large volumes of 800 to 1500 mL are delivered so that the patient can take as much air as desired for each breath to vary tidal volumes, speech volume, and cough flows as well as to air stack. Neck movement and lip function are needed to use mouthpiece NIV; otherwise, a nasal prongs system is used for daytime support [18]. Nasal NIV is also most practical for nocturnal and daytime use by infants. Other than perhaps for uncontrollable seizures and the inability to cooperate, there are no contraindications to the use of NIV long term.
Glossopharyngeal Breathing
Both inspiratory and, indirectly, expiratory muscle function can be assisted by GPB [23]. This is the glottis pistoning boluses of air into the lungs. One GPB breath usually consists of six to nine gulps of 40 to 200 mL each (Fig. 150.3). During the training period, its efficiency can be monitored by spirometrically measuring the milliliters of air per piston action, piston actions per breath, and breaths per minute. A training manual [24] and numerous videos are available [25], the most analytical of which was produced in 1999 [26]. GPB can provide an individual with no VC with ventilator-free breathing tolerance up to all day [23,27].
Severe oropharyngeal muscle weakness can limit or eliminate the usefulness of GPB. However, we have managed 13 Duchenne muscular dystrophy ventilator users who had no breathing tolerance other than by GPB [28]. Approximately 60% of ventilator users with no autonomous ability to breathe but with good bulbar muscle function can use GPB for ventilator-free breathing up to all day [23,27]. GPB is rarely useful in the presence of an indwelling tracheostomy tube. The safety and versatility afforded by GPB are key reasons for tube decannulation or avoidance of tracheostomy in favor of NIV and MAC. This frees patients from the fear of sudden ventilator failure or accidental ventilator disconnection [23,27,29].
Oximetry Monitoring and Feedback Protocol
For a hypercapnic patient with oxyhemoglobin desaturation due to chronic alveolar hypoventilation or for the patient being weaned from tracheostomy ventilation, introduction to and use of mouthpiece or nasal NIV are facilitated by oximetry feedback. An SpO2 alarm set at 94% signals the patient to maintain normal SpO2 by taking deeper breaths and to maintain SpO2 above 94% all day [10]. When it is no longer possible to achieve this by unassisted breathing, it is done by mouthpiece or nasal NIV. With advancing disease and muscle weakness, the patient can require increasing periods of daytime NIV to maintain normal SpO2 and intact central ventilatory drive.
Continuous SpO2 feedback is especially important during respiratory tract infections. The cough of infants and small children who can never sit is inadequate to prevent chest cold–triggered pneumonia and acute respiratory failure. MAC is used for any dip in SpO2 below 95%. When NIV is used continuously, such dips are usually due to bronchial mucous plugging rather than to hypoventilation, and if the mucus is not quickly cleared, atelectasis and pneumonia can quickly result. Thus, patients are instructed to use NIV and MAC to maintain normal SpO2 to avert pneumonia and respiratory failure. For adults with infrequent chest colds, rapid access to MAC may be all that is necessary.
Potential Treatment Complications
Abdominal distention tends to occur sporadically in NIV users. The air usually passes as flatus once the patient is mobilized in the morning. When it is severe, however, it can increase ventilator dependence and necessitate burping the air out by opening a gastrostomy or nasogastric tube.
Despite aggressive lung mobilization and expansion three times daily, often to above 60 cm H2O pressures and along with NIV support for more than 50 years in many cases, we have had one case of pneumothorax in more than 1000 NIV users [30]. Although it is often described as a complication of or limiting factor for NIV, secretion encumbrance most often results from failure to use MAC.