CHAPTER 15. MALIGNANCIES
Debra E. Heidrich and Peg Esper
The term cancer refers to a group of diseases characterized by uncontrolled growth of abnormal cells, with local tissue invasion and systemic metastasis. It is estimated that 1,399,790 Americans will be diagnosed with cancer in 2006 and 564,830 patients—approximately 1500 a day—are expected to die of malignant disease. Cancer is the second leading cause of death in the United States, accounting for one of every four deaths. The 5-year survival rate for patients diagnosed with cancer between 1995 to 2001 was 65%; this is an increase from 50% between 1974 to 1976. The increased survival is likely due to advances in early detection and treatment (American Cancer Society [ACS], 2006).
According to the National Hospice and Palliative Care Organization (NHPCO, 2006), approximately 46% of the 1,060,000 patients admitted to hospice programs in 2004 had a diagnosis of cancer. While there are potential flaws in using the data on cancer deaths from one report to compare with the number of cancer patients served by hospice programs based on a different report, it is reasonable to conclude that a large majority of patients who die with cancer are admitted to hospice programs. However, the average and median lengths of stay in hospice programs are only 57 days and 22 days, respectively. So, even though many cancer patients are referred to hospice programs, the referrals come late. In order to promote optimal quality of life, patients with advanced cancers require coordinated, individualized interdisciplinary palliative care and appropriate referrals to hospice programs. The question becomes, then, how can the clinician do a better job of identifying those who should be referred for palliative care in a timely manner?
Cancers of the lung, colon and rectum, breast, pancreas, and prostate, as well as non-Hodgkin’s lymphoma, have the highest mortality rates, accounting for approximately 60% of all cancer deaths in both males and females (ACS, 2006). These cancers are briefly discussed in this chapter. Among these cancers there is wide variability on survival based on tumor size, histological grade, response to treatment, and number of metastatic sites. In general, larger, poorly differentiated tumors and the presence of distant metastasis are poor prognostic indicators. A referral to a palliative care team is appropriate for all patients with stage IV disease. Also, those patients with other stages of cancer whose diseases progress while on treatment or with short progression-free intervals after completing a course of treatment have a worse prognosis and should be referred for a palliative care team consult.
Figure 15-1 shows the typical dying trajectory for cancer patients. This model is based on a study that monitored the number of independent activities of daily living performed over the last year of the lives of patients with cancer (Lunney, Lynn, Foley et al., 2003). As illustrated in this figure, performance status is a good indicator of length of life for cancer patients—the poorer the performance status, the shorter the survival. Multiple studies of patients enrolled in palliative care programs report that a Karnofsky Performance Status score of 50% suggests a life expectancy of less than 8 weeks (Lamont & Christakis, 2003). Patients with a declining performance status should be referred to a palliative team. Many patients with cancer are likely eligible for hospice care under the admitting criteria of the Hospice Medicare benefit (i.e., a life expectancy of 6 months or less) when their performance status begins to decline.
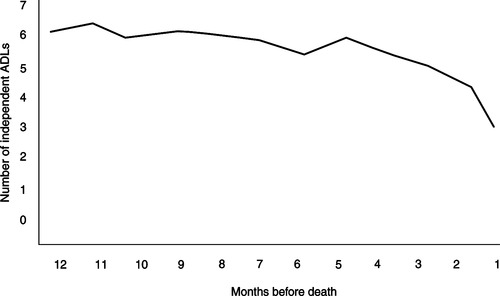 |
| Figure 15-1
(Adapted from Lunney, J.R., Lynn, J., Foley, D.J., et al. [2003]. Patterns of functional decline at the end of life. JAMA, 289[18], 2387-2392.)
|
The symptom profile of the patient also assists in identifying those patients appropriate for palliative care. Dyspnea, dysphagia, xerostomia, anorexia, and cognitive impairment are consistently identified as being associated with decreased length of life (Glare, 2005; Lamont & Christakis, 2003). Interestingly, pain is not predictive of poor survival, although increasing pain is reported to be more common in the last weeks of life (Glare, 2005). Also, increasing pain can be a sign of disease progression and must be evaluated thoroughly and managed optimally.
Predictions of survival by physicians are usually overly optimistic, but there is a correlation between predicted and actual survival up to 6 months. The most precise predictions are for those with less than 4 weeks to live, but predictions beyond 6 months show no relationship to actual survival (Glare, 2005). This helps explain why the median length of stay in hospice programs is only 22 days—physicians are most confident in predicting that a patient is in the last 6 months of life only about 4 weeks before the patient’s death.
Patients who should receive palliative care from an interdisciplinary team include those with a cancer diagnosis who have an advanced stage of disease; a tumor that progresses during treatment; a short interval between treatment and tumor progression; a poor functional status; a symptom profile that includes dyspnea, dysphagia, xerostomia, anorexia, and cognitive impairment; or a clinician’s prediction of survival of 6 months or less.
ETIOLOGY AND PATHOPHYSIOLOGY
Cancer is now understood to be caused by genetic mutations, including hereditary genetic factors and somatic genetic changes. It likely requires multiple accumulated oncogenic changes for complete transformation to malignancy. These genetic changes may lead to dysregulated growth, silencing of tumor-suppression capabilities, interference with normal cell death (apoptosis), and arresting of cell differentiation (Calvo, Petricoin, & Liotta, 2005). Together, these changes allow cancer cells to become self-renewing, immortal, and undifferentiated, as well as to possess the ability to be invasive. The familial cancers, such as certain retinoblastomas, breast cancers, colon cancers, and Wilms’ tumors, involve at least one, if not several, hereditary genetic factors that interact with somatic genetic changes before malignant cells develop. The somatic genetic changes may be caused by viruses, chemical agents (including tobacco), radiation, and ultraviolet light (Yuspa & Shields, 2005).
Cancer is primarily a disease of the older population. One reason for this may be that progression from normal tissue to invasive cancer takes place over 5 to 20 years (Calvo et al., 2005). The fact that many of the cancers of the elderly (e.g., breast, lung, colon, prostate) arise from epithelial tissue is of interest. Because the epithelial tissues tend to be in areas that have undergone continued renewal throughout the life span, it is possible that cancers arise from a cumulative mutational load (Wong & Depinho, 2005).
Metastasis is the process by which malignant cells are released from the primary tumor and travel to regional or distant sites where they adhere and grow. Metastasis is the leading cause of both treatment failure and death for most patients who die of cancer. The process of metastasis formation is called the metastatic cascade. A host of factors are involved that allow tumor cells to “detach from the primary tumor, invade the extracellular basement membrane and enter circulation, survive in circulation to arrest in the capillary bed, adhere to a subendothelial basement membrane, gain entrance into the organ parenchyma, respond to paracrine growth factors, proliferate and induce angiogenesis, and evade host defenses” (Stetler-Stevenson, 2005, p. 117).
Common sites of metastasis are the lymph nodes, bone, brain, liver, and lungs. Cancer diagnoses that most often lead to terminal illness are those that metastasize early and therefore are diagnosed at advanced stages. The six leading causes of death due to cancer are discussed below. Table 15-1 outlines the metastatic patterns of these diseases.
| XXX, Seen in almost all patients with advanced disease; XX, seen in most patients with advanced disease; X, seen in many patients with advanced disease. | |||||||||
| Cancer Diagnosis | Lung | Pleura | Brain | Bone | Liver | Adrenal Glands | Lymph Nodes | Skin | Bone Marrow |
|---|---|---|---|---|---|---|---|---|---|
| Lung | |||||||||
| Squamous | — | X | X | X | |||||
| Adenocarcinoma | — | XX | XX | XX | XX | XXX | XX | ||
| Large-cell | — | XXX | X | XX | XX | XXX | XX | ||
| Small-cell | — | XXX | X | XX | XXX | XXX | XXX | ||
| Colorectal | XX | X | X | XXX | XXX | ||||
| Breast | XXX | XX | XX | XXX | XXX | XX | XXX | X | |
| Prostate | X | XXX | X | XXX | |||||
| Pancreas | XX | XX | X | XX | XXX | XX | XXX | ||
| Non-Hodgkin’s lymphoma | XX | X | XX | X | XX | XX | — | X | XX |
Lung Cancer
Accounting for 13% of all new cancers and 29% of all cancer deaths, lung cancer is the predominant cause of cancer deaths across all race, gender, and ethnic groups in the United States. In 2006, an estimated 162,460 Americans will die from lung cancer (ACS, 2006). Squamous cell lung cancers tend to remain more localized than the other cell types and thus have the best chance for surgical cure. The other major cell types—adenocarcinoma and large-cell and small-cell lung cancers—tend to metastasize early and frequently (Murren, Turrisi, & Pass, 2005; Schrump, Altorki, Henschke et al., 2005).
In addition to the common sites of metastasis as outlined in Table 15-1, all cell types of lung cancer are highly likely to metastasize to mediastinal lymph nodes. Other potential sites of metastasis include the pericardium and the pancreas. Lung cancers may also lead to the following paraneoplastic syndromes (Murren et al., 2005; Schrump et al., 2005):
▪ Hypercalcemia occurs secondary to parathyroid hormone–related protein production and is most common with squamous cell lung cancer.
▪ The syndrome of inappropriate antidiuretic hormone (SIADH) develops due to ectopic natriuretic factor and is most common with small-cell lung cancer.
▪ Eaton-Lambert syndrome occurs due to a reduction in acetylcholine released at motor nerve terminals. This syndrome is most common with small-cell lung cancer. Symptoms include proximal muscle weakness, diplopia, bulbar symptoms, dry mouth, impotence, and constipation.
▪ Ectopic adrenocorticotropic hormone leads to Cushing’s syndrome and is seen in small-cell lung cancer. Symptoms include muscle weakness, edema, hypertension, mental changes, glucose intolerance, and weight loss.
The pattern of early and frequent metastasis in lung cancer often translates into patients being diagnosed when the disease has reached an advanced stage. Indeed, 60% of small-cell lung cancers and 40% of non–small-cell lung cancers present in stage IV (Smith & Khuri, 2004). The 5-year survival rate for individuals diagnosed with distant metastasis is only 2.1% (ACS, 2006). Most patients should be referred for palliative care from the time of diagnosis.
Essentially all chemotherapy for advanced lung cancer is palliative in that cure is not the aim. Survival may be prolonged with chemotherapy, but the potential for increased length of life must be balanced against the effect on quality of life, toxicity of the chemotherapy, and costs of treatment. It is important to note that patients who respond best to chemotherapy are those who have not been previously treated with chemotherapy and who have a good performance status. Patients who have disease progression while receiving chemotherapy, relapse soon after completion of chemotherapy, or have a compromised performance status have a much lower potential for favorable outcomes from chemotherapy.
Colorectal Cancer
Colorectal cancer is the third leading cause of cancer deaths for men and for women and is the overall second leading cause of cancer deaths (men and women combined) in the United States. An estimated 55,170 will die of colorectal cancer in 2006, accounting for 10% of all cancer deaths (ACS, 2006). As with all cancers, the stage at diagnosis is the primary determinant of prognosis. The 5-year survival rate for colorectal cancers with distant metastasis is 9.7% (ACS, 2006).
Sites of metastasis include the lymph nodes, liver, lung, brain, bone, and adrenal glands (Libuiti, Saltz, Rustgi et al., 2005). These tumors are also likely to cause obstruction of the bowel, leading to nausea and vomiting and abdominal distention; obstruction of the ureter, causing flank pain, hydronephrosis, and renal failure; and obstruction of the urethra, leading to urinary retention and risk of bladder infection.
Until the past few years, colorectal tumors were considered resistant to most antineoplastic agents. Newer chemotherapy drugs and molecularly targeted therapies are improving survival in colorectal cancer (Wilkes, 2005). There is some controversy regarding the optimal duration of chemotherapy for metastatic disease. It appears that continuation of chemotherapy until clinical deterioration or disease progression has no advantage over a planned discontinuation of therapy after a fixed time (e.g., 3 months or 6 months). Patients with good performance status, good bone marrow reserve, and good organ function appear to benefit more from chemotherapy than do those who do not meet these criteria (Libuiti et al., 2005).
Breast Cancer
In the United States, breast cancer is the most frequent new cancer diagnosis in women and the second leading cause of cancer deaths in women. An estimated 41,430 patients will die from breast cancer in 2006 (ACS, 2006). The mortality rate has declined over the past decade largely due to early detection and the use of aggressive multimodality treatments (Wood, Muss, Solin et al., 2005). The 5-year survival rate for patients with distant metastasis is 26.1% (ACS, 2006). Poor prognostic indicators include stage IV disease, young age, estrogen receptor (ER)–and progesterone receptor (PR)–negative tumors, and tumors with HER2 overexpression. Stage IV breast cancer is not considered curable. The median survival for patients with metastatic disease is 18 to 24 months. However, many treatments are available to control metastasis and prolong life considerably, and with newer therapies, it is estimated that approximately 10% of patients with metastasis will survive 10 years or longer (Wood et al., 2005).
Women who receive hormonal therapy and chemotherapy following surgery in early-stage disease demonstrate delayed recurrence and improved overall survival compared with those who do not receive chemotherapy (Wood et al., 2005). Women with ER- and PR-positive tumors tend to respond to hormone therapy. Women with ER- and PR-negative tumors and slowly progressive disease with minimal symptoms should be considered for hormone therapy as well. However, women with ER- and PR-negative tumors with rapidly progressive tumors should be considered for chemotherapy (Wood et al., 2005).
Women with ER-positive tumors with metastatic disease should be treated with hormone therapy before chemotherapy is considered (Wilcken, Hornbuckle, & Ghersi, 2003). Tamoxifen is still considered first-line hormonal therapy for premenopausal women with ER-positive metastatic disease. In postmenopausal women with metastasis, the ideal sequencing of hormonal therapy is not clear; tamoxifen, aromatase inhibitors, and fulvestrant are all considered reasonable choices for first-line therapy at this time (Wood et al., 2005). Improvement in bone pain is the best indication of response to hormone therapy in women with metastasis. Hormone therapy is generally continued as long as symptoms are controlled and side effects are tolerated. If hormone therapy is no longer indicated, the dose of the hormone is weaned slowly to prevent a withdrawal response.
When hormone treatment is no longer effective, chemotherapy is usually considered. Improved survival and symptom control may be possible in stage IV disease with combination chemotherapy, even in women who have previously received chemotherapy (Berruti, Sperone, Bottini et al., 2000; Ghersi, Wilcken, & Simes, 2005; Nagourney, Link, Blitzer et al., 2000; Wood et al., 2005). Taxane-containing regimens have fewer side effects than regimens with platinum-containing chemotherapeutic agents (Carrick, Ghersi, Wilcken et al., 2005; Ghersi et al., 2005). Response rates to initial chemotherapy regimens range from 25% to 60%, with a median time to progression of 6 months. Response rates for second- and third-line treatments diminish by half (Wood et al., 2005). The beneficial effect of chemotherapy, when given for symptom control, should be apparent by the second or third treatment. If the symptom being treated is not improving, carefully evaluate the benefits versus the burdens of continuing treatment should be carefully evaluated.
The lymph nodes are the most frequent site of metastasis. Other sites include the bone, lung, liver, pleura, adrenal glands, kidney, brain, skin, and chest wall. Women with ER- and PR-positive tumors are most likely to develop bone metastasis, and those with ER- and PR-negative tumors tend to have liver and other visceral metastasis (Wood et al., 2005). Due to the proximity of tumors to the skin surface, breast tumors may cause skin lesions that can progress to fungating tumor wounds.
Prostate Cancer
Cancer of the prostate is the most frequent cause of cancer in men, with 234,460 new diagnoses expected in 2006. It is the second leading cause of cancer deaths in men. Approximately 27,350 men will die of prostate cancer in 2006. The 5-year survival rate for patients with distant metastasis is 33.5% (ACS, 2006). Again, distant metastasis is not considered curable, but clearly, with a 5-year survival of 33.5%, men are living for years with metastatic prostate cancer.
Prostate cancers are often androgen dependent. Most tumors will respond to hormonal manipulation, providing symptomatic benefits and a moderate survival benefit (Scher, Leibel, Fuks, et al., 2005). However, the side effects of androgen suppression can reduce the quality of life for some patients (Schmitt, Bennett, Seidenfeld et al., 2000). These tumors are also radiosensitive and may be treated with external beam radiotherapy or implanted radioactive seeds. Systemic irradiation (e.g., strontium 89) may be helpful for managing widely metastatic bone pain, but it is more expensive and no more effective than local field radiotherapy for managing bone pain in a localized area (Finlay, Mason, & Shelley, 2005; Oosterhof, Roberts, de Reijke et al., 2003). Until recently, chemotherapy did not play a role in the management of prostate cancer. Mitoxantrone-based regimens were the first to show palliative benefits in hormone-resistant tumors, but docetaxel-based therapies are also showing promise (Petrylak, Tangen, Hussain et al., 2004; Tannock, de Wit, Berry et al., 2004). Keep in mind that most study subjects showing a good response were less symptomatic and had a good performance status at the initiation of treatment; the benefit and tolerability of chemotherapy regimens in patients with poorer performance status, widely metastatic disease, or some organ system compromise are not known. Clinical trials using vaccines derived from dendritic cells expressing prostatic acid phosphatase are showing promise (Arlen & Gulley, 2005; Vieweg & Dannull, 2005).
Local spread of prostate cancer to the pelvic and abdominal lymph nodes, seminal vesicles, bladder, and peritoneum is common. Frequent sites of metastasis beyond the pelvis are bone (most frequent), lungs, and liver (Scher et al., 2005). Men with prostate cancer are at risk for developing urinary outflow obstruction, leading to urinary frequency, hesitancy, and nocturia; this may progress to urinary retention. They are also at risk for urethral obstruction, causing bladder dilatation, hydronephrosis, and impaired renal function.
Pancreatic Cancer
Although pancreatic cancer ranks eleventh in incidence of new cancers in the United States, it is the fourth leading cause of cancer deaths. In 2006, an estimated 32,300 patients will die from this malignancy. The prognosis for patients with pancreatic cancer is poor. The 5-year survival rate for all stages is just 4.6%, and for those with distant metastasis, the 5-year survival is only 1.8% (ACS, 2006). The disease generally does not display early symptoms. By the time jaundice is present or pain becomes significant enough for an individual to seek medical evaluation, 80% of pancreatic cancers are metastatic (Yeo et al., 2005).
Treatment options are limited. In the rare instance that disease is localized, surgery offers the only potential for cure, but survival after resection is often still limited. Most often, however, surgery is used for palliation of symptoms by relieving or preventing obstructions of the biliary duct and duodenum. The median survival after palliative surgery is about 6 months (Yeo et al., 2005). Chemotherapy does appear to modestly improve survival and to palliate some symptoms (El-Rayes & Philip, 2003; Yeo et al., 2005). Patients in clinical trials with gemcitabine demonstrated less pain, improved functional ability, and weight gain (Burris, Moore, Anderson et al., 1997). Patients showing the best responses to chemotherapy are treated soon after diagnosis, having not previously received chemotherapy, and have a good performance status. Combination chemotherapy with gemcitabine-based protocols is showing promise but needs further evaluation (Reni, Cordio, Milandri et al., 2005). The role of radiation therapy is limited, but some studies show benefit when chemotherapy is combined with radiation, particularly to shrink tumors preoperatively (Yeo et al., 2005). Palliative uses include relief of intestinal obstruction or unresectable biliary obstruction.
Regional metastatic sites include the regional lymph nodes, major vessels, celiac nerve plexus, duodenum, stomach, bile duct, retroperitoneum, spleen, kidney, adrenal glands, and colon. Distant metastasis occurs most often to the liver. Jaundice due to biliary obstruction is common. Additional metastatic sites include lung, bone, and brain (Yeo et al., 2005). Patients with pancreatic cancer may also develop a sudden onset of diabetes due to destruction of the islet cells.
Non-Hodgkin’s Lymphoma
In 2006, approximately 58,870 people will be diagnosed with non-Hodgkin’s lymphoma and 18,840 will die of this disease, making it the sixth leading cause of cancer deaths. The 5-year survival rate is 60% (ACS, 2006). Patients over the age of 60 tend to have a poorer prognosis (Fisher, Mauch, Harris et al., 2005).
The major classifications of non-Hodgkin’s lymphoma are based on cell-line (B cell or T cell/NK [natural killer] cell) and histological grade (low, medium, and high grade). Each of these types of classification has a significant influence on both treatment options and prognosis. In addition to cell type and histology, the stage of the disease influences the outcome. Although the staging system is different than that for solid tumors, stage IV is, again, the most advanced stage. Patients with diffuse or disseminated involvement of one or more extralymphatic organs, bone marrow involvement, or liver involvement have stage IV disease (Fisher et al., 2005).
Symptoms are related to the organ systems affected by the lymphadenopathy. Abdominal lymphadenopathy causes pain, anorexia, nausea and vomiting, bleeding, diarrhea, and obstruction. Hilar and mediastinal lymphadenopathy lead to cough, dyspnea, chest pain, pleural effusion, and superior vena cava syndrome. Central nervous system lymphoma may cause headache, lethargy, focal neurologic symptoms, seizures, and paralysis. Night sweats, recurrent fevers, and weight loss tend to be poor prognostic indicators (Fisher et al., 2005).
Many patients receive treatment upon diagnosis, because even when cure is not possible, treatment can significantly prolong survival. Patients with low-grade lymphoma who relapse may be retreated with the same or second-line therapy for symptomatic relief. Chemotherapy, radiation therapy, monoclonal antibody therapy, and stem cell transplantation are all potential options depending on the cell type and stage of disease.
COMMON SYMPTOMS OF ADVANCED CANCERS
As noted in Table 15-1, patients with advanced cancers may have multiple sites of metastasis. Keep in mind that almost all cancers can metastasize to almost any site; the sites identified on the chart are the more common metastatic sites. The symptoms commonly experienced by patients when cancer invades these sites are outlined below.
In addition to the following symptoms, all patients with cancer are at risk for pain due to compression of tissues by the tumor itself, leading to local ischemia, nerve compression, and obstruction of organ systems. Tumors may also invade local blood vessels. Oozing from small vessels may cause anemia; invasion of a major vessel may lead to death from exsanguination. Another common symptom of many advanced cancers is cachexia and anorexia (see Chapter 20). In addition to the symptoms caused by the disease itself, the treatments for cancer lead to uncomfortable symptoms, such as nausea and vomiting, diarrhea, anemia, mucositis, fatigue, and chemotherapy-induced peripheral neuropathies.
Lymph Node Metastasis
Lymph node metastasis causes pain from inflammation and swelling. When lymph flow is blocked by a tumor, lymphedema results and the affected area is at risk for infection, massive edema, and pain. Enlarged lymph nodes can also compress organs and surrounding tissues, leading to a risk for superior or inferior vena cava syndrome, spinal cord compression, nerve plexus compression, and obstruction of the bowel, urinary tract, or esophagus.
Bone Metastasis
Bone metastasis causes sharp pain that ranges from moderate to severe in intensity. Bone destruction leads to hypercalcemia and risk for pathological fractures. Patients with extensive bone metastasis are also at great risk for spinal cord compression from vertebral collapse.
Brain Metastasis
Brain metastasis puts the individual at risk for increased intracranial pressure and its accompanying symptoms—headache, vomiting, and change in level of consciousness. Depending on the area of the brain involved, personality changes are possible. Patients with brain metastasis are also at risk for seizure activity.
Liver Metastasis
Liver metastases interfere with the functioning of the liver, leading to impaired drug metabolism, disturbances in hemostasis, malabsorption, pruritus, anorexia, ascites, jaundice, and hepatic encephalopathy. Pain along the right rib margin is caused by the enlarging liver and is often referred around to the back and to the right shoulder.
Lung Tumors and Metastasis
Lung tumors, whether primary or metastatic, put the individual at risk for cough from irritation; dyspnea from effusion, atelectasis, and pneumonia; and wheezing from bronchospasm and obstruction. Hemoptysis results when tumor erodes blood vessels in the lung. Large tumors can compress local organs and tissues: if the esophagus is compressed, the individual experiences dysphagia; neuropathic pain occurs if there is pressure on the brachial plexus.
Adrenal Gland Metastasis
Adrenal gland metastases are often asymptomatic until the tumor size is at least 5 cm. With increasing tumor size, the individual experiences abdominal or back pain, as well as the gradual onset of weakness, lethargy, and anorexia. Hyperpigmentation is observed, especially in buccal mucosa, skin creases, and sites of friction. Nausea, vomiting, and postural hypotension are late signs of adrenal gland metastasis.
Bone Marrow Involvement
Bone marrow involvement by tumor leads to depression in bone marrow function. The result may be infections related to neutropenia, bleeding resulting from thrombocytopenia, and fatigue and dyspnea from anemia.
HISTORY AND PHYSICAL EXAMINATION
A complete history and physical examination of the individual with advanced cancer is essential for disease and symptom treatment planning.
General Assessment
▪ Identify and evaluate anorexia/cachexia, weight loss, fatigue level, functional status, pain, temperature, and reports of night sweats.
Cardiac Assessment
▪ Evaluate pulse rate and rhythm, heart sounds, and blood pressure.
Respiratory Assessment
▪ Assess respiratory rate, effort, and patient reports of dyspnea.
▪ Auscultate the lungs to identify any wheezing, rales, crackles, pleural rub, decreased breath sounds, or distant breath sounds.
▪ Percuss the chest, noting any dullness over effusions or atelectasis.
▪ Observe sputum for hemoptysis and evaluate the color and consistency for signs of infection.
▪ Observe the color of nail beds and mucous membranes for signs of cyanosis.
Skin/Mucous Membrane Assessment
▪ Identify erythema, skin breakdown, dryness or lesions of mucous membranes, bruising/petechiae, jaundice, hyperpigmentation, and signs of itching.
▪ Evaluate any tumor nodules or wounds.
Abdominal Assessment
▪ Assess bowel status, bowel patterns, and any problems with nausea or vomiting.
▪ Auscultate bowel sounds; percuss abdomen for air, fluid, or consolidation.
▪ Palpate lightly to identify presence of lymphadenopathy or enlargement or tenderness of the liver, spleen, or kidneys.
▪ Look for signs of bleeding—occult or frank bleeding of upper or lower gastrointestinal tract, melena, and “coffeeground” emesis.
Genitourinary Tract Assessment
▪ Assess urinary output and observe the concentration and color of the urine.
▪ Evaluate for hematuria and dysuria.
▪ Identify any difficulties with urinary retention.
Musculoskeletal Assessment
▪ Evaluate range of motion and muscle strength.
▪ Note any lymphedema or sign of infection.
▪ Identify the potential for pathological fracture.
Neurologic Assessment
▪ Assess motor strength and coordination.
▪ Evaluate changes in sensation and mental status.
DIAGNOSTICS
Diagnostic testing should be considered if the identification or confirmation of the underlying cause of uncomfortable symptoms influences the course of action. If, for example, superior vena cava syndrome is suspected but the patient is clearly close to death, interventions to promote comfort (e.g., corticosteroids to reduce inflammation, opioids to treat dyspnea) can be instituted without confirmation of the diagnosis with a radiographic study or computed tomography scan. Likewise, serum aspartate aminotransferase and alanine aminotransferase levels, while supplying chemical evidence of liver dysfunction, are not necessarily helpful in managing symptoms.
▪ Chest radiographic studies identify bronchial obstruction, atelectasis, pneumonia, pleural effusion, and superior vena cava syndrome.
▪ Computed tomography scans may show metastasis to brain, liver, lungs, adrenal glands, and abdomen; pleural effusion; superior vena cava syndrome; and spinal cord compression.
▪ Bone scans show sites of bone metastasis.
▪ Sputum culture and sensitivity tests assist in selecting the appropriate antiinfective interventions if pulmonary infection recurs or is persistent after empiric antibiotic treatment.
▪ Blood count and serum chemistries, including complete blood count, calcium, sodium, and glucose, aid in the detection of bone marrow deficiencies and chemical imbalances.
INTERVENTIONS PARTICULAR TO METASTATIC DISEASE
All of the symptoms addressed in Unit IV are potential complications of advanced cancer. Refer to the guidelines provided in Unit IV for management of these symptoms. In addition, the following section presents interventions specifically helpful for symptom management in advanced cancer. These interventions are to be considered in combination with the interventions in Unit IV.
Bone Metastasis
The pain associated with bone metastasis often requires a combination of opioid and nonsteroidal antiinflammatory drugs. More recent data show that bisphosphonates help decrease the risk of skeletal complications and delay their onset in patients with bone metastasis. Zolendronic acid has better efficacy in solid tumors than other bisphosphonates (Lipton, 2005). Discussion must take place as to whether the benefit warrants the cost associated with these agents and, if initiated, when it is appropriate to discontinue them. Obtaining local control is thought to be paramount to ensuring the patient’s quality of life, making surgical intervention to prevent, stabilize, or repair a pathological fracture an alternative that should be considered in some circumstances (Manabe, Kawaguchi, Matsumoto et al., 2005). Radiation therapy is also very helpful for the pain of bone metastasis. It appears that there is no difference in pain relief using high-dose single fractions versus fractionated schedules (Wu et al., 2003). Consider that high-dose single fractions are less disruptive to daily living. Systemic radiopharmaceuticals (e.g., strontium 89) may be considered if the patient has widespread bone metastasis and has a prognosis of at least 3 months. Hormonal manipulation may be helpful for bone pain in patients with breast or prostate cancer.
A less frequently used measure may include embolization of metastatic lesions. This can be done with coils, alcohol, or other agents, resulting in significant pain relief (Kato, Tsuyuki, Kikuchi et al., 2005). Palliation using hyperthermia or cryotherapy continues to be explored (Uchida, Wakabayashi, Okuyama et al., 2004).
Brain Metastasis
Brain metastases are typically treated by one of the following modalities: surgery, whole brain irradiation, stereotactic radiosurgery, chemotherapy, or symptom management. Brain metastasis occurs in approximately 25% of all patients with cancer (Kaal, Niel, & Vecht, 2005). The diagnosis of brain metastasis generally portends an ominous prognosis, with those patients who decline any treatment having an estimated survival of approximately 1 month (Lassman & DeAngelis, 2003). Symptoms associated with brain metastasis are largely dependent on the site of disease within the brain and the degree of associated edema. Headache is noted to be the presenting symptom in approximately half of the patients. Chemotherapeutic strategies to control brain metastasis are very limited because most agents are unable to cross the blood-brain barrier. Progress has been made in glioblastoma and in anecdotal cases of metastatic melanoma to the brain using temozolamide. Whole brain irradiation remains a primary treatment when multiple brain lesions exist. Research continues to identify potential chemotherapeutic agents that may serve as radiation sensitizers in this setting.
Management strategies are best identified with an evaluation of the patient’s general clinical status. Aggressive treatment may not be appropriate in patients with declining performance status, multiple brain tumors, significant neurological deficits, and radioresistant histologies such as melanoma, renal cancer, and sarcoma (Pollock, Brown, Foote et al., 2003). Corticosteroids, while having no direct effect on tumors other than lymphomas, are often used for symptom palliation. However, long-term use is not without side effects; these side effects can also prove to diminish the patient’s quality of life and include proximal myopathies, bacterial and fungal infections, mood disorders, and blood sugar elevations, to name a few. Dosing may include an initial bolus of dexamethasone 10 mg to 24 mg orally followed by 2 mg to 6 mg orally every 6 hours (Lassman & DeAngelis, 2003).
The prophylactic use of anticonvulsant medications in patients with brain metastasis is no longer thought to be of benefit but is appropriate for patients with a history of seizures. Use of an anticonvulsant agent such as levetiracetan (Keppra) that is not metabolized via the P450 enzyme system is preferred (Barker, 2005).
In the event of a sudden increase in intracranial pressure with risk of herniation—and if reversal of this condition is in the patient’s interest—consider admission to an intensive care unit for assisted ventilation, administration of hyperosmolar agents (mannitol), and administration of high-dose intravenous corticosteroids. These intensive measures are rarely appropriate in the palliative care setting because reversal of the disease process is not possible.
Liver Metastasis
The liver is one of the most common sites of metastatic disease (Adam, 2002). A celiac plexus block should be considered for the pain associated with liver metastasis if it persists despite maximal pharmacological management. If the underlying disease process is small-cell lung cancer, chemotherapy may be appropriate after careful evaluation. Before initiating chemotherapy, the individual’s previous response to chemotherapy, length of time from last treatment to disease progression, and functional status must be considered.
Corticosteroids are sometimes helpful for anorexia and malaise associated with liver metastasis, and cholestyramine is helpful for pruritus. Short fractionation radiotherapy may provide palliation (10 Gy in two fractions over 2 days) (Bydder, Spry, Christie et al., 2003). A role may exist for the insertion of stents such as self-expanding metallic stents inserted endoscopically in patients with obstructions of the gastrointestinal and biliary tract (Holt, Patel, & Ahmed, 2004). Less data are available regarding the evidence for the use of radiofrequency ablation, cryotherapy, chemoembolization, and octreotide administration, although each may have a role in specific circumstances (Lau, Lo, & Tan, 2003; Parikh, Curley, Fornage et al., 2002; Patel & Jindal, 2001; Sotsky & Ravikumar, 2002; Valiozis, Zekry, Williams et al., 2000). Treat encephalopathy due to liver failure as a terminal event; do not institute unpleasant therapies (e.g., magnesium sulfate enemas and lactulose).
Pleural Effusions
Malignant pleural effusions are common in a number of malignancies and may be seen in any malignancy that involves metastasis to the lungs. Thoracentesis provides relief of the dyspnea and discomfort associated with pleural effusions. However, malignant effusions will almost always recur within a relatively short time frame. Large-bore chest tube placement requires hospitalization and can involve a significant amount of discomfort for patients, making it a less desirable choice in the palliative setting. Sclerotherapy to produce pleurodesis is recommended to decrease the incidence of recurrence. Doxycycline is the agent of choice for this, because it is relatively inexpensive and has reasonably good efficacy (Covey, 2005). More recently, a variety of small-bore tunneled or pigtail catheters have been used as a way to drain pleural fluid and relieve patient symptoms. These tubes are inserted using radiographic guidance in the outpatient setting, and patients can be sent home with the catheter in place. The patient and family are instructed to connect the pleural catheter to a vacuum drainage system at prescribed intervals and as needed. If drainage decreases significantly or stops altogether, the tube may be removed. In many cases, it appears that mechanical pleurodesis occurs (Musani, Haas, Seijo et al., 2004; Putman, Light, Rodriguez et al., 1999; Putman, Walsh, Swisher et al., 2000; Pollak, 2002). A more aggressive procedure involves the use of video-assisted thoracoscopic surgery (VATS). This technique is useful in draining loculated fluid collections but typically involves general anesthesia and a hospital stay of several days (Brega-Massone, Conti, Magnani et al., 2004).
Compression and Obstruction Due to Tumor or Lymphadenopathy
Excessive tumor growth can result in obstruction of airways; obstruction of urinary, gastrointestinal, and biliary passages; as well as compression of lymph and blood vessels. The associated symptoms depend on the site of obstruction.
Obstruction of the urinary system may require the use of stents, urinary catheters, or suprapubic catheters to maintain adequate flow. A surgical procedure to debulk the tumor or bypass the obstruction is an option if the patient is a good surgical candidate. Airway obstruction can be more difficult to manage and is certainly more anxiety producing for patients and families. Endobronchial surgical techniques, radiotherapy, chemotherapy, or embolization procedures should be considered for symptom palliation along with the use of oxygen or humidified air.
Venous compression, as seen in superior vena cava syndrome, may be initially managed with corticosteroids to decrease inflammation and edema. This can be followed by radiation therapy, raising the head of the bed, oxygen therapy, and diuretics (Jacobs, 2003). Measures to control lymphedema will be based on the patient’s activity level and prognosis.
CONCLUSION
The care of the patient with advanced cancer and his or her family requires a combination of in-depth knowledge of the underlying disease process and probable symptom progression as well as expertise in symptom management. As with all patients at the end of life, patients with cancer and their families have often experienced years of tiresome and perhaps ineffective treatments, uncomfortable symptoms (many of which are not adequately controlled), and an increasing burden on caregivers. Oftentimes patients and families have lost faith in the health care system. In addition to expertise related to the disease process and progression, the clinician needs to establish and maintain a relationship based on mutual trust and respect.
REFERENCES
Adam, A., Interventional radiology in the treatment of hepatic metastases, Cancer Treat Rev 28 (2) ( 2002) 93–99.
American Cancer Society (ACS), Cancer facts and figures 2006. ( 2006)Author, Atlanta.
Arlen, P.M.; Gulley, J.L., Therapeutic vaccines for prostate cancer: A review of clinical data, Curr Opin Investig Drugs 6 (6) ( 2005) 592–596.
Barker, F.G., Surgical and radiosurgical management of brain metastases, Surg Clin North Am 85 (2) ( 2005) 329–345.
Berruti, A.; Sperone, P.; Bottini, A.; et al., Phase II study of vinorelbine with protracted fluorouracil infusion as a second-or third-line approach for advanced breast cancer patients previously treated with anthracycline, J Clin Oncol 18 (2000) 3370–3377.
Brega-Massone, P.P.; Conti, B.; Magnani, B.; et al., Minimally invasive thoracic surgery for diagnostic assessment and palliative treatment in recurrent neoplastic pleural effusion, Thorac Cardiovasc Surg 52 (4) ( 2004) 191–195.
Burris III, H.A.; Moore, M.J.; Andersen, J.; et al., Improvements in survival and clinical benefits with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial, J Clin Oncol 15 (1997) 2403–2413.
Bydder, S.; Spry, N.A.; Christie, D.R.; et al., A prospective trial of short-fractionation radiotherapy for the palliation of liver metastases, Australas Radiol 47 (3) ( 2003) 284–288.
Calvo, K.R.; Petricoin, E.F.; Liotta, L.A., Genomics and proteomics, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 51–72.
Carrick, S.; Ghersi, D.; Wilcken, N.; et al., Platinum containing regimens for metastatic breast cancer, Cochrane Database Syst Rev ( 3) ( 2005); CD003374..
Covey, A.M., Management of malignant pleural effusions and ascites, J Support Oncol 3 (2) ( 2005) 169–173.
El-Rayes, B.F.; Philip, P.A., A review of systemic therapy for advanced pancreatic cancer, Clin Adv Hematol Oncol 1 (7) ( 2003) 430–434.
Finlay, I.G.; Mason, M.D.; Shelley, M., Radioisotopes for the palliation of metastatic bone pain: A systematic review, Lancet Oncol 6 (6) ( 2005) 392–400.
Fisher, R.I.; Mauch, P.M.; Harris, N.L.; et al., Non-Hodgkin’s lymphomas, In: (Editors: DeVita, V.T.; Hellman, S.; et al.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 1957–1997.
Ghersi, D.; Wilcken, N.; Simes, J., Taxane containing regimens for metastatic breast cancer, Cochrane Database Syst Rev ( 2) ( 2005); CD003366..
Glare, P., Clinical predictors of survival in advanced cancer, J Support Oncol 3 (5) ( 2005) 331–339.
Holt, A.P.; Patel, M.; Ahmed, M.M., Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: The treatment of choice?Gastrointest Endosc 60 (6) ( 2004) 1010–1017.
Jacobs, L.G., Managing respiratory symptoms at the end of life, Clin Geriatr Med 19 (1) ( 2003) 225–239.
Kaal, E.C.; Niel, C.G.; Vecht, C.J., Therapeutic management of brain metastasis, Lancet Neurol 4 (5) ( 2005) 289–298.
Kato, Y.; Tsuyuki, A.; Kikuchi, K.; et al., Dramatic relief of pain by transcatheter arterial embolization for bone metastasis from hepatocellular carcinoma, J Gastroenterol Hepatol 20 (2) ( 2005) 326–327.
Lamont, E.B.; Christakis, N.A., Complexities in prognostication in advanced cancer, JAMA 290 (1) ( 2003) 98–104.
Lassman, A.B.; DeAngelis, L.M., Brain metastases, Neurol Clin 21 (1) ( 2003) 1–23.
Lau, T.N.; Lo, R.H.; Tan, B.S., Colorectal hepatic metastases: Role of radiofrequency ablation, Ann Acad Med, Singapore 32 (2) ( 2003) 212–218.
Libuiti, S.K.; Saltz, L.B.; Rustgi, A.K.; et al., Cancer of the colon, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 1061–1109.
Lipton, A., Management of bone metastases in breast cancer, Curr Treat Opt Oncol 6 (2) ( 2005) 161–171.
Lunney, J.R.; Lynn, J.; Foley, D.J.; et al., Patterns of functional decline at the end of life, JAMA 289 (18) ( 2003) 2387–2392.
Manabe, J.; Kawaguchi, N.; Matsumoto, S.; et al., Surgical treatment of bone metastasis: Indications and outcomes, Int J Clin Oncol 10 (2) ( 2005) 103–111.
Murren, J.R.; Turrisi, A.T.; Pass, H.I., Small cell lung cancer, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 810–843.
Musani, A.I.; Haas, A.R.; Seijo, L.; et al., Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters, Respiration 71 (6) ( 2004) 559–566.
Nagourney, R.A.; Link, J.S.; Blitzer, J.B.; et al., Gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed breast cancer patients, J Clin Oncol 18 (2000) 2245–2249.
National Hospice and Palliative Care Organization (NHPCO), Hospice facts and figures, Retrieved February 20, 2006, from www.nhpco.org/files/public/Facts_Figures_for2004data.pdf ( 2006).
Oosterhof, G.O.; Roberts, J.T.; de Reijke, T.M.; et al., Strontium(89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: A phase III study of the European Organisation for Research and Treatment of Cancer, Genitourinary Group, Eur Urol 44 (5) ( 2003) 519–526.
Parikh, A.A.; Curley, S.A.; Fornage, B.D.; et al., Radiofrequency ablation of hepatic metastases, Semin Oncol 29 (2) ( 2002) 168–182.
Patel, N.H.; Jindal, R.M., The role of chemoembolization in the treatment of colorectal hepatic metastases, Hepatogastroenterology 48 (38) ( 2001) 448–452.
Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; et al., Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer, N Engl J Med 351 (15) ( 2004) 1513–1520.
Pollak, J.S., Malignant pleural effusions: Treatment with tunneled long-term drainage catheters, Curr Opin Pulm Med 8 (4) ( 2002) 302–307.
Pollock, B.E.; Brown, P.D.; Foote, R.L.; et al., Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease, J Neuro-oncol 61 (1) ( 2003) 73–80.
Putman, J.B.; Light, R.W.; Rodriguez, R.M.; et al., A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions, Cancer 86 (10) ( 1999) 1992–1999.
Putman, J.B.; Walsh, G.L.; Swisher, S.G.; et al., Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter, Ann Thorac Surg 69 (2) ( 2000) 369–375.
Reni, M.; Cordio, S.; Milandri, C.; et al., Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: A randomized controlled multicentre phase III trial, Lancet Oncol 6 (6) ( 2005) 369–376.
Scher, H.I.; Leibel, S.A.; Fuks, Z.; et al., Cancer of the prostate, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 1192–1259.
Schmitt, B.; Bennett, C.; Seidenfeld, J.; et al., Maximal androgen blockade for advanced prostate cancer, Cochrane Database Syst Rev ( 2) ( 2000); CD001526..
Schrump, D.S.; Altorki, N.K.; Henschke, C.L.; et al., Non-small cell lung cancer, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 753–810.
Smith, W.; Khuri, F.R., The care of the lung cancer patient in the 21st century: A new age, Semin Oncol 31 (2 Suppl. 4) ( 2004) 11–15.
Sotsky, T.K.; Ravikumar, T.S., Cryotherapy in the treatment of liver metastases from colorectal cancer, Semin Oncol 29 (2) ( 2002) 183–191.
Stetler-Stevenson, W.G., Invasion and metastases, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 113–127.
Tannock, I.F.; de Wit, R.; Berry, W.R.; et al.TAX 327 Investigators, Docetaxel plus prednisone or mitoxantrone plus prednisone for advance prostate cancer, N Engl J Med 351 (15) ( 2004) 1502–1512.
Uchida, A.; Wakabayashi, H.; Okuyama, N.; et al., Metastatic bone disease: Pathogenesis and new strategies for treatment, J Orthop Sci 9 (4) ( 2004) 415–420.
Valiozis, I.; Zekry, A.; Williams, S.J.; et al., Palliation of hilar biliary obstruction from colorectal metastases by endoscopic stent insertion, Gastrointest Endosc 51 (4 Pt 1) ( 2000) 412–417.
Vieweg, J.; Dannull, J., Technology insight: vaccine therapy for prostate cancer, Nature Clin Pract Urol 2 (1) ( 2005) 44–51.
Wilcken, N.; Hornbuckle, J.; Ghersi, D., Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer, Cochrane Database Syst Rev ( 2) ( 2003); CD002747..
Wilkes, G.M., Therapeutic options in the management of colon cancer: 2005 Update, Clin J Oncol Nurs 9 (1) ( 2005) 31–43.
Wong, K.; Depinho, R.A., Telomerase, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 105–112.
Wood, W.C.; Muss, H.B.; Solin, L.J.; et al., Malignant tumors of the breast, In: (Editors: De Vita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 1415–1477.
Wu, J.S.; Wong, R.; Johnston, M.; et al.Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group, Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases, Int J Radi Oncol Biol Physics 55 (3) ( 2003) 594–605.
Yeo, C.J.; Yeo, R.P.; Hruban, R.H.; et al., Cancer of the pancreas, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 945–986.
Yuspa, S.H.; Shields, P.G., Etiology of cancer: Chemical factors, In: (Editors: DeVita, V.T.; Hellman, S.; Rosenberg, S.A.) Cancer: Principles and practice of oncology7th ed. ( 2005)Lippincott Williams & Wilkins, Philadelphia, pp. 185–191.