CHAPTER 138
Neurogenic Bowel
Meena Agarwal, MD, PhD, MS, Dip Urol, FRCS, FRCS(Urol)
Definition
Within the nervous system, there are peripheral, somatic, and autonomic (sympathetic and parasympathetic) contributions to the various organ-based functions. Related to the gastrointestinal system, several end-organ problems resulting from neurologic dysfunction include prolonged colonic transit time, reduced anorectal sensibility, and lack of voluntary control of the external anal and urethral sphincters, often associated with a dyssynergic response. The severity of colorectal dysfunction depends on the degree of completeness and level of the spinal cord injury [1,2]. However, specific evaluation is required in individual cases. These problems have an extensive impact on quality of life. Our study based on interviews of 125 consecutive male patients and 2 female patients with spinal cord injury showed that 27% of patients had chronic disabling gastrointestinal problems requiring alteration of their lifestyle; symptoms usually appeared 5 to 10 years after injury (P < .05) [3]. Severity of bowel dysfunction correlated with high level of lesion, completeness of cord injury, and longer duration of injury (≥ 10 years) [4,5].
Bowel Innervations and Gastrointestinal Motility
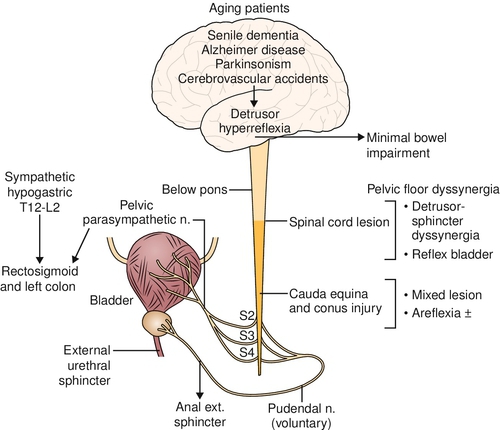
Unlike in the bladder, small and large bowel movements are mainly autonomous and may be influenced with some spinal cord lesions. The vagus nerve, which arises intracranially and provides parasympathetic innervations from the esophagus to the splenic flexure of the colon, is spared in spinal cord lesions (Fig. 138.1). The pelvic nerve carries parasympathetic fibers from S2-S4 to the descending colon and rectum. Some pelvic nerve branches travel proximally and innervate the transverse and ascending colon [6]. Sympathetic innervations are supplied by the superior and inferior mesenteric (T9-T12) and hypogastric (T12-L2) nerves. The somatic pudendal nerve (S2-S4) innervates the pelvic floor.
The intrinsic nervous system of the gastrointestinal tract, which includes Auerbach plexus, is situated in the colonic wall between the longitudinal and circular muscle layers. This nerve supply helps coordinate colonic wall movement and the advancement of stool through the colon. The behavior of the bowel can be controlled by the intrinsic innervations of the gut independently of input from the central nervous system.
The extrinsic nervous system also innervates the colon and includes the parasympathetic, sympathetic, and somatic nerves [4,5]. Peristaltic waves travel both toward and away from the ileocecal valve in the ascending colon; but in the descending colon, the waves travel mainly to push the contents to the anus [4]. The motility of the colon is performed by three primary mechanisms: myogenic, chemical, and neurogenic. The myogenic transmission of signals occurs between enteric smooth muscle cells that are interconnected by gap junctions, which produces transmission from cell to cell. Most intestinal muscle displays autorhythmicity that causes colonic wall contractions [4].
Chemical control is through the activity of neurotransmitters and hormones. The chemicals influence the promotion or inhibition of contractions through the action of the central nervous system or autonomic nervous system or by direct action on muscle cells. This activity can be triggered by luminal stimuli that are detected by nerves through epithelial intermediation. Epithelial enterochromaffin cells act as sensory transducers that activate the mucosal processes of both intrinsic and extrinsic primary afferent neurons through their release of 5-hydroxytryptamine (5-HT). Intrinsic primary afferent neurons are present in both the submucosal and myenteric plexuses. Peristaltic and secretory reflexes are initiated by submucosal intrinsic primary afferent neurons, which are stimulated by 5-HT acting at 5-HT1P receptors. Serotonergic transmission within the enteric nervous system and the activation of myenteric intrinsic primary afferent neurons are 5-HT3 mediated [6]. Signaling to the central nervous system is also predominantly 5-HT3 mediated. The gut is thus the only organ that can display reflexes and integrate neuronal activity even when it is isolated from the central nervous system.
The neurogenic mechanism of colonic control is through the enteric nervous system, which coordinates all segmental motility and some propagated movement. The number of intrinsic neurons in the gut greatly exceeds the number of fibers in the vagus and splanchnic nerves. In humans, the enteric nervous system contains up to 100 million neurons, compared with only 2000 efferent fibers in the vagus nerve, suggesting that intrinsic nerves may direct most reflex and control activities and that the extrinsic innervations may serve only a modulatory function [7,8].
Normal defecation is the result of a complex interaction between muscles, nerves, and central nervous system. For a normal defecation, there needs to be a mass movement of colonic contents associated with relaxation of internal and external anal sphincters. Mass movement of colonic contents before defecation associated with internal sphincter relaxation has recently been shown to result from high-amplitude propagating contractions in children even with sigmoid dysmotility [9]. The colon absorbs fluids, electrolytes, and short-chain fatty acids; provides for growth of symbiotic bacteria; secretes mucus for lubrication of feces; and slowly propels stool toward the anus [10]. The contents in the distal colon are retained until bowel evacuation. Transport of contents may take 12 to 30 hours from the ileocecal valve to the rectum [2].
Neurogenic Bowel
A neurogenic bowel occurs when there is a dysfunction of the colon or rectosigmoid due to the lack of nervous control [11–13]. The enteric nervous system remains intact after spinal cord injury. However, depending on the level of the injury, different bowel problems and complications may arise. The lower motor neuron bowel syndrome or areflexic bowel results from a lesion affecting the parasympathetic cell bodies in the conus medullaris, cauda equina lesions, or damage to the pelvic nerves. No spinal cord–mediated peristalsis occurs, and there is slow stool propulsion. Only the myenteric plexus coordinates segmental colonic peristalsis, and a dryer, rounder stool shape occurs. Because of the denervated external anal sphincter, there is increased risk for incontinence. The levator ani muscles lack tone, and this reduces the rectal angle and causes the lumen of the rectum to open. The lower motor neuron bowel syndrome produces constipation and a significant risk of incontinence due to the lax external anal sphincter. A lesion above the conus medullaris causes an upper motor neuron bladder and bowel syndrome or hyperreflexic bladder and bowel. There is increased colonic wall and anal tone. The voluntary control of the external anal sphincter is lacking, and the sphincter remains tight, thereby retaining stool. The nerve connections between the spinal cord and the colon, however, remain intact; therefore, there is reflex coordination and stool propulsion. The upper motor neuron bowel syndrome with supraconal lesions in the spinal cord produces constipation and fecal retention at least in part owing to the hyperactivity of the external anal sphincter.
Pathophysiology of Constipation in Neurologically Impaired Patients
In neuropathic bowel, constipation is usually a major consequence [11–13]. The pathophysiologic mechanisms of constipation are obstructed defecation, weak abdominal muscles, impaired rectal sensation, and delayed colonic transit time. Both incomplete and complete lesions can have an obstructed defecation or fecal incontinence [14]. The mechanism for fecal incontinence is due to areflexic or atonic anal sphincter, uninhibited rectal contractions, poor rectal sensibility, and lack of anal sphincter tone and contraction (conus and cauda equina lesions).
During attempts to defecate, in some able-bodied persons with chronic constipation, there is also an inappropriate contraction (or failed relaxation) of the puborectalis and of the external anal sphincter muscles. This paradoxical contraction of the pelvic floor musculature during straining at defecation is also called pelvic floor dysfunction [14,15] or pelvic floor dyssynergic response. This is not a true dyssynergia because it can be relaxed with volitional control. The diagnostic criteria were elucidated in the Rome II report and include those for functional constipation plus at least two of three investigations among manometry, electromyography, and defecography showing inappropriate contraction of or failure to relax the pelvic floor muscles [14,15]. Patients can learn to relax the pelvic floor musculature with biofeedback to manage functional obstructed defecation. This dyssynergic response, therefore, needs to be distinguished from true detrusor anal sphincter dyssynergia due to neurologic impairment, in which biofeedback may not have any role for the functional improvement.
Symptoms
There is a high prevalence and wide spectrum of gastrointestinal symptoms after spinal cord injury. Abdominal bloating and constipation are usually related to specific spinal cord levels of injury [16]. The limited manner through which spinal cord–injured patients can manifest symptoms resulted in complaints that were characteristically vague [3]. The most common problems that impaired quality of life were poorly localized abdominal pain (14%) and difficulty with bowel evacuation (20%), hemorrhoids (74%), abdominal distention (43%), and autonomic dysreflexia. Twenty-three percent of our population required at least one admission to the hospital for a gastrointestinal complaint after their injury. The prevalence of chronic gastrointestinal symptoms increased with time after injury [3].
Physical Examination
For the rehabilitation of neurogenic bowel, an individual evaluation [17,18] is important with a careful rectal examination and anorectal neurologic testing to document degree of neurologic impairment. A neurologic examination can reveal the extent of the nerve damage and the completeness of the spinal cord injury. The abdomen should be inspected and palpated for distention, palpable fecal masses, increased abdominal muscle tone indicative of spasticity, and bowel sounds. The rectal examination can provide information about external anal sphincter tone, stool in the rectal vault, presence of hemorrhoids, cystocele in women, or masses, and it assesses the tone and ability to produce voluntary contraction of the puborectalis muscles.
The bulbocavernosus reflex assesses the integrity of the local spinal reflex arc; its absence along with poor anal tone indicates a conus or cauda equina lesion (lower motor neuron). It is also important to assess the patient’s strength in the upper and lower extremities, hand function, sitting balance, and ability to transfer; the length of the patient’s arms, legs, and trunk; and the patient’s weight. These factors are helpful to determine whether the patient can perform his or her own bowel program or whether assistance will be needed. Berkowitz and colleagues [19] found that 37% of all patients with spinal cord injury need assistance with bowel care. People with tetraplegia are more likely to need assistance than are people with paraplegia.
Functional Limitations
There is some degree of loss of voluntary control for bowel evacuation, constipation, unpredicted incontinence, abdominal distention, and associated discomfort, depending on the degree and level of completeness of the neurologic lesion.
Diagnostic Studies
Colonic and anorectal dysfunctions are recognized as the principal pathophysiologic mechanism underpinning chronic constipation and particularly obstructed constipation in neurologically impaired patients [20].
Colonic motor activity comprises four main components: myoelectric activity [21], phasic contractile activity, tonic contractile activity, and intraluminal transit. Specific methods are available for the assessment of each separate component, but no single investigation gives information about all four types of activity. In current clinical practice, evaluation of colonic motor function is almost exclusively limited to assessment of intraluminal pressure and transit time [22,23]. Although the direct assessment of colonic contractile activity can be achieved through colonic manometry, this procedure is only slowly gaining clinical acceptance, notably in pediatrics. Other novel methods are also available; two techniques exist for the routine assessment of colonic (or whole gut) transit, both of which involve irradiation of the subjects: radiopaque markers [24] and radionuclide scintigraphy [25]. Wireless (telemetric) motility capsules [26] with magnetic markers to obviate irradiation are currently being tried, but they need further validation before being incorporated into general clinical practice. Together with assessment of rectal evacuation and rectal sensation, studies of colonic transit should form the cornerstone of investigation of chronic idiopathic constipation in patients with functional or partial neurologic impairment. These investigations have led to the conceptualization of constipation in three broad and overlapping categories: normal-transit constipation, slow-transit constipation, and evacuation disorders.
Anorectal Dyssynergia
For the precise diagnosis of anorectal dyssynergia, particularly in incomplete or functional lesions, anorectal manometry along with simultaneous electromyography of the external anal sphincter is important to distinguish between functional constipation [15,16] and obstructed constipation due to a neurologic lesion. It is also important to evaluate impairment due to an incomplete lesion (e.g., multiple sclerosis, pudendal nerve lesion after childbirth, lumber disc disease, back injury, or spinal tumor). Defecography, nerve stimulation and pudendal latency, ultrasonography, and magnetic resonance imaging may also be required for better understanding of gastrointestinal dysfunction. A rectal examination should be performed to look for a rectocele, voluntary anal contraction, and bulbocavernosus reflex to evaluate sacral nerve root lesions. Defecography detects structural abnormalities and assesses functional information on the movement of the pelvic floor and the organs that it supports; conversely, excessive descent (descending perineum syndrome) can also be a pathophysiologic mechanism of constipation. Defecography can also help complement anorectal manometry studies in ruling out slow transit and other causes of constipation. Magnetic resonance imaging or pelvic floor sonography can further complement the studies. This will help reduce morbidity rates and will improve the quality of life for the neurologically impaired patient.
Treatment
Initial
Unless there is an associated acute abdomen, the small bowel and the bulk of the colon are functional and are not paralyzed. Management of bowel evacuation will also depend on level of injury. In cauda equina lesions and also during the shock phase after spinal cord injury, there is a flaccid bowel; the management generally involves manual removal (disimpaction) of stool and use of a suppository. Digital stimulation may also be helpful with intact bulbocavernosus reflex. In patients with a supraconal lesion with spastic bowel, routine use of stool softeners, suppository insertion, and digital stimulation help evacuation of the fecal matter. Digital stimulation with and without suppository for about 20 to 30 minutes usually evokes bowel evacuation. Glycerin or Microlax suppository has been commonly used.
Rehabilitation
Bowel Management after Spinal Cord Injury
In the rehabilitation of the spinal cord–injured patient, adequate bowel evacuation in less than 60 minutes is an ideal goal; however, a large number of patients may require up to 180 minutes. It is therefore important to individualize the bowel program for adequate evacuation on the basis of the neurologic and physical status with a set time, diet control, and digital stimulation with or without a glycerin suppository [11]. Additional help to regulate the bowel with bulking agents by increasing water content (e.g., Metamucil) or stool softeners by increasing water penetration of stool (e.g., Coloxyl) has been useful in children. Iso-osmotic laxative (e.g., Movicol) and osmotic laxative (e.g., lactulose) are also widely used to help individualize the bowel program.
The addition of bisacodyl to aid myoelectric propagation activity, transit in the ascending colon, and rectal tone in humans has been reported. Internal anal sphincter relaxation associated with bisacodyl-induced colonic high-amplitude propagating contractions in children with constipation has also been reported: a coloanal reflex [27,28]. Bisacodyl has been widely prescribed for the management of neurogenic bowel [29]. The dosage is normally 5 or 10 mg, but up to 30 mg can be taken for complete cleansing of the bowel before a procedure. If it is taken at the maximum dosage, there will likely be a sudden, extremely powerful, uncontrollable bowel movement, and so precautions should be taken. When it is administered rectally in suppository form, it is usually effective in 15 to 60 minutes. Two suppositories can be inserted at once if a very strong, purgative, enema-like result is needed. A few hours after the initial evacuation, there can be a secondary action that will continue as long as there is unexpelled bisacodyl present in the rectum.
For design of the bowel program, a variety of factors need to be considered. Is this an upper motor neuron or lower motor neuron bowel dysfunction? Is it a complete or an incomplete lesion? Is this associated with anorectal dyssynergia? A detailed history is needed to find out any bowel problems antedating spinal cord injury, such as diabetes, irritable bowel syndrome, lactose intolerance, inflammatory bowel disease, or past rectal bleeding. These disorders may affect the management and choice of medications used in the bowel regimen. Other medications frequently used by patients with spinal cord injury for other problems, such as anticholinergics for treatment of neurogenic bladder, antidepressants, narcotics, and antispasticity medications, also affect the bowel.
In addition, the person’s dietary habits and the amount of fluid intake need to be documented as part of bowel management. It is helpful to evaluate psychosocial and family circumstances to provide guidelines to modify convenient timing for the bowel program and to develop rehabilitation strategies through diet, pelvic floor exercises, and biofeedback (in partial lesions).
Patient Education and Awareness of Risk Factors
It is critical to develop a comprehensive, individualized, and structured education program for prevention of incontinence, bowel accidents, and skin breakdown during sitting on a toilet seat. It is also important to identify other risk factors for negative outcomes: colonic overdistention, irritable bowel syndrome, bladder dysfunction, or autonomic dysreflexia in high spinal cord lesions. Equipment essential during bowel care also has been studied, although new technology in bowel chair design and manufacture has been slow to evolve [30,31]. There are flaws in commode-shower chair design, as reported by consumers, which increase the risk of falls during transfers and risk of pressure ulcers due to inadequate padding as well as the long duration of the bowel care process.
The current and most frequently used neurogenic bowel management strategies in some persons with only digital stimulation or a suppository insertion may be associated with incomplete evacuation, some incontinence, increased risk of pressure-induced tissue damage resulting from longer duration of commode sitting, and more damage to the mucosal tissue than with other methods available to persons with spinal cord injury. The use of the docusate mini enema may be an option in neurogenic bowel management because it has been shown to reduce the occurrence of bowel incontinence; it reduces the duration of commode sitting and thus reduces the risk of pressure ulcers, and it does not cause inflammation or seepage of the mucosal lining of the lower bowel [32]. All of these may improve quality of life and social and community integration of persons with spinal cord injury.
Use of Prokinetic Drugs
When conservative management is not effective, prokinetic agents, such as cisapride, prucalopride, metoclopramide, neostigmine, and fampridine, have been used and are supported by strong evidence for the treatment of chronic constipation in spinal cord–injured patients. They need to be used carefully for their side effects. Serious cardiac arrhythmias including ventricular tachycardia, fibrillation, and QT prolongation have been reported in patients taking cisapride. Cisapride has therefore been removed from the U.S. market [33–35]. The gastroprokinetic effects make metoclopramide useful in the treatment of gastric stasis and in gastroesophageal reflux disease. Because of the risk of tardive dyskinesia with chronic or high-dose use of the drug, the U.S. Food and Drug Administration recommends that metoclopramide be used for short-term treatment, preferably less than 12 weeks [35]; in 2009, it required all manufacturers of metoclopramide to issue a black box warning [35].
Procedures
Botulinum Toxin in Gastrointestinal Disorders
Botulinum neurotoxin inhibits contraction of gastrointestinal smooth muscles and sphincters; it has also been shown that the neurotoxin blocks cholinergic nerve endings in the autonomic nervous system, but it seems not to block noradrenergic responses mediated by nitric oxide. This has attracted use of botulinum neurotoxin for overactive smooth muscles, such as the anal sphincters for treatment of anal fissure and the lower esophageal sphincter for treatment of esophageal achalasia [36]. It is critical to appreciate the anatomic and functional organization of the denervation of the gastrointestinal tract for neuropathic bowel dysfunctions, particularly in patients with long-term constipation and incomplete bowel evacuation for whom a bowel rehabilitation program has failed. An early resolution of obstruction may be with botulinum neurotoxin to control the pelvic floor dyssynergic response. It might thus prevent back pressure effects on the rectosigmoid and colon in a neuropathic bowel.
Despite uncontrolled data, botulinum toxin is now being used for a variety of spastic disorders of gastrointestinal smooth muscle. Its usefulness has been exploited in anismus patients with pelvic floor dyssynergia. This is one of the common causes of constipation, in which the pelvic floor muscles contract too much or do not relax enough during a bowel movement. In one study [37], either 100 units of botulinum toxin (Botox; Allergan, Irvine, Calif) or 500 units (equivalent dose) of Dysport (Ipsen Ltd; Slough, United Kingdom) was diluted in 2 mL of normal saline, and a 21-gauge needle was passed cranially through the external sphincter to the level of the puborectalis muscle. The needle was then gradually withdrawn, injecting small amounts of mixture along the length of the puborectalis–external sphincter muscle; 1 mL was given bilaterally at the 3- and 9-o’clock positions. In these patients, initially 39% (21 patients) benefited, but after the second injection, 95% had resolution of symptoms. At a median follow-up of 19.2 (7.0-30.4) months, 20 (95%) of 21 patients had a sustained response and required no further treatment [37]. One unit of Botox is equivalent to about 3 units of Dysport. Most others have used only about 50 mg of Botox for anorectal injections.
So far, few placebo-controlled trials have been performed despite widespread use of the toxin for the past 10 years. Botulinum toxin appears to be safe, and side effects are uncommon. The short-term efficacy of intrasphincteric injection of botulinum neurotoxin in achalasia is now well established. The U.S. Food and Drug Administration has not approved Botox for any of these conditions.
Surgery
In patients with severe bowel dysfunction and markedly prolonged colon transit time [24], we evaluated indications for transverse colostomy [38]. In a follow-up study [39], the effect on quality of life of this procedure was evaluated with 100% satisfaction and reduction of bowel care from 117 minutes to 12.8 minutes per day (P < .00001). Indications for and usefulness of intestinal diversion have also been reported with positive results in patients with severe bowel dysfunctions after spinal cord injury [40]. In an another study [41], the difference in the long-term outcome among left-sided colostomies, right-sided colostomies, and ileostomies was evaluated; The average daily time to bowel care was significantly shortened in all groups (right-sided colostomies, 102 to 11 minutes, P < .05; left-sided colostomies, 123 to 18 minutes, P < .05; and ileostomies, 73 to 13 minutes, P < .05). The successful outcome noted in all groups suggests that preoperative symptoms and colonic transit time studies may have been helpful in optimal choice of stoma site selection. However, the choice of colostomy in the descending colon was considered better because of the lesser fluid content in the fecal matter (more solid fecal matter) and the easy management of the collecting devices. In a systematic review of electronic databases (MEDLINE and CINAHL) from January 1960 to November 2007, colostomy in selected patients provided equivocal or superior quality of life outcomes compared with conservative bowel management strategies [11].
Surgical interventions, such as colostomy, Malone antegrade continence enema, and implanted stimulators [1,42], are not routinely used, although all are supported by lower levels of evidence (pre–post studies) in reducing bowel-related complications and improving quality of life. Overall, more intervention trials are needed to assess management programs for neurogenic bowel among individuals with spinal cord injury, especially trials involving multiple centers [1]. The use of common and validated scoring systems, such as the Neurogenic Bowel Dysfunction score and those found in the International Bowel Function data sets [43,44], will be helpful if they are implemented so that comparison of results and meta-analyses may be conducted to further our knowledge on the treatment and management of neurogenic bowel after spinal cord injury.
Potential Disease Complications
In spinal cord lesions above T6 level, one of the serious complications is autonomic dysreflexia. It usually accompanies poor bladder drainage and impacted fecal matter in the rectum. It needs immediate attention with gentle bowel evacuation after lidocaine jelly (4%) insertion in the rectum and use of alpha blockers to control blood pressure [11].
In a slow-transit bowel, marked abdominal distention with chronic constipation and dilated colon further aggravates bowel evacuation. A barium contrast enema will delineate an obstructing lesion, if it is present, or may reveal a huge colon with redundant bowel. Although this finding will not delineate the specific cause, it will indicate the magnitude of the anatomic abnormality. If the impaction is located more proximally in the bowel, oral stimulants, such as magnesium citrate solution or bisacodyl tablets, may be required. Caution in the use of oral medication is needed if a bowel obstruction is suspected. Intestinal perforation could result. In addition, oil retention enemas may be helpful in combination with oral agents to loosen the stool. The decision to proceed with colonoscopy depends on the individual’s clinical history and findings as well as on whether the physician is satisfied with the results of the contrast enema. To clear the contrast material and to prevent constipation, oral laxatives and frequent bowel care should be used for a few days after studies that require barium [11].
Fecal incontinence can lead to overgrowth of microorganisms around the anus, which weakens the skin, and skin sores can develop. Also, sitting on an unpadded bowel care seat for a long time without frequent pressure relief could result in skin sores.
Hemorrhoids occur frequently [3] and may become more symptomatic as they increase in size; they may be exacerbated by physical interventions, such as suppositories, enemas, or digital stimulation, to regulate the bowels in individuals with spinal cord injury. When hemorrhoids become clinically significant, they may cause pain (if sensation is present), bleeding, mucus incontinence secondary to prolapsed mucosa, or symptoms of autonomic dysreflexia. Persistent bleeding and autonomic dysreflexia that are not responsive to changes in bowel care routine are indications for consideration of banding [45] or hemorrhoidectomy.
Potential Treatment Complications
In 27% of spinal cord–injured patients, chronic gastrointestinal problems appeared usually 5 to 10 years after the initial injury. This seems to be related mostly to anorectal dyssynergia with resultant obstructed constipation and incomplete evacuation with the back pressure effect on the colon [3], suggesting that these problems are acquired and may therefore be avoided by the adoption of certain chronic care routines to manage obstructed constipation with anal stretch, high-fiber diet, and adequate fluid intake. Chronic use of stimulant laxatives can lead to damage of the myenteric plexus with aggravated colonic dysmotility.