CHAPTER 137
Neurogenic Bladder
Ayal M. Kaynan, MD, FACS; Meena Agarwal, MD, PhD, MS, Dip Urol, FRCS, FRCS(Urol); Inder Perkash, MD, FRCS, FACS
Definition
The term neurogenic bladder describes a process of dysfunctional voiding as the result of neurologic impairment. This can interfere with urine storage at low bladder pressures, disrupt voluntary coordinated voiding, and lead to varying degrees of incontinence. Neurologic control of bladder function is at multiple levels throughout the central nervous system and subject to multiple pathophysiologic processes. Voiding dysfunction occurs in most of the neurologically impaired patients [1].
The micturition reflex center has been localized to the pontine mesencephalic reticular formation in the brainstem [2,3]. Efferent axons from the pontine micturition center travel down the spinal cord in the reticulospinal tract to the detrusor motor nuclei located in the S2, S3, and S4 segments in the sacral gray matter (vertebral levels T12 to L2). Parasympathetic nerves take their origin from nuclei at the intermediolateral gray column of the spinal cord at S2, S3, and S4 and travel by the pelvic nerve and pelvic plexus to ganglia in the bladder wall. The predominant parasympathetic nerve root supplying the bladder is usually S3. Acetylcholine is released from the postganglionic nerves, which in turn excites muscarinic receptors [4,5].
Preganglionic sympathetic neurons originate in the intermediolateral gray column of the spinal cord from spinal segments T10 to L2. These nerves course to the sympathetic chain ganglion and through the pelvic plexus to the bladder neck and fundus of the bladder. Receptors at the bladder neck are primarily α-adrenergic [6], stimulation of which results in closure of the internal sphincter during urinary storage and, in men, during ejaculation as well. In contrast to the bladder neck, the fundus of the bladder is populated with β-adrenergic receptors, which contribute to bladder relaxation (and therefore urinary storage) during sympathetic activation.
The external urethral sphincter (striated muscle, voluntary) surrounds the membranous urethra and extends up and around the distal part of the prostatic urethra. The pudendal nerves, which innervate the external sphincter, take their origin from the somatic motor nuclei in the anterior gray matter of the sacral cord (conus, S2-S4); however, it is the S2 spinal segment that provides the principal motor contribution. Toe plantar flexors also have S1 and S2 innervations. Thus, the preservation of toe plantar flexors after spinal cord injury suggests that the external urethral sphincter is intact.
The central control of the bladder is a complex multilevel process. Advances in functional brain imaging have allowed research into this control in humans. The regions of the brain that have been implicated in the central control of continence include the pontine micturition center, periaqueductal gray, thalamus, insula, anterior cingulate gyrus, and prefrontal cortices. The pontine micturition center and the periaqueductal gray are thought to be crucial in the supraspinal control of continence and micturition. Higher centers, such as the insula, anterior cingulate gyrus, and prefrontal regions, are probably involved in the modulation of this control and cognition of bladder sensation. Further work should aim to examine how the regions interact to achieve urinary continence [7].
Symptoms
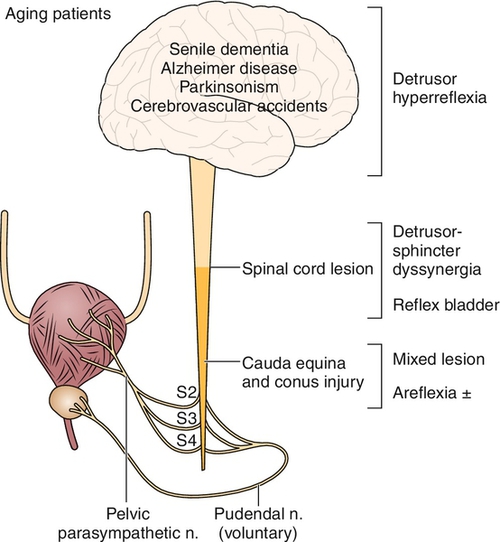
The symptoms of neurogenic bladder have a wide spectrum of presentation and include urinary incontinence, urinary retention, suprapubic or pelvic pain, incomplete voiding, paroxysmal hypertension with diaphoresis (autonomic dysreflexia), recurrent urinary tract infections, and occult deterioration in renal function. The symptoms vary according to the level of spinal cord injury and pathophysiologic basis of the neurologic disorder.
Abnormalities in the midbrain (e.g., Parkinson disease) lead to detrusor hyperreflexia due to loss of dopamine. Lesions in segmental areas of the spinal cord lead to detrusor-sphincter dyssynergia (DSD). Cortical lesions (lesions above the pontine micturition center) usually result in loss of voluntary inhibition of the micturition reflex. Lesions in the forebrain, such as cerebrovascular accidents with change in blood flow to the cingulate gyrus, can lead to hyperreflexic bladder because of reduced dopamine D1 with increased glutamate activity. Thus, the cingulate gyrus plays an important role in urine storage. Patients with Parkinson disease have less severe urinary dysfunction with little evidence of internal or external sphincter denervation. By contrast, in multiple system atrophy, patients have more symptoms and wide-open bladder neck. The result is a hyperreflexic bladder with coordinated (synergic) sphincter function [8,9]. In the absence of outflow obstruction (e.g., urethral stricture, benign prostatic hyperplasia, large uterine leiomyoma, fecal impaction), complete bladder evacuation with some incontinence is the outcome. The findings of postmicturition residuals of more than 100 mL, detrusor–external sphincter dyssynergia, and open bladder neck at the start of bladder filling, with significant postural hypotension and neurogenic sphincter motor unit potentials, are highly suggestive of multiple system atrophy [8,9]. It seems DSD reported in such patients may be a voluntary contraction of external sphincter to avoid leakage and is therefore not true DSD. The patient can have unstable blood pressure, which is aggravated with a postural change, indicating some degree of autonomic failure. Similarly, after a severe head injury, an autonomic failure can result in unstable postural hypotension and wide-open bladder neck. The insula and anterior cingulate seem to be responsible for the modulation of autonomic function [10].
All lesions from the pons to spinal cord level S2 result in a loss of cortical inhibition and loss of coordinated sphincter activity during reflex voiding. The micturition reflex is without an inhibitory or coordinated control from higher centers. This results in a hyperreflexic bladder with dyssynergic sphincter function, which often results in incomplete voiding and high bladder pressures; it can lead to vesicoureteral reflux. Urinary retention from functional obstruction occurs, and overflow incontinence may occur with an overdistended bladder.
Spinal cord lesions above T5-T6 result in autonomic dysreflexia above the key level (T5-T8) that innervates the splanchnic bed and regulates blood supply to control blood pressure. Accentuated visceral activity (e.g., full bladder, fecal impaction), which causes sympathetically mediated vasoconstriction, is normally inhibited by secondary output (a negative feedback) from the medulla and is countered by vasodilation in the splanchnic bed through the greater splanchnic nerve. Without the proper inhibitory reflexes or control of the splanchnic bed to redistribute circulating blood volume, blood pressure rises sharply. With the carotid bodies and vagal nerves intact, bradycardia results. The full syndrome is characterized by paroxysmal and extreme elevation in blood pressure, facial flushing, perspiration, goose pimples, headache, and some degree of bradycardia. This is virtually always seen in conjunction with detrusor-sphincter dyssynergia [11].
Spinal cord lesions in the conus at S2 or below result in lower motor neuron injury to the bladder and external sphincter. The effect on the bladder is predictable: areflexia. Because the parasympathetic ganglia reside in or near the bladder wall, bladder tone is generally maintained. Bladder compliance therefore tends to decrease with time as a result of neural decentralization (or infection-related fibrosis) [1,12]. The result on the bladder neck and external sphincter is not as intuitive. An atonic synergic sphincter system might be expected; the external sphincter usually retains some fixed tone, although not under voluntary control; and the bladder neck is often competent because of the intact sympathetic innervations (α-adrenergic activity) but is nonrelaxing. Even though bladder pressures are generally low during filling and storage, obstructive physiology is often the case during voiding [1,13]. Overflow incontinence is possible. A small, titrated dose of alpha blockers can maintain some continence and improve voiding.
In the acute phase of injury, most central nervous system lesions result in a temporarily areflexic bladder [14,15]. This phase, termed central nervous system shock, is variable and can last several weeks. Reappearance of knee jerks heralds recovery from the shock phase. The specific patterns of voiding dysfunction with the most common neurologic abnormalities in the chronic phase are detailed in Table 137.1 and Figure 137.1.
Confounding medical problems, such as diabetes and many cardiovascular drugs (Table 137.2), will profoundly affect bladder function. Patients who catheterize themselves intermittently should be asked about the size of catheter used and whether there is any resistance or trauma during catheterization—clues to the presence of a urethral stricture. Patterns of voiding should be elicited, and changes in voiding habits should be scrutinized. Patients with suprasacral spinal cord injury, for example, often give a history of intermittent stream coinciding with spasticity of their lower extremities, a strong clue to detrusor-sphincter dyssynergia. Spinal cord–injured patients with incomplete lesions can void with excessive Valsalva maneuver and can produce very high intra-abdominal pressures. This can lead to vesicoureteral reflux, upper tract changes, repeated pyelonephritis, and even bladder and kidney stone disease. They therefore need to be monitored frequently with urodynamics and managed appropriately to achieve low-pressure voiding. Approximately 50% of men ultimately have benign prostatic hyperplasia. Thus, even in the case of stable neurologic disease, these men may develop difficulty in voiding from progressive outflow obstruction. Typical symptoms include nocturia, decreased force of stream, hesitancy, and postvoid dribbling. However, patients with outflow obstruction frequently have irritative voiding symptoms as well. It is important to make sure that these symptoms are not due to symptomatic infection: back pain, suprapubic pain, fever, dysuria, urgency, frequency, or hematuria. These symptoms are not specific and can reflect many of the processes discussed. Their presence must therefore be interpreted according to context.
Table 137.2
Pharmacologic Action on the Bladder
| Drug | Indication | Mechanism | Side Effects and Cautions |
| Cholinergics Bethanechol |
Areflexic bladder | Muscarinic receptor agonists Bladder has M2 and M3 receptors; M3 receptors are responsible for normal detrusor contraction |
Bronchospasm, miosis |
| Anticholinergics Hyoscyamine Oxybutynin Tolterodine Trospium chloride (quaternary amine) Darifenacin Solifenacin |
Hyperreflexic bladder | Muscarinic receptor antagonists | Constipation, dry mouth, tachycardia |
| Sympathomimetics Norepinephrine Pseudoephedrine |
Open bladder neck | α-Receptor antagonists | Arrhythmia, hypertension, coronary vasospasm, excitability, tremors |
| Antiadrenergics (alpha blockers) Phenoxybenzamine Phentolamine Terazosin Doxazosin Tamsulosin Alfuzosin |
Smooth sphincter dyssynergia (competent, nonrelaxing bladder neck) | α-Receptor agonists | Orthostatic hypotension, dizziness, rhinitis, retrograde ejaculation |
| Tricyclic antidepressants Amitriptyline Imipramine |
Hyperreflexic bladder with stress incontinence | Anticholinergic and sympathomimetic properties | Myocardial infarction, tachycardia, stroke, seizures, blood dyscrasias, dry mouth, drowsiness, constipation, blurred vision |
| Benzodiazepines Chlordiazepoxide |
Extremity spasticity with detrusor-sphincter dyssynergia | γ-Aminobutyric acid (GABA) channel activator, centrally acting muscle relaxant | Dizziness, drowsiness, extrapyramidal effects, ataxia, agranulocytosis |
| Baclofen | Extremity spasticity with detrusor-sphincter dyssynergia | GABAB channel activator (?); exact mechanism unknown; centrally acting muscle relaxant | Central nervous system depression, cardiovascular collapse, respiratory failure, seizures, dizziness, weakness, hypotonia, constipation, blurred vision* |
| Dantrolene | Extremity spasticity with detrusor-sphincter dyssynergia | Direct muscle relaxant by calcium sequestration in the sarcoplasmic reticulum | Hepatic dysfunction, seizures, pleural effusion, incoordination, dizziness, nausea, vomiting, abdominal pain |
| Botulinum toxin | Detrusor-sphincter dyssynergia | Inhibits release of acetylcholine | Repeated injections necessary |
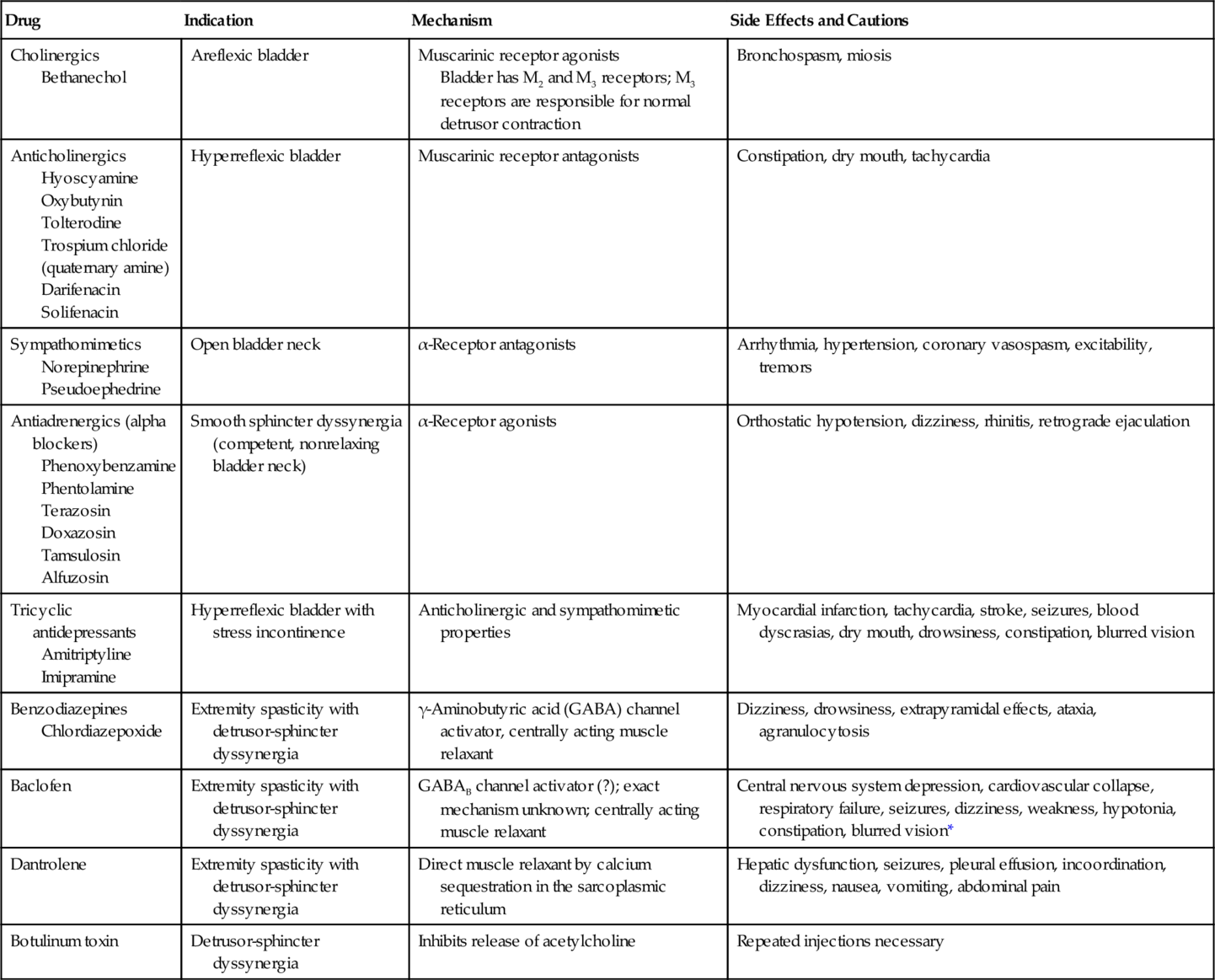
* Note that baclofen is also administered intrathecally by an implanted pump in these patients, and adverse effects are primarily limited to the central nervous system. Bladder contractility may also be reduced. No significant change occurs in detrusor-sphincter dyssynergia.
Physical Examination
General considerations include the level of disability and the capability to use upper and lower extremities. The neurologic examination focuses on the strength and dexterity of the upper extremities and the tone and reflexes of the lower extremities. Neurourologic examination includes perianal sensation for evidence of sacral sparing, anal sphincter voluntary contraction, and bulbocavernosus reflex.
The genitalia are examined for the condition of the penis: whether it is circumcised, its size, and adequacy of the meatus; attention is also paid to the presence of meatal erosion due to an indwelling catheter. In women, it is important to note the appearance of the urethral meatus; this structure erodes quite readily with long-standing catheterization. Pelvic examination will identify confounding factors to voiding dysfunction, such as uterine prolapse or leiomyoma. Rectal examination yields information on anal tone, size of prostate, and presence of fecal impaction. Voluntary contraction of the anal sphincter indicates control over the perineal muscles; in the presence of quadriplegia, it indicates an incomplete central cord–type lesion. To determine voluntary contraction, the clinician places a finger in the patient’s anal canal. The bulbocavernosus reflex should be tested. Because deep tendon reflexes at the patella reflect status of the spinal cord at L3-L4, hyperreflexia at the knee almost certainly indicates increased tone at the pelvic diaphragm and thus detrusor-sphincter dyssynergia. Absence of the toe plantar flexors reflects either damage to S2 or a supraconal lesion, and it therefore predicts damage to the external urinary sphincter and possible involvement of the bladder. For patients with spinal cord injury, the return of deep reflexes below the level of injury heralds the recovery from spinal cord shock. The first and most important determination with regard to bladder function is the establishment of good bladder evacuation. The abdomen must therefore be examined for bladder palpability after a trial of voiding. Either a bladder ultrasound scan or urinary catheterization should be performed to determine the postvoid residual.
Functional Limitations
Functional limitations are typically due to incontinence and include social rejection and isolation. Incontinence may also affect a patient’s ability to work, to participate in recreational activities, and to sustain interpersonal relationships.
Diagnostic Studies
All patients with neural injury should have blood chemistries at baseline and periodically during follow-up for blood urea nitrogen and creatinine concentrations. Renal and bladder ultrasound examination should be performed to assess the status of the urinary tract: size, shape, and echogenicity of the kidneys; presence of hydronephrosis or hydroureter; presence of renal or bladder stones; change in hydronephrosis during and after voiding; and completeness of the void. If vesicoureteral reflux or urethral strictures are suspected, voiding cystourethrography may be performed.
If renal stones are suspected, non–contrast-enhanced computed tomography (renal stone protocol) is an excellent tool for identification of the size and location of the stones. In dealing with renal stones, it is sometimes important to assess calyceal or ureteral anatomy or differential renal function, in which case intravenous urography may be performed. Finer qualitative assessment of differential renal function may be performed by technetium Tc 99 m mertiatide (99mTc-MAG3) nuclear renal scan. When compromise of renal function is suspected, this test may be useful.
Transrectal linear array ultrasonography may be performed to assess prostate size, prominent median prostatic lobe, ledge at the bladder neck [16], or proximal urethral and bladder neck strictures. Voiding videofluoroscopy or ultrasonography can show abrupt cessation of urine flow.
Cystoscopy is an excellent tool for studying bladder and urethral anatomy; it quickly identifies the presence of a urethral stricture (although it may not determine its length or depth), provides an assessment of internal prostatic size, provides indirect evidence of high intravesical pressures (bladder trabeculation), and readily demonstrates bladder stones. Cystoscopy is essential in the assessment of hematuria (more than five red blood cells per high-power field on two or more urine specimens). Whereas hematuria in neurally injured patients is commonly related to infection or traumatic catheterization, the use of indwelling catheters for a long time places these patients at a significantly higher risk for bladder cancer [17]. It has been suggested, therefore, that these patients undergo surveillance cystoscopy annually, particularly if they have other risk factors for bladder cancer, such as smoking or a family history with bladder cancer. After bladder irrigation with normal saline, a urine specimen for malignant cell cytology may be helpful as a screening test. No test is as sensitive for the detection of bladder tumors as the cystoscopic examination and biopsy of a suspicious area.
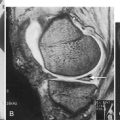
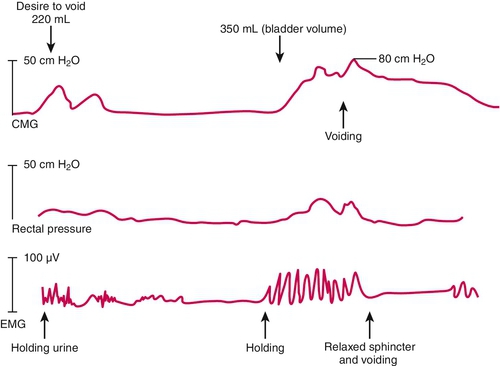
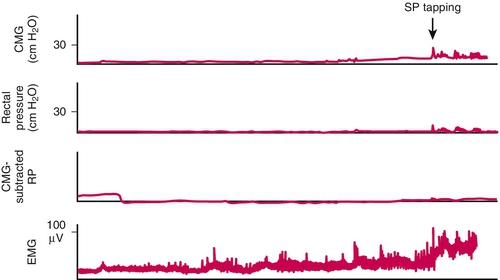
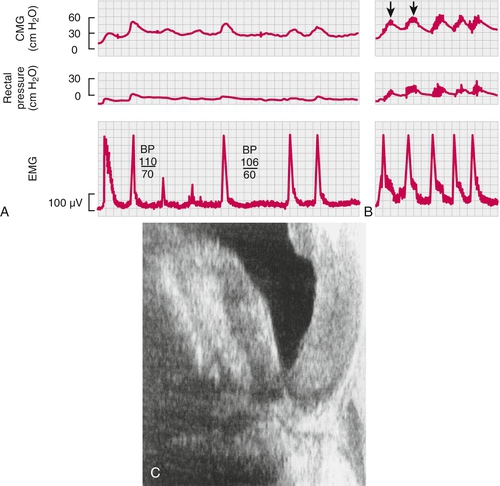
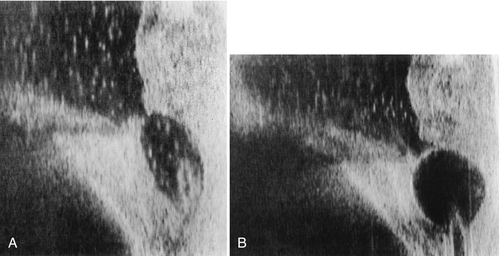
Note that cystoscopy does not provide functional information. The best single test for bladder function is urodynamic testing. Formal urodynamic testing, including cystometrography and electromyography, is crucial to the proper documentation of bladder and outlet function. The test is subject to inherent errors in technique, interpretation, and cooperation of the patient; however, the astute clinician must interpret the results in light of the entire clinical scenario. Properly performed, it will elucidate and quantify postvoid residual of urine, bladder capacity, bladder compliance, bladder pressures during filling and voiding, and external sphincteric coordination. In conjunction with videofluoroscopic monitoring, ureteral reflux, bladder position, and internal and external sphincteric function may be visualized. Urodynamics should be performed at baseline for all patients with neurologic disease. Because of central nervous system shock in the acute phase of injury, it is best to perform urodynamics after the shock resolves—on return of distal reflexes. Figure 137.2 illustrates a normal voiding pattern. Figure 137.3 illustrates the urodynamic findings of an areflexic bladder. Figure 137.4A, B shows detrusor-sphincter dyssynergia. The sonographic correlate of detrusor-sphincter dyssynergia is shown in Figure 137.4C (nonrelaxation of the external urethral sphincter is demonstrated).
Treatment
Initial
The priorities of bladder management relate first to preservation of renal function and abolition of infection and second to social concerns. Overflow incontinence, ureteral reflux, or high bladder pressures in the presence of renal insufficiency or active infection must be managed aggressively by ensuring proper egress for urine. A source for persistent infection, such as urinary lithiasis, must be sought and, if found, eliminated. High bladder pressure, particularly if it is sustained (> 40 cm H2O), ultimately results in deterioration of renal function and should therefore be addressed actively, even if renal function is normal [18].
Rehabilitation
Patients at risk for degenerative neurogenic bladders, particularly those with (or at risk for) sensory neuropathies (e.g., diabetic patients, phenytoin users), should have a timed voiding schedule to prevent overdistention and progression to bladder areflexia. A 24-hour voiding diary, including fluid intake, time and quantity voided, and postvoid residual (by catheterization or ultrasound evaluation), should be recorded periodically. These patients should void every 6 hours, void again immediately after the first void, and adjust their fluid intake and voiding frequency according to the voiding diary. Patients with diabetes should be careful to maintain good glycemic control, not only for global prevention of related degenerative disease but also to prevent osmotic diuresis.
Most neurogenic bladder lesions are associated with impaired bowel function. Fecal impaction and obstructed constipation may also cause mechanical obstruction to the passage of urine. Further, many of the medications used to reduce bladder contractility, particularly the anticholinergics, exacerbate bowel motility dysfunction. It is therefore important that these patients be routinely prescribed high-fiber diets, stool softeners (e.g., docusate, 100 mg orally, three times daily), laxatives (e.g., psyllium, 1 packet [3.4 g] orally every day), and suppositories (e.g., bisacodyl, 10 mg per rectum every day) and undergo digital stimulation either daily or every other day. Digital stimulation is best performed after either a meal or coffee or tea to take advantage of the gastrocolic reflex.
The Credé method (suprapubic pressure) alone can lead to high intravesical pressures and even vesicoureteral reflux. Such pressure, or persistent tapping of the suprapubic region for 2 minutes at a time, should be performed only when methods to relieve bladder outlet obstruction have been ensured. This should not be performed in patients with active detrusor-sphincter dyssynergia and detrusor hyperreflexia because it will only exacerbate already high bladder pressures, and urine will not be completely evacuated.
Acute Phase and Central Nervous System Shock Phase
This phase usually lasts days to weeks. The bladder is areflexic during this period, and adequate bladder drainage should be secured to prevent the areflexic bladder from developing overdistention and myogenic failure. Indwelling continuous Foley catheterization (14 F) is the easiest way to ensure bladder drainage. Alternatively, intermittent catheterization may be performed (after the initial phase of diuresis) and, when it is used from the onset, reduces the incidence of infection and stone disease [19].
Patients do need training or assistance in self-catheterization. Rehabilitation nurses may also be involved in this educational process. Catheterization is performed every 4 to 6 hours and fluid is restricted to a maximum of 2 liters per day, if possible. The frequency of catheterization should be adjusted so that residuals are no more than 300 to 400 mL. For patients with a hyperreflexic bladder, long-term intermittent catheterization requires mitigation of the detrusor reflex with anticholinergics (Table 137.2).
Anticholinergic Drugs (Drugs to Increase Bladder Capacity)
In the human being, bladder (detrusor muscle) has muscarinic receptors (M2 and M3 receptors). M3 receptors compared with M2 receptors are small in number but are mainly responsible for bladder contraction. The antimuscarinic drugs oxybutynin, tolterodine, darifenacin, solifenacin, and trospium are the five major drugs (Table 137.3) currently available to modulate detrusor hyperreflexia, to increase bladder capacity, and to reduce bladder voiding pressures. Comparative clinical studies have shown that oxybutynin and solifenacin may be marginally more effective than tolterodine, although tolterodine seems to be better tolerated; but dry mouth and constipation are still major problems for compliance of patients with all of them because of the widespread existence of M3 receptors, particularly in the salivary glands. Except for trospium chloride, most of the other drugs shown in Table 137.3 are tertiary amines and cross the blood-brain barrier, enhancing the anticholinergic factor. There is evidence that they may lead to some loss of memory, particularly in elderly patients [20].
Table 137.3
Summary of Anticholinergic Agents
| Anticholinergic* | T1/2 (h) | Typical Side Effects | Comments |
| Oxybutynin Ditropan |
2-3 | Dry mouth, constipation, blurry vision, headaches Elderly: cognitive impairment, hallucinations |
Typical dose is 5 mg orally tid |
| Ditropan XL | 13 | Same as oral oxybutynin | Slower absorption with more stable blood concentration Typical dose is 10 mg orally daily, but doses up to 40 mg daily are generally safe |
| Oxytrol (oxybutynin patch) | 2-5 | Same as oral oxybutynin, plus local skin irritation | Bypasses first pass of the liver, which reduces the concentrations of active metabolites that are thought to contribute to side effects Dosed at 3.9 mg/day, 1 patch 2 times/week, alternating skin sites |
| Tolterodine Detrol |
2-10 | Same as oral oxybutynin | Possibly more selective to urinary M receptors, resulting in fewer side effects Typical dose is 2 mg orally bid |
| Detrol LA | 2-10 | Same as oral oxybutynin | Slower absorption with more stable blood concentration Typical dose is 4 mg orally daily |
| Darifenacin (Enablex) | 13-19 | Same as oral oxybutynin | Typical dose is 7.5 mg or 15 mg orally daily |
| Trospium | 20 | Same as oral oxybutynin | Quaternary amine does not cross the blood-brain barrier well, resulting in minimal central nervous system anticholinergic effect, and it may limit cognitive side effects (particularly in the elderly) Typical dose is 20 mg orally bid |
| Sanctura XR | 36 | Same as oral oxybutynin | More stable blood concentration Typical dose is 60 mg orally daily |
| Solifenacin (VESIcare) | 45-68 | Same as oral oxybutynin | Typical dose is 5 mg or 10 mg orally daily |
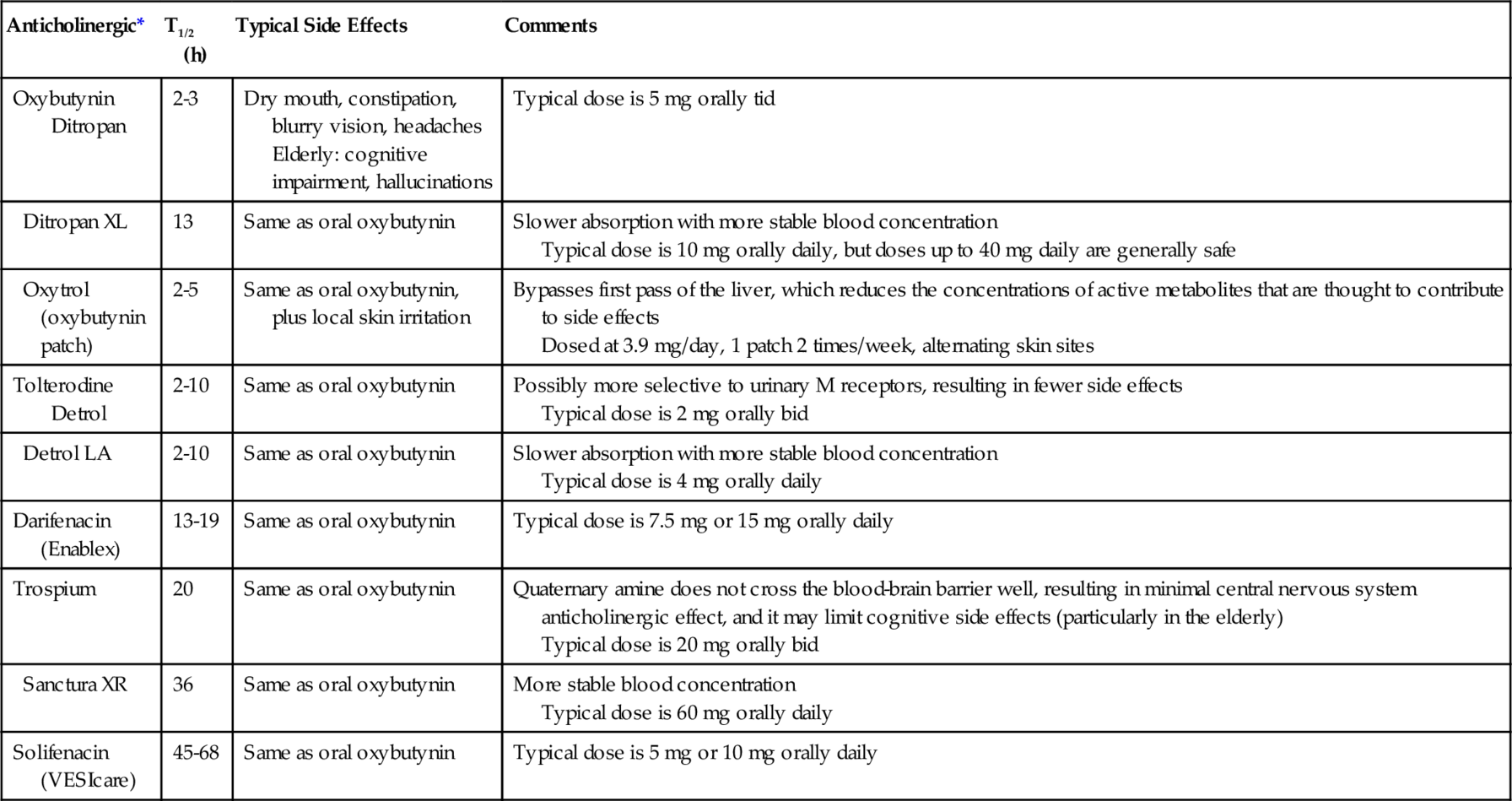
T1/2 (h), pharmacokinetic half-life (hours).
* Unless patients with urinary retention are managed with a catheter, anticholinergic therapy is contraindicated for them. Anticholinergics are also contraindicated in patients with gastric retention and patients with uncontrolled narrow-angle glaucoma.
Anticholinergic drugs have been, on the whole, a disappointment in the treatment of incontinence associated with neurogenic detrusor overactivity. Whereas urodynamic parameters often improve and the number of incontinence episodes is often reduced by anticholinergics, incontinence often remains a problem. Mirabegron (Myrbetriq; Astellas Pharma US, Inc), a selective β3-receptor agonist that causes detrusor fundus relaxation, has emerged recently as a pharmaceutical alternative for patients failing to respond to anticholinergic therapy in the able-bodied idiopathic overactive bladder population [21]. It has not yet been studied in the neurogenic bladder population, although a trial is certain for this in the near future. According to the Food and Drug Administration, its most important side effect is significant hypertension, noted in 11.3% (compared with 7.6% in the placebo arm), which is reversible with discontinuation of the drug. It is dosed at 25 mg or 50 mg orally once daily. Botox injections are used in the bladder wall to reduce bladder pressures to safe levels (< 40 cm H2O) and to achieve continence between catheterizations.
Procedures
Another alternative to long-term management of bladder drainage is placement of a suprapubic catheter. This is preferable to chronic transurethral catheterization because it eliminates the risk of urethral or meatal erosion and is less often the cause of epididymitis or orchitis. Urinary tract infections, however, are just as likely, and these catheters require changing once a month. They are best placed either in the operating room under cystoscopic guidance or, better, through suprapubic incision to ascertain that the catheter ultimately resides as superiorly as possible, far from the bladder neck. This helps prevent irritation at the bladder neck, which often causes reflex bladder contractions, particularly if the catheter balloon (also in an indwelling Foley catheter) drops down into the posterior urethra (Fig. 137.5).
Botulinum Toxin
Patients with neurogenic overactive bladder failing to respond to anticholinergic therapy may benefit from periodic administration of intravesical onabotulinumtoxinA, the leading manufactured agent being Botox. Its purported mechanism of action is the inhibition of release of acetylcholine at the presynaptic cholinergic junction [22], which effectively suppresses detrusor contraction [23]. Botox is typically administered by submucosal injection cystoscopically in 0.5-mL aliquots; the total dose ranges from 100 to 300 units through a rigid or flexible cystoscope.
Prospective randomized double-blind placebo-controlled trials showed that Botox is efficacious and safe in patients with neurogenic detrusor overactivity and incontinence, with clinical benefits seen up to 9 months after injection [24,25]. Botox reduced incontinence episodes and improved quality of life in patients with multiple sclerosis and in patients with spinal cord injury (T1 or lower). Median time to a request for re-treatment was 42 weeks [24]. In a 7-year retrospective review of 216 patients given Botox or Dysport in 365 sessions, significant improvement was seen in maximum detrusor pressure, detrusor compliance, and bladder capacity at 6 weeks and 6 months; a quarter of the patients never stopped anticholinergic therapy; half discontinued after 6 weeks; and after 6 months, 35% still had not resumed anticholinergic therapy [26]. In a randomized placebo-controlled study [27], similar improvement was demonstrated in urodynamic outcomes. Reitz and colleagues [28] showed that 73% of neurogenic detrusor overactivity incontinent patients achieved continence with Botox at 12 weeks.
After Botox injection, postvoid residuals may be significantly elevated in a dose-dependent fashion; a third of patients had urinary retention and required intermittent catheterization [24]. Another drawback of this agent is need for repeated treatment periodically. Rarely, generalized weakness, difficulty in swallowing, or dysarthria ensues, although these side effects reverse spontaneously in several weeks [26].
Sacral Nerve Stimulation
For patients with hyperreflexic bladders, electrical stimulation through peripheral patch electrodes or implantation of a sacral nerve root stimulator may be of some benefit [29–31]. The treatment is dependent on an intact sacral reflex and works by modulation of signaling from somatic and afferent nerves involved in the micturition reflex pathway. Patients must be able to empty the bladder voluntarily (or by Valsalva maneuver) when the device is turned off or be capable of performing intermittent self-catheterization.
Installation of a sacral nerve stimulator may be done in two stages: a test phase with temporary wires, typically lasting about 1 week; and pending a successful outcome with the test phase, installation of a permanent system. Correct placement of the lead is done under local anesthesia with the patient awake and conversant in the prone position. Its placement in the correct foramen level (typically S3) and depth of penetration to the anterior nerve root are confirmed by real-time fluoroscopic imaging, assessment for a tapping sensation in the vagina or perineum, dorsiflexion of the ipsilateral great toe, and bellowing contraction of the perineal area. For those having a permanent implant, the patient is then typically sedated for subcutaneous placement of the implantable pulse generator and tunneling of the wire.
The studies reported are primarily in able-bodied populations with idiopathic detrusor hyperreflexia. Limited evaluation thus far in the neurogenic population, however, has shown promise. In a review and meta-analysis of 26 studies, including 357 patients, and a mean follow-up of 26 months concerning the safety and efficacy of sacral nerve stimulation in neurogenic lower urinary tract dysfunction, the pooled success rate was 68% for the test phase and 92% for permanent sacral nerve stimulator implantation [32]. Adverse events were seen in none in the test placement group and 24% in the permanent placement group. Adverse effects include pain, infection, and lead migration. In one study, 33% required surgical revision [33]. The life span of these implantable pulse generators varies with intensity of use but averages about 5 years.
Sacral nerve stimulator implantation is relatively contraindicated in multiple sclerosis patients to the extent that these patients are frequently studied by magnetic resonance imaging over time. It is also not appropriate for patients with significant spinal and sacral bone abnormalities.
Surgery
Transurethral sphincterotomy has been used in suprasacral lesions in the past and has fallen into disfavor because of intraoperative and delayed bleeding potential. Use of a laser in a contact mode causes virtually no intraoperative bleeding [11,34]. This procedure results in incontinence postoperatively and requires the use of an external condom catheter.
There are rare circumstances in which bladder management has aggravated renal function, evidenced by recurrent ascending urinary tract infections or a bladder that is too contracted to store sufficient volumes. Some patients find it socially unacceptable to be incontinent and are willing to perform intermittent self-catheterization, but the body habitus precludes them from this. In such cases, there are certain reconstructive options that should be considered. An incontinent urinary diversion with a stoma in abdomen that drains urine in a bag may be performed [35,36]. Cystectomy may be combined with this procedure or the bladder may be left in situ with a small risk of pyocystis.
In patients willing and able to perform intermittent self-catheterization, the most common reconstructive alternative is an augmentation cystoplasty. The bladder is augmented by a segment of ileum, and the ureters remain in their native locations. Bladder pressure is reduced and capacity increased, thus protecting the upper tracts from side effects of high pressure bladder. The drainage of urine is performed by intermittent self-catheterization. This procedure may be combined with an artificial rrinary sphincter in patients with incompetent sphincter.
For bladders that have low pressures and good compliance but leak through fixed sphincters, the Mitrofanoff and bladder neck closure may be an excellent option. Spina bifida patients with conus lesions might be good candidates for this procedure. Egress through the bladder neck is eliminated, and the appendix is interposed between the bladder and the umbilicus where it is opened. In the common event that the bladder has poor compliance, an ileal bladder augmentation will raise bladder volume and lower bladder pressure. Patients would then catheterize their augmented bladders through the umbilicus.
Potential Disease Complications
Urinary tract infections, kidney stones, and autonomic dysreflexia are common disease complications associated with neurogenic bladder. Social isolation due to incontinence may lead to depression.
Patients with spinal cord injury who have a urinary tract infection commonly may not present with symptoms [4]. Fevers, chills, back pain, suprapubic pain, dysuria, frequency, and testicular swelling in the setting of positive urine cultures should be regarded as a urinary tract infection. Patients without overt symptoms of pyelonephritis, prostatitis, epididymitis-orchitis, or cystitis are more difficult to diagnose, particularly if they have an indwelling catheter or are being managed by intermittent catheterization. Those using catheters are virtually always colonized with bacteria, and the injudicious use of antibiotics will only select out resistant strains. Factors indicating a need for treatment include urinary lithiasis, as this is most often related to infection (struvite, magnesium ammonium phosphate), and pyuria (8 to 10 white blood cells per high-power field, or 100 white blood cells per milliliter) in the setting of bacteriuria (more than 10,000 colony-forming units per milliliter). Urine pH should be checked periodically; pH above 7 is invariably associated with infection from urea-splitting organisms, which may lead to struvite stone formation.
Patients with detrusor-sphincter dyssynergia or urinary retention of any kind should have the bladder drained expeditiously with a fresh Foley catheter during the course of their treatment to ascertain good egress of infected urine. If prostatitis is suspected, transurethral insertion of a Foley catheter is relatively contraindicated, and drainage should be ensured suprapubically. Fluoroquinolones are an excellent first choice for most urinary tract infections; therapy may then be tailored to culture and sensitivity results. Three to 5 days is sufficient for cystitis. Pyelonephritis requires 2 weeks of therapy. Epididymitis requires at least 3 weeks of therapy, and prostatitis often requires 6 weeks. A higher incidence of bladder cancer (squamous cell) has been reported in patients with long-term indwelling bladder drainage [37].
Autonomic Dysreflexia
The control of widespread sympathetic activity below the spinal lesion is the key factor in the management of autonomic dysreflexia, and prevention is the first concern. Noxious stimuli, such as overdistention of the bladder, should be reversed immediately by catheter drainage. Local instillation of 20 mL of lidocaine jelly of 0.3% tetracaine through the Foley catheter or suprapubic tube may provide topical anesthesia of the vesical mucosa and reduce triggering impulses to the spinal cord. Consideration of procedures for patients at risk (spinal lesions above T6) should include spinal anesthesia, use of ganglion blockers, and use of adrenergic blockers.
In the acute episode, if reversal of the noxious stimulus fails to control symptoms, administration of nifedipine (10-30 mg sublingually [38]) or nitropaste (about 1 inch applied on the body surface) is usually adequate to reduce blood pressure. Note that normal blood pressure for a patient with spinal cord injury is less than 100 mm Hg systolic. If nifedipine fails, hydralazine (10 to 20 mg intravenously or 10 to 50 mg intramuscularly) may be administered. Use lower doses initially and repeat every 20 to 30 minutes as necessary to maintain a low blood pressure. Other useful drugs include alpha blockers (such as prazosin, terazosin, guanethidine, and clonidine) and anticholinergics (such as oxybutynin and tolterodine). For long-term management of autonomic dysreflexia, clonidine (0.1 to 0.3 mg orally twice daily) is useful. Note that the chronic form of this syndrome is often related to active detrusor-sphincter dyssynergia, and methods aimed at control of this phenomenon, such as transurethral sphincterotomy, may alleviate the patient of autonomic dysreflexia [11].
Potential Treatment Complications
For those who have had indwelling Foley catheters for an extended period (more than several days) and require catheter removal or exchange, antibiotics should be administered prophylactically before, during, and after removal of the existing catheter. Gentamicin (80 mg intramuscularly once just before removal of the catheter) is appropriate for most patients with stable renal function, even if function is impaired, and has both gram-positive and gram-negative coverage. Those with prosthetics, aortic stenosis, or other risk factors for seeding should have coadministration of ampicillin (1 g intravenously) for enterococcus coverage. Alternatively, amoxicillin–clavulanic acid (750 mg) or ciprofloxacin (500 mg) may be given orally twice daily before and 24 hours after placement of the new catheter. In addition, the bladder should be irrigated gently through the catheter with 50 mL of Neosporin G.U. irrigant (DSM Pharmaceutical, Inc., Greenville, North Carolina) (at body temperature) containing 40 mg/L Neomycin and 200,000 units/L Polymixin B. Irrigation should be avoided in patients with a defect/ulceration in the bladder mucosa or in postoperative patients for the toxic absorption of these drugs; ideally, a new catheter is placed and urine for culture is obtained to avoid culturing bacteria in the biolayer around the catheter [32]. Bladder is irrigated with GU irrigant every 4 hours for at least 48 hours. Irrigation is continued while the catheter is being pulled through the urethra to remove urethral pathogens. This method has been shown to significantly reduce urinary tract infection related to catheter changes [33, 34].
Attention to hygiene is paramount in the prevention of urinary tract infections in the spinal cord–injured population. Those on condom catheter drainage should change it once a day. Leg bags should be routinely disinfected with 6% Clorox solution or bleach (most cost-efficient) and then washed well with running water. Wheelchair seat cushions should be changed and cleaned, and the patient should take a shower daily to reduce colony counts at the perineum. Suppressive treatment should be considered for patients who demonstrate recurrent infections. Nitrofurantoin, 100 mg orally daily, is sufficient. Methenamine hippurate (1 g orally twice a day) and ascorbic acid (500 mg orally daily) acidify the urine and may be good for prophylaxis of urinary tract infection [40,41].
Stoma care after colostomy is a source of great consternation for many who have it because of frequent appliance leaks and skin irritation. Also, the stoma must be situated properly on the abdomen according to the patient’s habitus and positioning in the wheelchair. Bladder augmentations of any kind are susceptible to perforations and life-threatening infections (as much as 10%). These patients have a 3% chance of small bowel obstruction from adhesions during their lifetime. Chronic indwelling Foley catheters carry the potential for urinary infection, meatal erosion, epididymitis-orchitis, stone disease, and urethral fistula. In women, the urethra becomes patulous in time and incontinence ensues with or without a catheter. Finally, with time and repeated infections, indwelling catheters put patients at risk for development of squamous cell cancer of the bladder [37]. Although the incidence is low, gross hematuria should be evaluated with great suspicion in these patients because this disease is often advanced at the time of discovery and is often fatal [39].