CHAPTER 136
Neural Tube Defects
Jess Nordin, DO; Glendaliz Bosques, MD
Definition
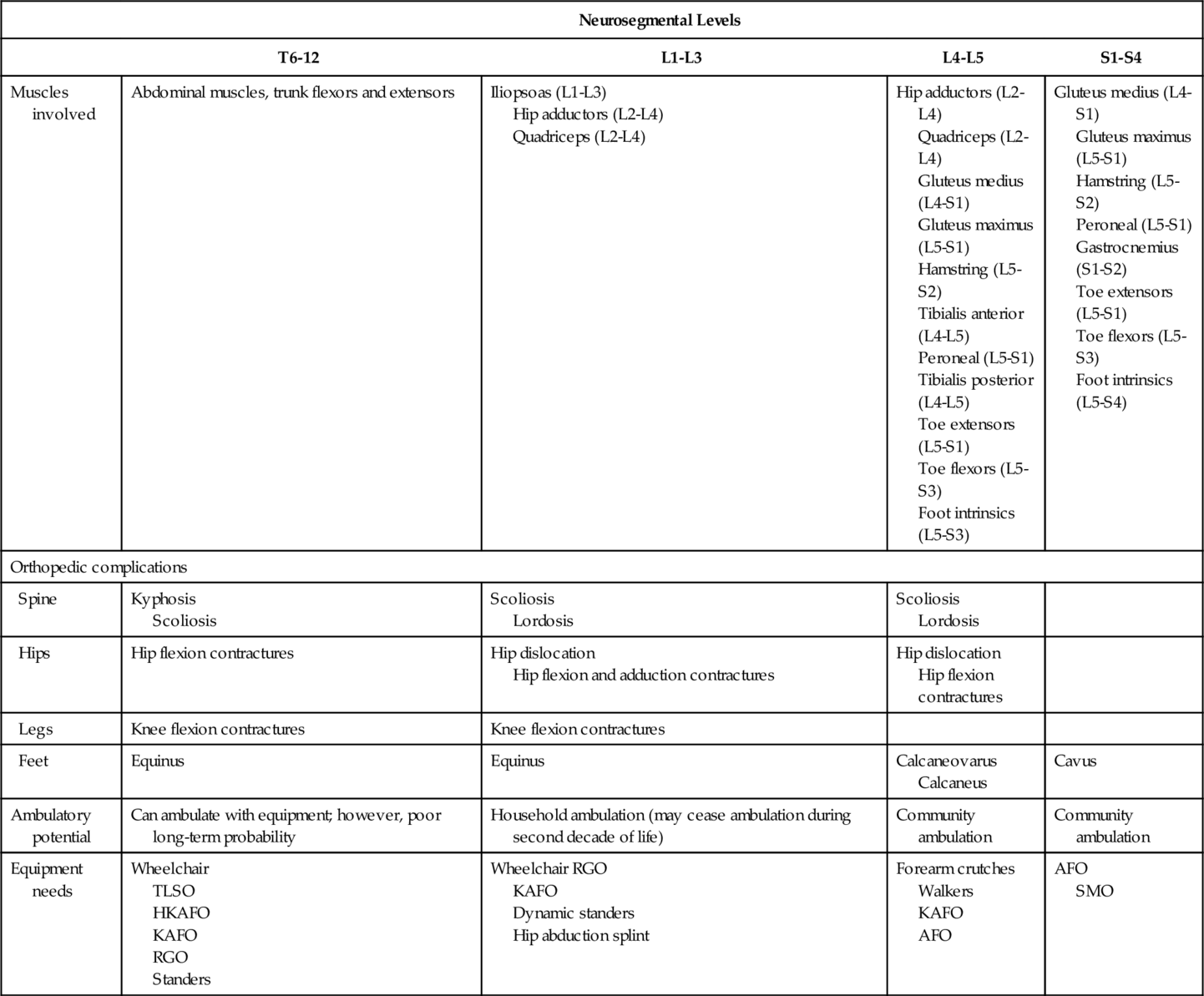
A neural tube defect occurs when the neural tube, the embryologic precursor to the brain and spinal cord, fails to close between the third and fourth weeks of embryogenesis. Neural tube defects are a subset of central nervous system congenital anomalies leading to varying degrees of neurologic impairment from complete failure of neurulation to spina bifida occulta, a posterior vertebral defect without protrusion of neural tissue. Myelomeningocele (MMC), a form of spina bifida cystica, is the most common spinal dysraphism. MMC occurs when the meninges and the spinal cord project through a vertebral defect, forming a sac most commonly located in the lumbosacral region.
The etiology of neural tube defects is multifactorial. Genetic factors have been implicated; there is a 4% risk of recurrence of MMC after having one affected child [1,2]. Associated environmental factors include low socioeconomic status, parental occupation, maternal diabetes mellitus, maternal hyperthermia, maternal obesity, and drug exposures, such as to valproic acid [1]. Since the mandatory fortification of grain products with folate in the United States in 1998, the prevalence rate of spina bifida declined 31% to a rate of 3.49 per 10,000 [1]. The highest prevalence, in the United States, is among women of Hispanic ethnicity [1].
Symptoms
Neurologic
Clinical presentation (Table 136.1) will vary by the extent of involvement of sensory, motor, and autonomic nerves. Dysphagia, aspiration, stridor, vocal cord paralysis, nystagmus, spasticity, bradycardia, and sleep apnea are all symptoms of brainstem dysfunction. A high index of suspicion is sometimes necessary for early assessment and management because severe apnea may lead to respiratory arrest, which could in turn result in death.
The symptoms of hydrocephalus will vary by age. In an infant, symptoms include lethargy, poor appetite, vomiting, accelerated head enlargement, bulging anterior fontanelle, dilated scalp veins, spasticity, and clonus. In an older child, headache is a prominent symptom. Mechanical obstruction of a ventriculoperitoneal shunt (VPS) is manifested acutely with signs of hydrocephalus. VPS infections are associated with fever, headache, and meningismus. Adults with VPS can develop chronic idiopathic headaches.
Tethered cord syndrome can result in progressive scoliosis, decline in lower extremity strength, development of lower extremity contractures, spasticity, change in gait or urinary symptoms, low back pain, and skin ulcers. Patients with spina bifida occulta may present to providers with tethered cord syndrome symptoms beginning during adulthood [3].
A noted increase in spasticity can be caused by hydrocephalus, syringomyelia, tethered cord, urinary tract infection, and decubitus ulcers. Seizures may be common in children with MMC.
Orthopedic
Symptoms from progressive scoliosis can include pain, cardiopulmonary dysfunction, and severe disability interfering with sitting balance and ambulation. Hip deformities, including contractures and dislocations, may result in popping sensation, leg length discrepancy, and pain. Pelvic obliquity can lead to progression of scoliotic curve and difficulties with sitting and positioning. Presence of knee flexion or extension contractures may interfere with functional tasks, such as transfers. Foot deformities are common and may depend on which spinal segments are affected. Pathologic fractures in the lower limbs may be manifested with localized erythema and swelling [4]. Musculoskeletal pain in the shoulder area may be extremely common in wheelchair users, especially in adult patients.
Urinary
Patients may present with urinary retention or incontinence from neurogenic bladder. Urinary tract infections can be associated with fever, changes in urinary control like new-onset leakage between catheterizations, and change in urine quality (color, foul odor, or sediment).
Gastrointestinal
Stool incontinence and constipation are symptoms of neurogenic bladder. Chronic constipation may lead to incontinence due to overflow.
Endocrine
Short stature due to growth hormone deficiency is the most common endocrine symptom [5].
Reproductive
Many men with MMC are infertile [6]. Fertility among women with MMC is thought to be normal [7]. Change in urologic status (i.e., new-onset incontinence) or back pain may be a presenting sign of pregnancy or labor.
Pulmonary
Brainstem dysfunction associated with Chiari II malformation can lead to hypoventilation, apnea, and respiratory failure. Restrictive lung disease can be seen with severe progression of neuromuscular scoliosis. In thoracic-level MMC, partial innervations of abdominal and intercostal musculature may result in respiratory insufficiency.
Cardiovascular
Lower extremity swelling may be due to positional edema or lymphedema. There is evidence showing increased prevalence of lymphedema [8]. Hypertension may be prevalent as well with associated metabolic syndrome and early-onset arteriosclerosis from sedentary lifestyle and obesity.
Allergy and Immunology
There is an increased prevalence of latex allergy. Careful precautions should be taken as a severe reaction can include angioedema and anaphylaxis. This can be life-threatening.
Dermatology
Patients with MMC are at risk for pressure ulcers because of insensate skin, incontinence, impaired mobility, orthopedic abnormalities, and orthoses.
Nutrition
Obesity is common, especially in older adolescents and adults. Significant dysphagia, on the other hand, may lead to failure to thrive and difficulty in gaining weight from brainstem dysfunction.
Psychosocial
Difficulties with motivation and academic performance may be reported by parents. Depression may be common in older adolescents and adults [9].
Physical Examination
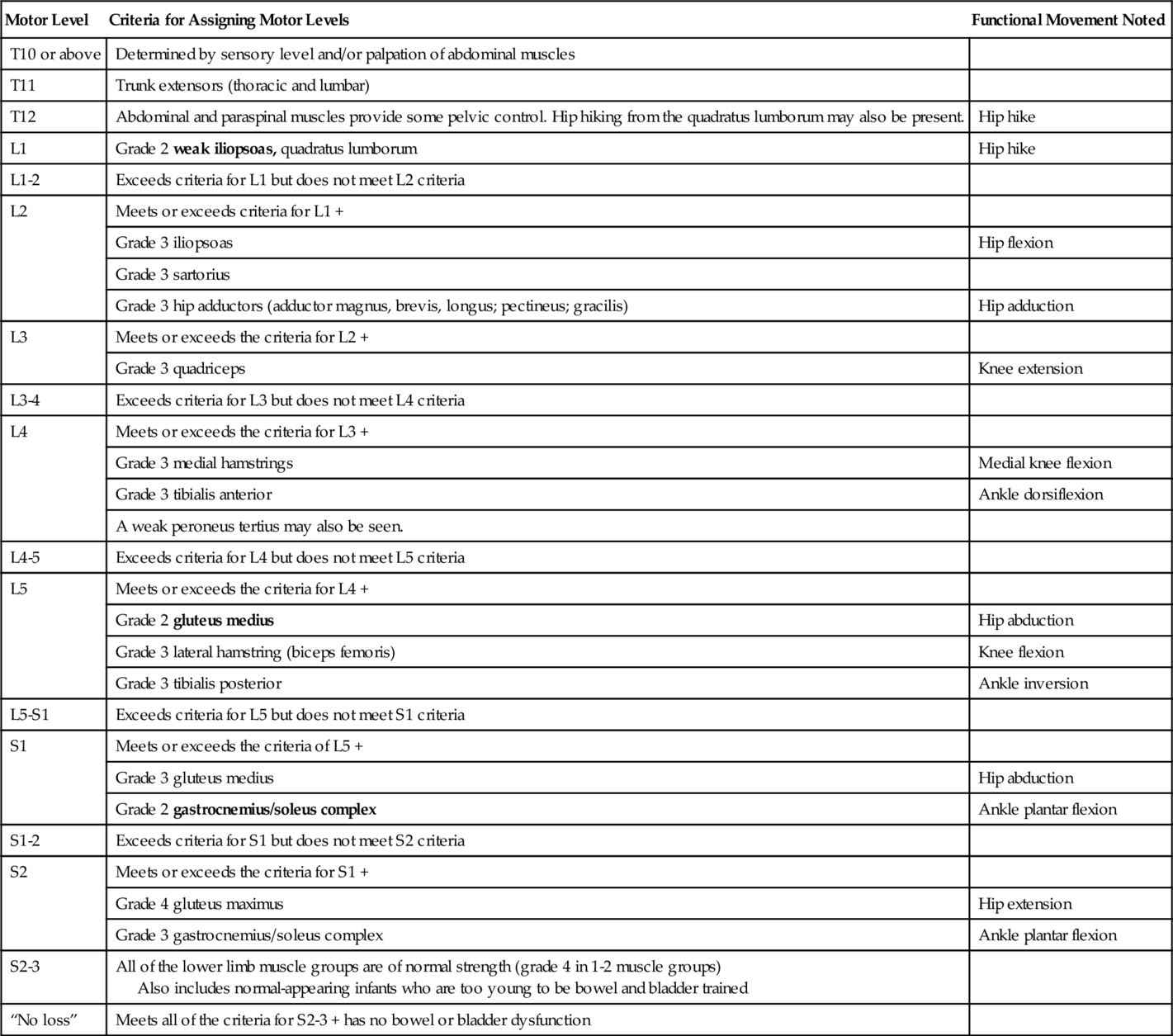
A full physical examination should be performed and will vary on the basis of the level of the lesion. Particular focus for the physiatrist lies in the neurologic and musculoskeletal examination and its relation to functional status. Criteria for assessment of motor level in patients with MMC should be used (Table 136.2). Examination should include assessment of the cranial nerves, mental status, motor strength, sensation, reflexes, range of motion in joints, muscle tone, spinal alignment, and gait. Developmental screening includes assessment of gross and fine motor, language, and cognitive abilities. Anthropometric measurements should be monitored in the MMC population because of a high incidence of decreased height or linear length from associated musculoskeletal deformities [10]. Arm span measurements allow a more accurate assessment of body mass index [10]. The Centers for Disease Control and Prevention growth charts used for able-bodied children may not provide all the information necessary for assessment of appropriate growth and nutrition. Evaluation for skin breakdown and for cutaneous midline lesions of the spine, which may signal an underlying form of occult dysraphism, should be performed. The patient’s equipment should be routinely evaluated.
Functional Limitations
Functional limitations vary by the degree of associated neurologic impairments and orthopedic abnormalities. Children with MMC generally have delay in ambulation [11]. The strength of the iliopsoas, quadriceps, and gluteus medius muscles is an important predictor of ambulatory potential. In general, patients with thoracic lesions are wheelchair dependent. Patients with upper lumbar lesions usually ambulate household distances with variable wheelchair dependency. Patients who have lower lumbar and sacral lesions ambulate longer community distances. These patients may still require some bracing or assistive devices.
Other factors, such as presence of hydrocephalus, seizures, muscle tone, contractures, fractures, weight, cognition, and motivation, among others, have been implicated with ambulation and mobility [11,12].
Diagnostic Studies
Prenatal
Elevated serum levels of α-fetoprotein detected in the second trimester suggest a neural tube defect. Fetal ultrasonographic imaging and magnetic resonance imaging can aid in determining prognosis and parental decision-making [13]. Amniocentesis may show elevated levels of α-fetoprotein and acetylcholinesterase, also consistent with a neural tube defect.
Postnatal
At birth, the determination of neurologic status is clinical. A head ultrasound examination is performed to assess for ventricular size. Echocardiography can be performed before surgical repair to rule out congenital heart disease. Cranial or spinal ultrasound examination can be used for noninvasive assessment of neurologic status in an infant. Beyond infancy, magnetic resonance imaging is performed to assess the spinal cord, and computed tomography is used to assess for hydrocephalus. A shunt series can be performed if there is concern for shunt malfunction. A VPS tap may be performed to rule out infection. Plain film radiography or computed tomography is used when there is concern for a joint or spine deformity or pathologic fracture.
Urologic studies include renal and bladder ultrasonography, voiding cystourethrography, urodynamic studies, DMSA nuclear scan, and postvoid residual measurement [14]. Cystoscopy is performed for bladder cancer surveillance, especially if the patient has undergone bladder augmentation procedures [15]. Routine blood work may include a complete blood count, comprehensive metabolic profile, lipid panel [16], 25-hydroxyvitamin D level, and cystatin C level [14]. Depending on clinical presentation, other diagnostic studies may include determination of endocrine hormone levels and prealbumin concentration, electroencephalography, dual-energy x-ray absorptiometry scan, polysomnography, and latex allergy assay. Throughout childhood and adulthood, health care providers should continue to perform routine health maintenance. Neuropsychological testing should be performed before entrance into school.
Treatment
Initial
Surgical closure of MMC within 48 hours is customary. Fetal surgery can be performed before 26 weeks of gestational age. Lower incidences of hindbrain abnormalities and shunt-dependent hydrocephalus as well as improved motor outcomes have been reported for in utero compared with postnatal surgical closure [17]. However, fetal surgery has not shown improvement in lower urinary tract function [18].
First-line management of hydrocephalus is placement of a VPS. Endoscopic third ventriculostomy combined with choroid plexus cauterization represents an alternative technique by addressing both the communicating and noncommunicating mechanisms of hydrocephalus without introducing shunt dependency [19].
Infants who are unable to void or who have large postvoid residuals should begin clean intermittent catheterization programs. Children can be taught to perform clean intermittent catheterization as early as 5 years of age, although it may take longer because of the high executive function impairments related to hydrocephalus. Patients with detrusor-sphincter dyssynergia or hydronephrosis may be treated with anticholinergic medications. Children with vesicoureteral reflux are often prescribed prophylactic antibiotics. Various treatments exist for hyperactive sphincter function.
A bowel management program may consist of stool softeners, bulking agents, suppositories, digital stimulation, manual removal, or enemas. Clinicians often recommend performing bowel programs after a meal to take advantage of the gastrocolic reflex.
An acute change in the respiratory status of a patient may warrant posterior fossa decompression or treatment of hydrocephalus. If this is unsuccessful, tracheostomy and long-term mechanical ventilation may be considered.
Other treatment modalities may include antiepileptics, growth hormone or gonadotropin-releasing hormone analogue treatment, bisphosphonates, and calcium and vitamin D. Recommendations for daily physical activity and dietary management must be included. Genetic counseling and appropriate folic acid supplementation are advised for affected women considering pregnancy. Current guidelines for folic acid supplementation are 0.4 mg/day for any woman of childbearing age and 4 mg/day for women with a previous neural tube defect pregnancy or who are at high risk [20].
Rehabilitation
Rehabilitation and comprehensive medical care for patients with MMC involve a family-centered approach by a multidisciplinary team to include physiatrists, physical therapists, occupational therapists, speech-language pathologists, recreational therapists, behavioral psychologists, neuropsychologists, teachers, social workers, and child life specialists in collaboration with other physicians and surgeons. Educational needs should be assessed for implementation of an individualized education plan or 504 plans. Vocational rehabilitation should be considered as well. Educating the patient about the condition and promoting independence in self-care and maintenance of mobility status are essential to improve quality of life and to decrease preventable conditions [21]. Advancements in the medical and surgical management of children with MMC, especially after 1975, have resulted in increased life expectancy [22,23]. Survival to 16 years of age is estimated at 54% for those treated before 1975 and 85% for those treated after 1975 [22]. If a shunt is in place, there is a risk of decreased survival after 34 years of age [22]. Effective transition to adult health care providers remains an area of further research.
The general goals of the treatment plan include maintaining range of motion, strengthening, promoting functional independence through development, encouraging weight-bearing exercises, and maximizing mobility and cardiovascular fitness. It is important to establish a home exercise program. Equipment should be optimized for functional level and modified for proper fit. Wheelchair users may require pressure mapping. A reciprocating gait orthosis may be considered in higher level lesions and requires active hip flexion for use. Other equipment may include walkers, crutches, canes, standers, parapodiums, resting splints, adaptive equipment, and orthoses, depending on the functional level. Patients may cease ambulation during the second year of life as a result of weight gain and axial growth [11].
Procedures
Spasticity may be treated with onabotulinum toxin or phenol injections. Functional electrical stimulation may be beneficial. Nonoperative management of various orthopedic abnormalities may include bracing and casting. Management of neurogenic bladder may include intravesical onabotulinum toxin injections or anticholinergics.
Surgery
Children with a VPS may develop obstruction requiring shunt revision. Studies show that shunted patients, presenting with shunt malfunction, may be freed from shunt dependence by an endoscopic third ventriculostomy, instead of a shunt revision [19]. A symptomatic Chiari II malformation warrants craniovertebral decompression. Severe brainstem involvement may lead to placement of a gastrostomy tube and tracheostomy because of respiratory insufficiency and dysphagia. Tethered cord syndrome with neurologic decline may require surgical intervention for release. Surgical options for spasticity and contracture management include contracture release, tendon lengthening, joint capsule release, targeted rhizotomy, and intrathecal baclofen pump placement.
Patients who have progressive scoliosis should undergo a spinal arthrodesis or fusion. Surgical treatment of hip contractures and dislocations is controversial [24]. Surgery for hip dislocation may not improve walking ability and should mostly be considered in unilateral dislocation in lesions below L4 [24]. Surgical correction of various foot deformities is performed to allow a functional position for ambulation and to prevent pressure ulceration.
Surgical procedures for neurogenic bladder and bowel include an artificial urinary sphincter, bladder augmentation, bladder neck sling or closure, continent catheterizable stoma, vesicotomy, antegrade continence enema, and colostomy.
Potential Disease Complications
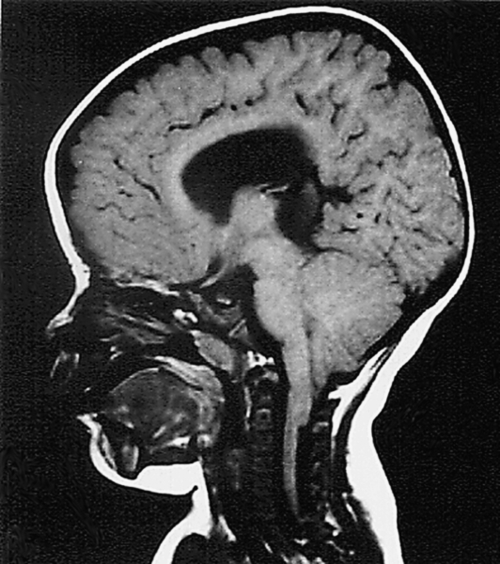
MMC is associated with Arnold-Chiari type II malformation, a posterior fossa malformation in which the caudal vermis, cerebellar tonsils, and medulla can herniate through the foramen magnum, resulting in obstructive hydrocephalus (Fig. 136.1). As a result, brainstem dysfunction can occur. In spinal dysraphisms, the spinal cord is generally anatomically fixed at the site of the anomaly after neonatal repair. Neurologic decline can occur secondary to traction, or tethering, on the cord from aggravating factors such as growth spurts, trauma, and existing deformities. An increase in spasticity can be caused by hydrocephalus, syringomyelia, tethered cord, urinary tract infection, and decubitus ulcers. Seizures may be common in children with MMC. Other complications may include supratentorial cerebral malformations and visuospatial dysfunction.
Scoliosis, kyphosis, or lordosis may result from muscle imbalances and congenital spinal deformities. Vertebral congenital abnormalities are associated with MMC and can cause worsening of spinal deformities. Orthopedic deformities of hips, knees, and ankles, including contractures and dislocations, are related to positioning and muscle imbalance. Some orthopedic deformities, such as foot deformities and early hip dislocation, may be congenital. Others, such as late hip subluxation, contractures, osteoporosis, and fragility fractures, may be acquired. Foot deformities are common in all MMC levels. Charcot joints from poor sensation, pressure, weight bearing, and repetitive microtrauma can be seen.
Neurogenic bladder may lead to chronic kidney disease from vesicoureteral reflux, recurrent urinary tract infections, and urinary incontinence or retention. Patients with neurogenic bladder and chronic use of indwelling urinary catheters or bladder augmentation procedures may be at higher risk for development of bladder cancer. Early surveillance is recommended.
Neurogenic bowel may lead to fecal impaction or incontinence. Early-onset diverticulosis and complications with megacolon have been reported as well.
Hypothalamic-pituitary dysfunction may be present because of hydrocephalus or other central nervous system abnormalities. However, underdevelopment of the lower limbs, from neurogenic atrophy, and spinal deformities present in MMC also play a role in short stature. Other reported complications include precocious puberty, cryptorchidism, sexual dysfunction, and male infertility. Fertility in women is mostly normal; however, a higher risk of having a child with MMC has been reported. Instead of 400 μg of folic acid recommended for the general female population, these patients should supplement with 4 mg of folic acid during childbearing age.
Lack of sensation, orthopedic deformities, equipment or medical device malfunction (including poor fit), and obesity may lead to pressure ulcers. Wound healing may be impaired because of autonomic dysfunction. Chronic nonhealing ulcers may require specialized medical or surgical management. Chronic occult osteomyelitis may be seen.
Obesity rates are higher among adults with spina bifida compared with the general population, especially affecting adult women [25]. This can have an impact on mobility and equipment needs.
Patients with thoracic-level lesions and hydrocephalus are at greater risk for cognitive impairment [26]. Other factors implicated in intellectual outcome include incidence of shunt revision and infection, epilepsy, and associated cerebral malformations [27]. Common findings include neuropsychological deficits in visual perception, academic difficulties in mathematics, and better verbal skills than written skills [26]. Mood disorders, such as depression, may be common in older adolescents and adults [9].
Potential Treatment Complications
VPS complications can include obstruction, infection, and intellectual delay. Other treatment complications include deterioration in neurologic status, cerebrospinal fluid leak, surgical wound dehiscence or infection, spinal cord injury, pain, osteoporotic fractures during therapy, development of latex allergy, and death.