CHAPTER 130
Heterotopic Ossification
Amanda L. Harrington, MD; Jeffrey B. Eliason, MD; William L. Bockenek, MD
Definition
Heterotopic ossification is the formation of mature, lamellar bone in nonskeletal tissue, usually in soft tissue surrounding joints [1,2]. Its exact etiology is unknown. Heterotopic ossification is commonly seen in patients with traumatic brain injury, spinal cord injury, cerebrovascular accident, burns, fractures, trauma, or muscle injuries and after total joint arthroplasty. Heterotopic ossification has also been described in medically complex patients after prolonged sedation, ventilation, critical illness, and immobilization [3,4]. In addition, heterotopic ossification has been found to be a complication after both cervical and lumbar disc replacement [5,6]. Riedel first described heterotopic ossification after trauma to the spinal cord in 1883 [7]. The term neurogenic heterotopic ossification has been commonly used for heterotopic ossification in patients with traumatic brain injury, spinal cord injury, and cerebrovascular accident [2,8]. The bone formation in heterotopic ossification differs from that in other disorders of calcium deposition in that heterotopic ossification results in encapsulated bone between muscle planes, which is not intra-articular or connected to periosteum [9].
The incidence rate reported in the literature varies from 11% to 75% in patients with severe traumatic brain injury and spinal cord injury [10,11]. Approximately 33% of patients with traumatic brain injury and spinal cord injury diagnosed with heterotopic ossification show a loss of joint range of motion; 10% to 16% progress to complete joint ankylosis [2,10]. The incidence of heterotopic bone formation after total hip arthroplasty and acetabular fracture is estimated at 16% to 53% and 18% to 90%, respectively [12].
Heterotopic ossification is both more common and more extensive in patients with severe spasticity. Increased spasticity and lower level of limb function increase the risk for development of heterotopic ossification and the rate of recurrence after surgical resection [8]. In addition, when other factors are controlled for, the incidence of heterotopic ossification increases with body mass index in patients with acetabular fracture [13]. In patients with spinal cord injury, the number of pressure ulcers and duration of time since injury are also associated with the development of heterotopic ossification [14]. When ectopic bone is discovered in patients with paraplegia, it is never found above the level of injury. Heterotopic ossification is rarely seen in flaccid limbs [8]. Interestingly, heterotopic ossification is infrequently reported in cerebral palsy or in children with anoxic brain injury [2].
The pathogenic mechanisms of heterotopic ossification are still being investigated. Whether genetic factors or local phenomena (trauma, tissue hypoxia, venous insufficiency, edema) are triggering factors, the final common pathway is inflammation and increased blood flow in the tissues [1,8,15]. Undifferentiated mesenchymal cells in connective tissue surrounding muscle or vasculature are transformed by bone morphogenetic proteins into osteoblasts, which lay down new bone matrix [4,12,13,15–17]. The scientific literature suggests that overactive bone morphogenetic protein signaling is involved in the development of both acquired heterotopic ossification and congenital heterotopic ossification, which is seen in fibrodysplasia ossificans progressiva.
The temporal relationship between injury and initiation of ossification is not clear. However, clinical signs, symptoms, and positive diagnostic test results may appear as early as 2 weeks after injury [12]. Mineralization and true bone formation are usually completed by 6 to 18 months [8]. The extent of bone formation has been described in the Brooker classification [2,11,17–19] for heterotopic ossification of the hip (Fig. 130.1). Only class III and class IV are clinically significant. Although modifications to the Brooker classification have been suggested, many clinicians continue to use this system to describe the extent of heterotopic bone formation (Table 130.1).
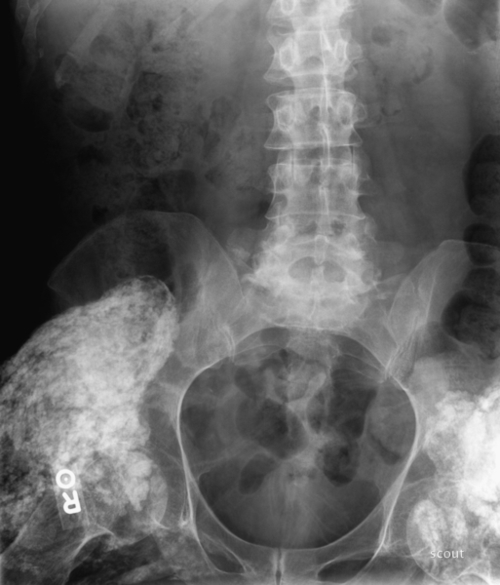
Table 130.1
Brooker Classification for Heterotopic Ossification of the Hip
| Class | Description |
| I | Islands of bone within soft tissue |
| II | Bone spurs from pelvis or proximal femur, at least 1 cm between bone surfaces |
| III | Bone spurs from pelvis or proximal femur with space between bone surfaces of less than 1 cm |
| IV | Apparent bone ankylosis of the hip (Fig. 130.1) |
Symptoms
Individuals demonstrate great variability in the initial symptoms and degree of heterotopic bone involvement. Patients are often asymptomatic [1,20]. In neurogenic heterotopic ossification, the most common symptom is pain (although this is often absent in spinal cord injury because of sensory deficits). Limitation to joint range of motion is commonly reported [8,12]. Symptoms range in onset from 2 weeks to 12 months after the inciting event, and patients may report warmth, swelling, and tenderness [2,11,21,22]. Heterotopic ossification may trigger autonomic dysreflexia in patients with spinal cord injury at or above the T6 level [23].
Physical Examination
Time at onset, location, and degree of heterotopic bone formation vary between individuals. Therefore, joints should be examined frequently in those at risk to assess range of motion and to assist in early diagnosis. The clinician should also inspect each joint for erythema and palpate for point tenderness or masses. The most common physical finding is decreased range of motion of the joint.
Distal joints of the hands and feet are almost never involved. Heterotopic ossification is typically limited to hips, knees, shoulders, and elbows [8]. In neurogenic heterotopic ossification secondary to traumatic brain injury or spinal cord injury, the hip is the most common joint affected [15]. Ossification is usually found inferomedial to the joint and is typically associated with adductor spasticity [2].
Functional Limitations
The loss of range of motion secondary to heterotopic ossification interferes with hygiene, transfers, and daily activities [22]. Pain from heterotopic ossification can be a significant cause of functional limitation.
Diagnostic Studies
The three-phase bone scan is the current “gold standard” for early detection of heterotopic ossification. It is possible to discover increased metabolic activity as early as 2 to 4 weeks after injury. This procedure involves intravenous injection of technetium Tc 99 m–labeled polyphosphate, which is known to accumulate in areas of active bone growth. The three phases are as follows [8,22] (Fig. 130.2):
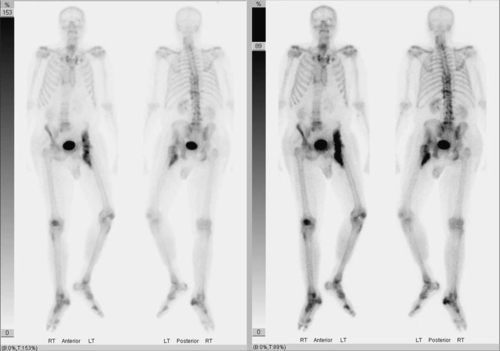
Phase 1: Dynamic blood flow occurs immediately after injection.
Phase 2: Immediate static scan detects areas of blood flow after injection.
Phase 3: Static phase involves a repeated bone scan after several hours.
A disadvantage of the three-phase bone scan is its lack of specificity. It therefore may be difficult to differentiate bone tumor, metastasis, or osteomyelitis from heterotopic ossification [8]. In addition, false-negative bone scans have been described after spinal cord injury [24].
Radiographs are readily available and economical but may not show calcification until after clinical signs and symptoms have developed or until 4 to 6 weeks after an abnormality is detected on the bone scan [11]. Heterotopic ossification has been described in three radiologic stages with variable time frames [2,25]:
1. early: increased activity on bone scan, no radiologic evidence;
2. intermediate: radiographically appearing immature bone; and
3. mature: well-developed, mature-appearing bone.
Both mature and immature bone can coexist. It is not uncommon for mature ossification to radiographically obscure immature bone [2]. Therefore, radiographic determination of the maturity of heterotopic bone is often unreliable [2,8].
Computed tomographic scanning is rarely used in detection of heterotopic ossification. There has been some evidence to suggest that magnetic resonance imaging of the knee may help to confirm an early diagnosis of heterotopic ossification [26]. Both computed tomography and magnetic resonance imaging have proved especially helpful in preoperative planning for resection to establish relationships of bone to muscle and neurovascular bundles [8,11].
Ultrasonography and angiography are not often used for the diagnosis of heterotopic ossification. However, cases have been reported in which ultrasound examination has been used to help differentiate heterotopic ossification from primary bone tumor, hematoma, or abscess. Similarly, angiography has been used to differentiate traumatic myositis ossificans from tumor and to define anatomy before surgical resection [8,12].
Many laboratory tests have been investigated for use in the diagnosis of heterotopic ossification. No one test has been found to be completely reliable with high sensitivity and specificity for heterotopic ossification. Although it is nonspecific, a widely used laboratory test for monitoring of heterotopic bone formation is the alkaline phosphatase (ALP) level. ALP has been shown to be elevated during the active bone formation of heterotopic ossification (normal range is 38 to 126 U/L). ALP levels rise as early as 2 weeks after injury, reaching a peak around 10 weeks [8]. ALP is helpful for diagnosis because it may be elevated up to 7 weeks before development of clinical symptoms [4]. Because the specificity of ALP elevation is low, it is recommended that three-phase bone scan be used to confirm suspected cases of heterotopic ossification [27].
Serum and urinary calcium concentrations, frequently nonspecific responses to trauma, do not provide any information about the ongoing ossification process and are therefore not used for diagnosis and monitoring of heterotopic ossification [15]. Other urinary markers, including hydroxyproline, deoxypyridinoline, and prostaglandin E2, have been suggested for use in detection of heterotopic ossification [3,4,8,12]. Serum intact osteocalcin, C-reactive protein, erythrocyte sedimentation rate, and creatine kinase have been used as markers for heterotopic bone formation in some studies. In patients with spinal cord injury, the inflammatory phase of heterotopic bone formation can be monitored by C-reactive protein levels, and creatine kinase can be used to estimate the severity of ossification [3,16]. Both C-reactive protein and creatine kinase levels may be used to assist in therapeutic and treatment decisions. When markers suggest active formation of heterotopic ossification, medical and rehabilitation treatments may be indicated; however, if markers suggest ongoing inflammation, surgical resection for definitive treatment may be delayed [16].
Treatment
Initial
Nonsurgical strategies for treatment of heterotopic ossification include mobilization of the joint, medications to decrease inflammation or bone formation, and prophylactic low-dose radiation therapy. These therapies and preventive strategies may be combined to improve outcome [2,20]. Spasticity and pain can be barriers to providing proper range of motion. Both should be managed appropriately to ensure that mobilization can occur.
Nonsteroidal anti-inflammatory drugs (NSAIDs) can be used not only for analgesia of the patient but also to reduce bone formation by the inhibition of prostaglandin synthetase. NSAIDs inhibit arachidonic acid metabolism, thereby inhibiting prostaglandin production, reducing inflammation, and slowing bone metabolism.
Studies have shown that indomethacin, ibuprofen, and other NSAIDs have been effective for the prevention of heterotopic ossification in total hip arthroplasty and in high-risk spinal cord–injured patients [2,8,12,16,21,28]. A recent review suggested that NSAIDs have the greatest efficacy in preventing heterotopic ossification when they are given early after a spinal cord injury [29,30]. Indomethacin has been the most widely used NSAID in prophylaxis for heterotopic ossification and is commonly used after surgical resection to prevent recurrence. Traditionally, indomethacin is started on postoperative day 1 at a dose of 25 mg three times a day for 3 weeks and is continued for up to 2 months [2,8,17]. Shorter treatment durations have been found effective for prophylaxis [31].
Etidronate disodium (EHDP), a bisphosphonate, has been shown to delay the aggregation of apatite crystals into large, calcified clusters in patients with traumatic brain injury and spinal cord injury [2,8,10]. Although EHDP does not eradicate bone that has already formed, it reduces further progression of ossification [16]. EHDP is not routinely used for prophylaxis, but some clinicians advocate prophylactic use in high-risk spinal cord–injured patients. Bisphosphonates are thought to be most effective for treatment once the diagnosis of heterotopic ossification is established, particularly if they are given early in the disease course before there is radiographic evidence of bone formation [29,30]. The treatment recommendation for established heterotopic ossification in spinal cord injury is 20 mg/kg daily orally for 6 months [32]. For patients with traumatic brain injury, it has been suggested that oral treatment be initiated at 20 mg/kg per day for 3 months, then reduced to 10 mg/kg per day for 3 months [10].
Observational evidence suggests that warfarin is associated with a reduced incidence of heterotopic ossification in patients with spinal cord injury; however, it is not used solely for prevention of heterotopic ossification, in part because of the side effect profile [30]. After elective total hip arthroplasty, aspirin thromboprophylaxis has been shown to reduce prevalence of heterotopic ossification [33]. At the molecular level, treatments aimed at the reduction of bone morphogenetic protein signaling may help reduce formation of heterotopic bone. Although not used in clinical treatment at this time, bone morphogenetic protein receptor antagonists, retinoic acid receptor agonists, and free radical scavengers are current research targets [34].
Rehabilitation
Comprehensive physical and occupational therapy can be considered both preventive and a first-line treatment modality. Several studies have shown that early range of motion exercises are beneficial in the prevention and treatment of heterotopic ossification [9]. Some have suggested that joint manipulation may increase the inflammatory response, thereby increasing production of heterotopic bone, but there is no objective evidence to show this to be true [2]. Joint manipulation may not alter bone formation, but it can prevent soft tissue contractures and maintain functional range of motion. Because physical therapy is often difficult secondary to pain or spasticity, forceful manipulation under anesthesia has been tried but is not a standard treatment modality. Exercises to maintain joint range of motion are still considered the mainstay for prevention of heterotopic ossification [2,21]. Continuous passive motion machines are beneficial in increasing range of motion after surgical resection of heterotopic bone [4,12].
Procedures
Radiation therapy has been shown to help prevent heterotopic ossification after total hip arthroplasty and after resection of mature heterotopic bone [1,27]. It is the only therapy for heterotopic ossification that acts locally [8]. Although opinions differ as to the most effective treatment method, single doses of 5 to 8 Gy are more frequently used than fractionated doses, and similar results are found when preoperative and postoperative treatments are compared [1]. Radiation therapy has been used successfully after heterotopic bone resection in patients with traumatic brain injury and spinal cord injury to help prevent recurrence and for local treatment of heterotopic ossification in spinal cord injury [2,16,17,35]. Pulse low-intensity electromagnetic therapy uses magnetic fields to improve blood flow to the target area and has been shown in one study to prevent the formation of heterotopic ossification after spinal cord injury [30,36].
Surgery
Although heterotopic ossification may recur after surgical resection, it remains the only definitive treatment for mature heterotopic bone. In fact, surgical excision is the most common treatment of heterotopic ossification after traumatic brain injury [29]. Surgical indications include joint immobility causing difficulty in activities of daily living, ankylosed joints leading to pressure ulcers or skin breakdown, and conditions in which heterotopic ossification contributes to focal peripheral neuropathy [2]. Preoperative planning often requires three-dimensional computed tomography and magnetic resonance imaging to assess the relationship between heterotopic bone and neurovascular structures at risk. Careful dissection with isolation of neurovascular bundles reduces risk of morbidity associated with hemorrhage, sepsis, or repeated ankylosis [2]. Functional range of motion can typically be reached with a wedge resection of the ectopic bone [2].
Controversy exists as to the timing of the procedure because bone maturity is difficult to assess. It has been shown that immature heterotopic bone has a greater incidence of recurrence [16,27]. Traditionally, three-phase bone scan and ALP levels have been used to monitor bone maturity. However, because both may remain abnormal indefinitely, many clinicians no longer wait for normalization of bone scans and ALP levels to proceed with resection [16]. Still, it is not uncommon for surgery to be delayed for up to 2 to 2.5 years in patients with traumatic brain injury or spinal cord injury; however, the suggested optimal timing is 12 to 18 months [29,37]. After heterotopic bone resection, radiation therapy and NSAIDs are often used to prevent recurrence [2,8].
Potential Disease Complications
Heterotopic bone deposition impairs normal joint range of motion and causes secondary soft tissue contractures, which involve surrounding skin, muscles, ligaments, and neurovascular bundles [2]. The resulting restrictive position predisposes the patient to the development of pressure ulcers and subsequent infections. Direct pressure or chronic spasticity can cause nerve ischemia and compression, resulting in focal peripheral neuropathy [2]. Vascular compression, deep venous thrombosis, and lymphedema may also result. Decreased range of motion predisposes to osteoporosis and subsequent pathologic fracture during transfer or lifting of the patient [2].
Potential Treatment Complications
NSAIDs have well-known side effects that most commonly affect the gastric, hepatic, and renal systems. There is also a risk of defective bone-prosthesis union and poor bone healing in orthopedic populations when NSAIDs are used for the prevention of heterotopic ossification [8,31].
EHDP is generally a safe method for treatment of heterotopic ossification. The most common side effects are nausea and diarrhea. Twice-daily divided dosing of EHDP helps alleviate these symptoms. EHDP also carries a potential risk of bone fracture secondary to osteomalacia; if it is withdrawn after a short treatment duration, a rebound ossification secondary to prolonged osteoclast inhibition may result [10,32].
Radiation therapy is rarely associated with malignant neoplasia and does not disrupt wound healing, provided wounds are not in the field of irradiation [8,38]. In orthopedic populations after total hip arthroplasty, increased rates of trochanteric nonunion have been described with both high-dose fractionated and single-dose protocols [31,38].
Surgical complications, which carry a high morbidity and are not uncommon, include hemorrhage, sepsis, wound infection, repeated ankylosis, and heterotopic bone recurrence [8,27]. Chronic pain and recurrent contracture may also result. The distorted anatomy in heterotopic ossification makes dissection visibility difficult, endangering neurovascular structures [2]. Postoperative blood loss requiring transfusion is not uncommon despite good surgical hemostasis [2].