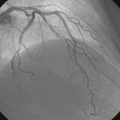
CASE 13 Complex Coronary Disease
Case presentation
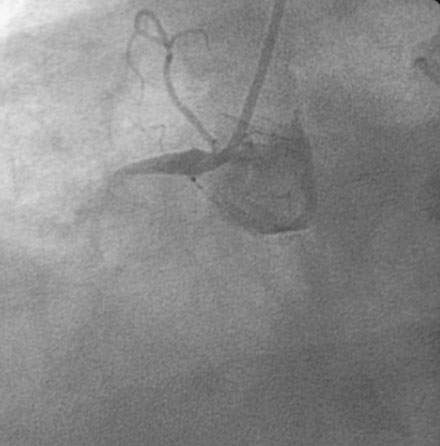
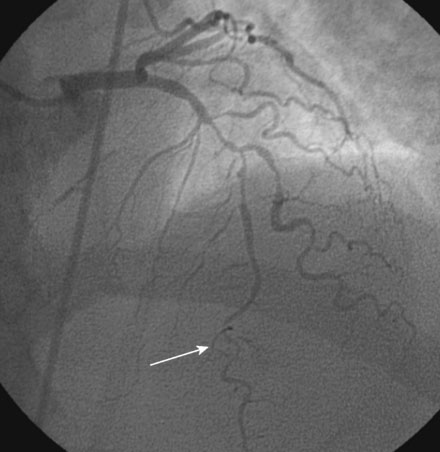
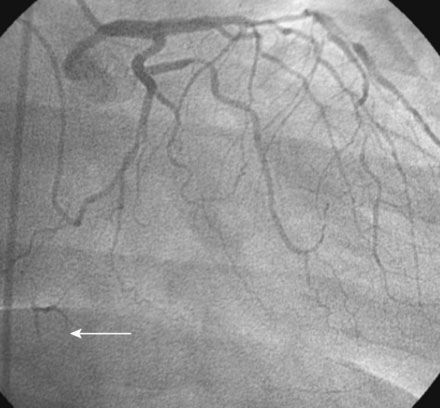
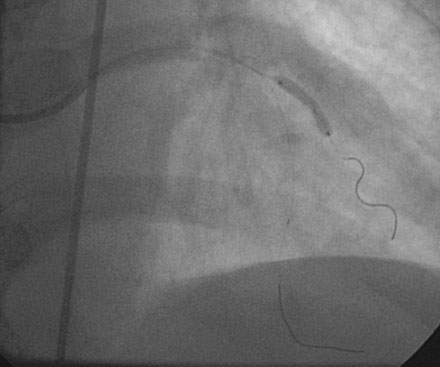
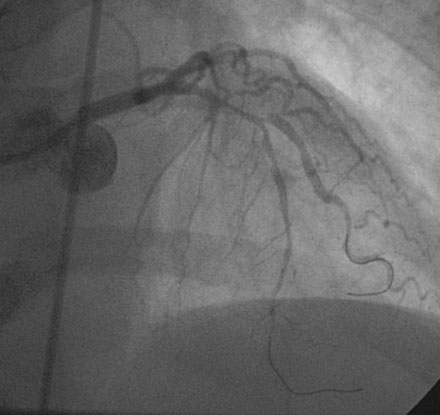
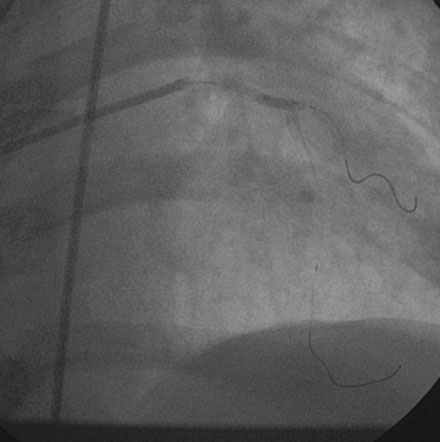
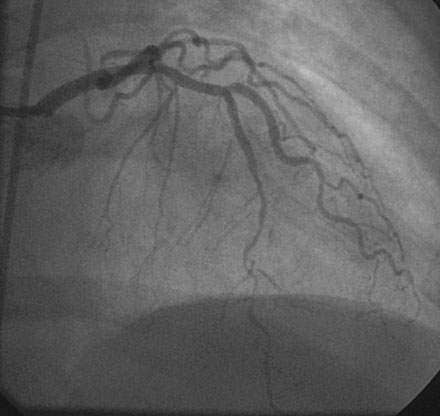
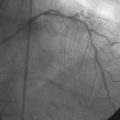
The onset of severe substernal chest pain awakened a 50-year-old diabetic woman without prior cardiac history from her sleep. The pain waxed and waned all morning until she finally presented to the emergency room 9 hours later. She was found to have an inferior wall ST-segment elevation myocardial infarction and was taken emergently to the cardiac catheterization laboratory. As suspected, based on the electrocardiographic changes, the right coronary artery was completely occluded (Figure 13-1); however, the operator was surprised to find severe and complex disease affecting the left anterior descending (LAD) and diagonal arteries (Figure 13-2 and Video 13-1) and moderate disease in the left circumflex artery (Figure 13-3 and Video 13-2). Lush collaterals filled the distal right coronary artery from the left coronary injections.
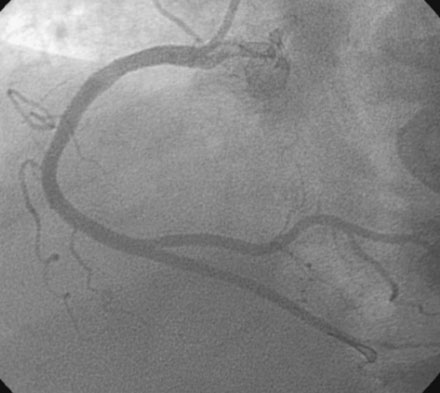
With the goal of achieving rapid reperfusion of the infarct-related artery, the operator decided to open the right coronary artery. This was promptly and successfully accomplished with balloon angioplasty followed by deployment of two 4.5 mm diameter bare-metal stents. The operator obtained an excellent luminal result and TIMI-3 flow (Figure 13-4 and Video 13-3).
Cardiac catheterization
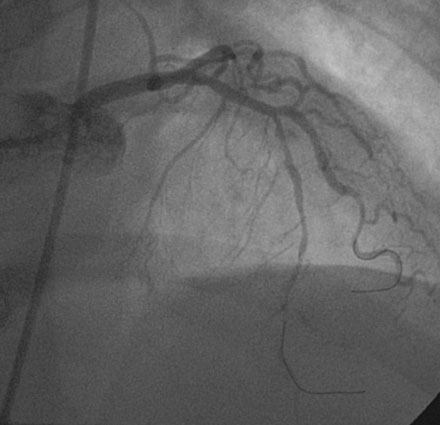
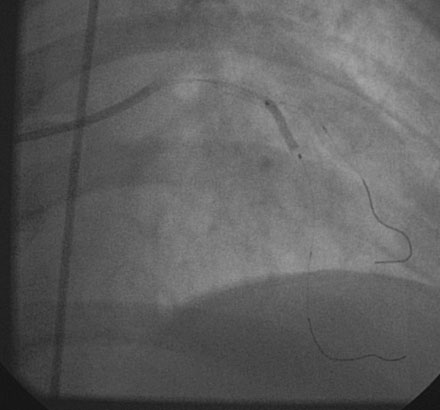
She returned to the cardiac catheterization laboratory for an elective complex intervention of the LAD and diagonal. After achieving therapeutic anticoagulation, the operator advanced 0.014 inch floppy-tipped guidewires in both the LAD and diagonal and performed balloon angioplasty in the LAD with a 2.0 mm diameter by 20 mm long compliant balloon (Figure 13-5). The first diagonal was then treated with two everolimus-eluting stents; the first one (2.5 mm diameter by 14 mm long) placed in the mid-diagonal (Figures 13-6, 13-7) and a second one (also 2.5 mm diameter by 14 mm long) placed in the diagonal but extended into the proximal part of the LAD (Figures 13-8, 13-9 and Video 13-4). The operator withdrew the LAD wire to the guide catheter because the wire was trapped behind the latter stent. This guidewire was then repositioned in the distal LAD through the stent struts. Balloon angioplasty was performed and a single 2.5 mm diameter by 18 mm long everolimus-eluting stent positioned in the LAD using a “T” configuration just distal to the diagonal bifurcation (Figure 13-10). Following the deployment of this stent, the ostium of the diagonal was postdilated with a 2.5 mm diameter noncompliant balloon (Figure 13-11). The final angiographic result, shown in Figure 13-12 and Video 13-5, appeared satisfactory to the operator.
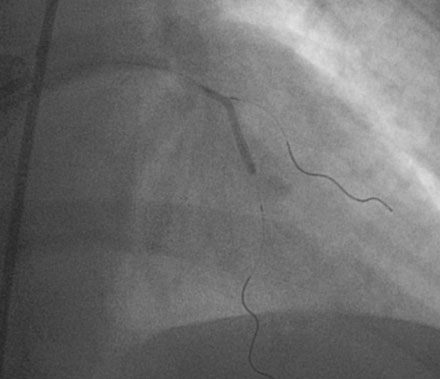
FIGURE 13-5 First, the LAD was treated with balloon angioplasty after a guidewire was positioned in the diagonal branch.
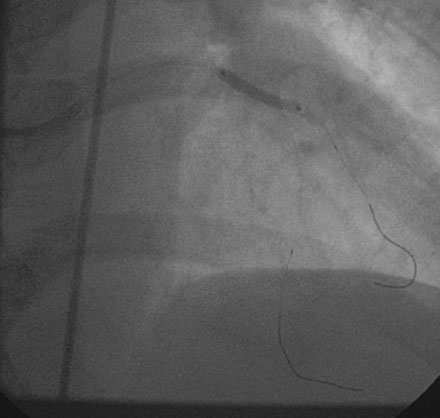
FIGURE 13-8 The proximal end of the diagonal was treated with another stent that extended into the LAD.
Discussion
First, it was not clear if and when revascularization of this other disease was indicated. Multivessel coronary disease is found in up to 60% of patients undergoing emergent cardiac catheterization for ST-segment elevation myocardial infarction.1–3 The optimal management of these “bystander” lesions is unclear and controversial. Because of the concern about the risks of intervening upon a non-infarct–related artery when there is acute infarction in another vascular territory, current practice guidelines discourage the performance of an intervention on obstructive lesions in the non-infarct–related artery at the time of acute infarct angioplasty. It is not known if these lesions should be treated during the hospital phase while the patient is still recovering from the infarction, in the weeks to months following the event when the infarction has healed, or deferred indefinitely unless there is objective evidence of ischemia. In this case, the complexity of the disease led to the decision to pursue medical therapy as the initial strategy. During follow-up, her ongoing symptoms despite appropriate medical therapy caused her physicians to recommend revascularization.
Finally, once the option of percutaneous revascularization was chosen, the operator was faced with several crucial technical decisions regarding treatment of the complex disease. The small caliber of the involved arteries (<2.5 mm diameter) is associated with an increased risk of procedural complication and an increased risk for restenosis. Bare-metal stents are superior to balloon angioplasty in small arteries but are associated with at least a 25% restenosis rate4; this rate approaches 50% in diabetics. Drug-eluting stents reduce the rate of restenosis in small arteries compared to bare-metal stents.5,6 In the present case, it was not clear to the operator that the involved arteries were large enough to accommodate a drug-eluting stent; fortunately, 2.5 mm diameter drug-eluting stents were successfully placed without creating distal edge dissections or other complications. Management of the bifurcation represented another significant technical challenge. Because of the diffuse disease in the distal LAD, the operator decided to prioritize the large diagonal branch and considered the LAD the “side branch” vessel. Choosing a relatively simple approach to stenting the bifurcation and avoiding overlapping stents at the bifurcation in this diabetic patient with small-caliber vessels likely contributed to the excellent outcome.
1 Muller D.W., Topol E.J., Ellis S.G., et al. Multivessel coronary artery disease: a key predictor of short-term prognosis after reperfusion therapy for acute myocardial infarction. Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. Am Heart J. 1991;121(4 Pt 1):1042-1049.
2 Jaski B.E., Cohen J.D., Trausch J., et al. Outcome of urgent percutaneous transluminal coronary angioplasty in acute myocardial infarction: comparison of single-vessel versus multivessel coronary artery disease. Am Heart J. 1992;124:1427-1433.
3 Kahn J.K., Rutherford B.D., McConahay D.R., et al. Results of primary angioplasty for acute myocardial infarction in patients with multivessel coronary artery disease. J Am Coll Cardiol. 1990;16:1089-1096.
4 Moreno R., Fernandez C., Alfonso F., Hernandez R., Perez-Vizcayno M.J., Escaned J., Sabate M., Banuelos C., Angiolillo D.J., Azcona L., Macaya C. Coronary stenting versus balloon angioplasty in small vessels: a meta analysis from 11 randomized studies. J Am Coll Cardiol. 2004;43:1964-1972.
5 Menozzi A., Solinas E., Ortolani P., Repetto A., Saia F., Piovaccari G., Manari A., Magagnini E., Vignali L., Bonizzoni E., Merlini P.A., Cavallini C., Ardissino D. SES-SMART Investigators: Twenty-four months of clinical outcomes of sirolimus-eluting stents for the treatment of small coronary arteries: the long-term SES-SMART clinical study. Eur Heart J. 2009;30:2095-2101.
6 Hermiller J.B., Fergus T., Pierson W., Su X., Sood P., Sudhir K., Stone G.W. Clinical and angiographic comparison of everolimus-eluting and paclitaxel-eluting stents in small coronary arteries: a post hoc analysis of the SPIRIT III randomized trial. Am Heart J. 2009;158:1005-1010.