CHAPTER 129
Dysphagia
Koichiro Matsuo, DDS, PhD; Jeffrey B. Palmer, MD
Definition
Dysphagia generally refers to any difficulty with swallowing, including occult or asymptomatic impairments. It is a common problem, affecting one third to one half of all stroke patients [1] and about one sixth of elderly individuals [2]. It is frequent in head and neck cancer, traumatic brain injury, degenerative disorders of the nervous system, gastroesophageal reflux disease, and inflammatory muscle disease (Table 129.1). Dysphagia is classified according to the location of the problem as oropharyngeal (localized to the oral cavity or pharynx, not just the oropharynx) or esophageal. It may also be classified as mechanical (due to a structural lesion of the foodway) or functional (caused by a physiologic abnormality of foodway function) [3].
Table 129.1
Selected Causes of Oral and Pharyngeal Dysphagia
| Neurologic Disorders and Stroke | Structural Lesions | Connective Tissue Diseases |
| Cerebral infarction Brainstem infarction Intracranial hemorrhage Parkinson disease Multiple sclerosis Amyotrophic lateral sclerosis Poliomyelitis Myasthenia gravis Dementias |
Thyromegaly Cervical hyperostosis Congenital web Zenker diverticulum Caustic ingestion Neoplasm Post–ablative surgery Radiation fibrosis |
Polymyositis Muscular dystrophy Psychiatric disorders Psychogenic dysphagia |
Sudden onset is suggestive of stroke. Concomitant limb weakness suggests a neurologic or neuromuscular disorder. Medication-induced dysphagia is commonly overlooked. Medications that impair level of consciousness (such as sedatives and tranquilizers), have anticholinergic effects (tricyclics, propantheline), or can damage mucous membranes (nonsteroidal anti-inflammatory drugs, aspirin, quinidine) may also cause dysphagia [4].
Symptoms
The most common symptoms of dysphagia are coughing or choking during eating [5] and the sensation of food sticking in the throat or chest. Some of the many symptoms and signs of dysphagia are listed in Table 129.2. A history of drooling, significant weight loss, or recurrent pneumonia suggests that the dysphagia is severe. The history is most useful for identification of esophageal dysphagia; the complaint of food sticking in the chest is usually associated with an esophageal disorder. In contrast, the complaint of food sticking in the throat has little localizing value and is often caused by an esophageal disorder. Coughing and choking during swallowing suggest an oropharyngeal origin and may be precipitated by aspiration (penetration of material through the vocal folds and into the trachea). However, some patients have impaired cough reflexes, resulting in silent aspiration (without cough) [5,6]. Silent aspiration occurs in 28% to 94% of people with dysphagia, depending on the population of patients [7,8]. Patients with neurologic disorder have a higher incidence of silent aspiration. Pain on swallowing (odynophagia) may occur transiently in pharyngitis, but persistent pain is unusual and is suggestive of neoplasia. Heartburn is a nonspecific complaint that is usually not associated with swallowing but occurs after meals. Heartburn may occur in gastroesophageal reflux disease, but a more specific symptom of gastroesophageal reflux disease is regurgitation of sour or bitter-tasting material into the throat after eating.
Physical Examination
An examination of the oral cavity and neck may identify structural abnormalities, weakness, or sensory deficits. The finding of dysarthria (abnormal articulation of speech) or dysphonia (abnormal voice quality) is often associated with oropharyngeal dysphagia. However, the examination is primarily useful for finding evidence of underlying neurologic, neuromuscular, or connective tissue disease. The examination should always include trial swallows of water [9–11]. During the swallow, there should be prompt elevation of the hyoid bone and larynx. Changes in voice quality and spontaneous coughing after swallowing suggest pharyngeal dysfunction. The history and physical examination are limited in their ability to detect and to characterize dysphagia, so instrumental studies are usually necessary [12].
Neurologic examination is important in the evaluation of dysphagic individuals because neurologic disorders commonly cause dysphagia. Disorders of either upper or lower motor neurons may produce dysphagia. The findings of atrophy or fasciculations of the tongue or palate suggest lower motor neuron dysfunction of the brainstem motor nuclei. In contrast to the prevailing wisdom, the gag reflex is not strongly predictive of the ability to swallow. It may be absent in normal individuals and normal in individuals with severe dysphagia and aspiration [13].
Functional Limitations
Functional limitations depend on the nature and severity of the dysphagia. Many individuals modify their diets to eliminate foods that are difficult to swallow; others require special postures or respiratory maneuvers. Some require inordinate amounts of time to consume a meal. In severe cases, tube feeding is necessary. These alterations in the ability to eat a meal can have a profound effect on psychological and social function [14]. Interaction with family and friends often centers on mealtime—family dinners, “going out” for a drink or for dinner, “coming over” for a snack or for dessert. Difficulty in eating a meal may disrupt relationships and result in social isolation. Some patients may require supervision during meals or feel unsafe when they eat alone, causing further disruption of social and vocational function.
Diagnostic Testing
Because the mechanics of swallowing are largely invisible to the naked eye, diagnostic studies are commonly needed. The sine qua non for diagnosis of oropharyngeal swallowing disorders is the videofluorographic swallowing study (VFSS) [15]. In this test, the patient eats and drinks a variety of solids and liquids combined with barium while images are recorded with videofluorography (x-ray videotaping). The VFSS is usually performed jointly by a physician (physiatrist or radiologist) and a speech-language pathologist. A unique benefit of the VFSS is that therapeutic techniques (such as modification of food consistency, body position, or respiration) can be tested and their effects on swallowing observed during the study. A routine barium swallow study is frequently sufficient if the problem is clearly esophageal.
If a VFSS cannot be performed because of the physical limitation of the patient, the fiberoptic endoscopic evaluation of swallowing is useful to visualize the anatomy of the pharynx and larynx and vocal fold function during eating with no x-ray exposure [16]. It is also highly sensitive for detection of aspiration [8]; but it does not visualize essential aspects of swallowing, such as the oral and esophageal stages of swallowing, or critical events of pharyngeal swallowing including opening of the upper esophageal sphincter, elevation of the larynx, and contraction of the pharynx.
In cases of esophageal dysphagia, esophagoscopy is frequently necessary to detect mucosal lesions or masses. Biopsy is indicated when mucosal abnormalities are detected. Manometry is useful for detection and characterization of motor disorders of the esophagus and is sometimes performed on the pharynx as well. Electromyography is indicated when neuromuscular disease is suspected and is useful for detection of lower motor neuron dysfunction of the larynx and pharynx.
Treatment
Initial
The treatment of dysphagia depends on its causes and mechanism. Common treatments are listed in Table 129.3. Whenever possible, initial treatment should be directed at the underlying disease process; for example, steroids for inflammatory muscle disease. Esophageal dysphagia necessitates evaluation and treatment by a gastroenterologist. When no therapy exists for the underlying disease or the therapy is ineffective or contraindicated, rehabilitative strategies are appropriate. Patients and their family members are encouraged to learn the Heimlich maneuver; this is important because airway obstruction is potentially fatal.
Rehabilitation
Many patients benefit from a structured swallowing therapy provided by a speech-language pathologist, including instruction and supervision about diet, compensatory maneuvers, and exercise [17]. The goals of therapy are to reduce aspiration, to improve the ability to eat and drink, and to optimize nutritional status. Therapy is individualized according to the patient’s specific anatomic and structural abnormalities and the initial responses to treatment trials observed at the bedside or during the VFSS [18]. A fundamental principle of rehabilitation is that the best therapy for any activity is the specific activity itself; swallowing is generally the best therapy for swallowing disorders, so the rehabilitation evaluation is directed at identifying the circumstances for safe and effective swallowing for each individual patient.
Diet modification is a common treatment of dysphagia [19]. Patients vary in ability to swallow thin and thick liquids, and that determination is usually best made by VFSS. A patient can usually receive adequate oral hydration with either thin (e.g., water or apple juice) or nectar-thick liquids (e.g., apricot nectar, tomato juice). Rarely, a patient may be limited to honey-thick or pudding consistency if thin and thick liquids are freely aspirated. Most patients with significant dysphagia are unable to safely eat meats or similarly tough-textured foods and require a mechanical soft diet. A pureed diet is recommended for patients who exhibit oral preparatory phase difficulties, pocket food in the buccal recesses (between the teeth and the cheek), or have significant pharyngeal retention after swallowing with chewed solid foods. Maintenance of oral feeding often requires compensatory techniques to reduce aspiration or to improve pharyngeal clearance. A variety of behavioral techniques are used, including modifications of posture, head position (Fig. 129.1), and respiration, as well as specific swallow maneuvers [20].
Exercise therapy for dysphagia is indicated when the problem is related to weakness of the muscles of swallowing [21]. The choice of exercises must be individualized according to the physiologic assessment. The full range of exercises is beyond the scope of this chapter, but several examples illustrate the principles.
• Tongue weakness can be treated with lingual resistance exercise [22].
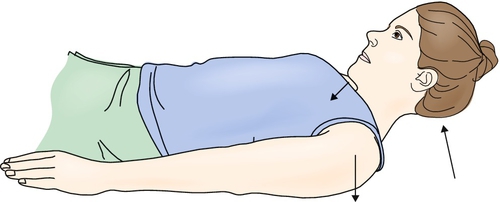
• Strengthening of the anterior suprahyoid muscles is useful when the upper esophageal sphincter opens poorly. Flexing the neck against gravity while lying supine can strengthen these muscles (Fig. 129.2) [23,24].
• Vocal fold adduction exercises may be useful in cases of aspiration due to weakness of these muscles. These exercises are done several times a day whenever possible.
Procedures
VFSS functions as both a diagnostic and a therapeutic procedure for dysphagia, especially oropharyngeal dysphagia, because it can be used to test the effectiveness of modifying food consistency and other compensatory techniques [25]. Endoscopy with dilation of the esophagus is often indicated in cases of partial esophageal obstruction due to stricture or web. Dilation is also appropriate in stenosis of the upper esophageal sphincter. Endoscopy can be used for biofeedback, especially to demonstrate movements of the larynx during swallowing maneuvers. Electromyography is also used for biofeedback. Activities of the infrahyoid and suprahyoid muscles are recorded with surface electrodes during swallowing therapy. Biofeedback itself is not a dysphagia therapy but can be a useful adjunct to therapy. Surface electrical stimulation on the submental or anterior cervical muscles is a new treatment of dysphagia, but the evidence for its efficacy is weak [26].
Surgery
Surgery is rarely indicated in the care of patients with oral or pharyngeal dysphagia. The most common procedure for pharyngeal dysphagia is cricopharyngeal myotomy, during which the upper esophageal sphincter is disrupted to reduce the resistance of the pharyngeal outflow tract. However, the effectiveness of myotomy is highly controversial [27]. Esophagectomy may be necessary in case of esophageal cancer or obstructive strictures. Feeding gastrostomy (usually percutaneous endoscopic gastrostomy) is indicated when the severity of the dysphagia makes it impossible for adequate alimentation or hydration to be obtained orally, although intravenous hydration or nasogastric tube feedings may be sufficient on a time-limited basis [28]. Orogastric tube feedings have been used successfully by patients who have absent gag reflexes and can tolerate intermittent oral catheterization. Laryngectomy or surgical closure of the larynx is rarely performed in cases of recurrent, intractable aspiration pneumonia.
Potential Disease Complications
Severe dysphagia may result in aspiration pneumonia [29], airway obstruction, bronchiectasis, dehydration, or starvation and is potentially fatal. Severe dysphagia often causes social isolation because of the inability to consume a meal in the usual manner. This can lead to clinical depression. Suicide has been reported.
Potential Treatment Complications
The VFSS is safe and well tolerated. Prescription of a modified diet often means the substitution of thick for thin liquids. Some patients find these unpalatable and reduce fluid intake to the point of dehydration and malnutrition. Failure to reevaluate patients in a timely manner can lead to unnecessary prolongation of dietary restrictions, increasing the risk of malnutrition and adverse psychological effects of dysphagia. Dilation of the esophagus or sphincters can result in perforation, but this complication is uncommon. Percutaneous endoscopic gastrostomy can have direct or indirect sequelae. Direct sequelae, such as pain, infection, and obstruction of the feeding tube, are common. Percutaneous endoscopic gastrostomy tube feeding can promote aspiration pneumonia in individuals with severe gastroesophageal reflux disease.