CHAPTER 123
Cancer-Related Fatigue
Definition
Cancer causes one in four deaths in the United States and is a major public health problem worldwide. Projections for 2013 were that more than 1,660,290 new cancer cases would be diagnosed and that more than 580,305 patients would die of cancer [1]. Cancers vary widely in prognosis, natural history, management, treatment responsiveness, adverse sequelae, and associated physical impairments. As a consequence, cancer rehabilitation is not amenable to one-size-fits-all treatment approaches. Physical impairments vary with the location and stage of cancer as well as by the type of treatment. For example, cancers of the head and neck may require neck dissection and irradiation. Common sequelae include shoulder dysfunction and fibrosis of cervical soft tissue. In contrast, primary breast cancer treatment may cause myofascial pain and upper extremity swelling. The reader is referred to chapters specific to these conditions and anatomic locations (e.g., scapular winging, lymphedema).
A more uniform approach can be applied to the management of cancer-related fatigue (CRF). The National Comprehensive Cancer Network defines CRF as a distressing, persistent, subjective sense of physical, emotional, or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning [2]. CRF is the most common cancer-associated symptom among the more than 12 million cancer survivors in the United States [3]. Its effects can be overwhelming and, depending on the nature and treatment of the cancer, can limit the lives of as many as 70% of disease-free survivors [4–7]. Whereas severe post-treatment CRF is particularly common among lung cancer survivors [8,9], high levels of persistent CRF are also reported among the survivors of most malignant neoplasms, including but not limited to those of the breast, brain, head, and neck and childhood malignant neoplasms [7–21]. CRF, even at it mildest levels, affects all health-related quality of life domains [4,22–24]. On a vocational level, CRF intensity predicts failure to return to work [4,25–27], and it is estimated that 1 million cancer survivors may be receiving disability payments as a consequence of its persistence [28]. On a general level, multiple investigators have found that increased CRF is considered an important unmet care need by patients [29–31] and explains a significant proportion of performance and mobility degradations [32–38] across a wide range of cancers [39–42]. Not surprisingly, the indirect costs associated with patients with CRF are increased, with research showing that patients with CRF visit general practitioners 50%, specialists 350%, physical therapists 130%, and complementary caregivers 520% more often than those without the condition do [4,43,44]. Fortunately, effective CRF treatment has been shown to improve vocational productivity and performance of activities of daily living while reducing family stress and caregiver burden [45].
Symptoms
Before formal evaluation is undertaken, it is important to establish the patient’s current place on the cancer trajectory, which influences all elements of the history and physical examination. Three important distinctions must be made:
1. Is the patient receiving active treatment?
2. Does the patient have residual cancer?
3. Is the patient deemed curable?
The willingness and capacity of patients to engage in the rehabilitation process will be reflected in the answers to these questions.
A characteristic constellation of symptoms should not be anticipated in patients’ reporting of CRF. As emphasized before, patients’ neoplasms, treatment regimens, and disease trajectories are variable. CRF may therefore be manifested differently, contingent on the particulars of each case. Patients’ descriptions of their fatigue may be inconsistent and, at times, puzzling to clinicians. Any of the following subjective complaints should raise concern about possible CRF: weakness (generalized or proximal), dyspnea on exertion, orthostatic hypotension, sedation, hypersomnolence, exertional intolerance, or cognitive compromise (e.g., attention or concentration deficits, short-term memory dysfunction). Patients may report the sensation that their legs are leaden or that they are walking through water. Validated self-report fatigue scales (e.g., Brief Fatigue Inventory, Functional Assessment of Cancer Treatment—Fatigue, Profile of Mood States) can be exceedingly useful to quantify severity of symptoms and to monitor treatment response [46]. A brief screen for depression or other mood disorders is essential, and validated screening tools are widely available.
Patients’ cancer histories warrant attention, including prior and ongoing radiation therapy and chemotherapy as well as any surgical procedures. Awareness of a patient’s primary cancer will shift the focus toward particular causes of symptoms. Information comparable with that solicited through a good pain history should be elicited for fatigue: acuity of fatigue onset, activity- or treatment-related precipitants, diurnal fluctuation, associated symptoms (e.g., pain, nausea), progressive worsening or improvement, exacerbating and alleviating factors, and prior treatments and degree of response. Questions about sleep patterns, sleep hygiene, and daytime napping are useful. Reports suggest that frequent daytime napping may actually worsen fatigue [47].
The extent to which fatigue limits vocational, avocational, and familial pursuits as well as autonomous mobility and self-care should be comprehensively reviewed. Because fatigue most commonly interferes with activities requiring stamina and exertional tolerance, changes in a patient’s comfortable walking distance, duration of physical activity, and willingness to climb stairs will help characterize the impact of fatigue.
Physical Examination
Special tests are rarely indicated on physical examination. Rather, clinicians should perform a comprehensive evaluation with emphasis on musculoskeletal and neurologic elements. Assessment of range of motion, gait (including tandem), static and dynamic balance, and ability to squat repetitively may identify potential contributing factors amenable to therapeutic exercise. Examination may reveal evidence of congestive heart failure or pulmonary compromise. Signs of hypothyroidism should be sought, particularly in patients irradiated for head and neck cancers. For patients without evidence of cancer, the neurologic examination findings should be normal beyond chemotherapy-related peripheral neuropathy. Weakness in proximal hip and shoulder musculature suggests steroid myopathy. Identification of new neurologic deficits should trigger evaluation for malignant progression or emerging treatment toxicity. The mental status examination may reveal evidence of compromised arousal, attention, memory, or concentration, particularly in patients who have received whole-brain radiation therapy or intrathecal chemotherapy.
Functional Limitations
Although fatigue is rarely so severe that it undermines basic mobility or performance of activities of daily living apart from the palliative context [48], evaluation of these functional domains is integral to comprehensive evaluation. Severe functional compromise may be a red flag, contingent on the clinical context that triggers evaluation for significant comorbidity or recurrent cancer. Ambulation for moderate distances may produce limiting dyspnea in patients with cancer-related cardiac or pulmonary dysfunction. Patients with steroid myopathy or generalized muscle weakness may have difficulty in rising from low surfaces, such as a toilet, soft chair, or car seat. These patients may also demonstrate decreased ability to independently complete their activities of daily living in a reasonable time frame. As suggested previously, many patients describe generalized heaviness of the limbs and global decrements in activity level without precise functional limitations.
Dysfunction in social, vocational, psychological, and sexual domains may be present. Patients should be questioned about compromised social interactions, sleep, and intimacy as well as work-related and leisure pursuits. Many patients abandon their avocational activities as a consequence of fatigue, with the potential for isolation and secondary depression. Patients with cognitive deficits related to radiation therapy or chemotherapy may experience difficulty in maintaining their vocational productivity. Financial and domestic management skills may be compromised as well.
Diagnostic Studies
Diagnostic tests should be informed by patients’ symptoms and findings on clinical examination. Dyspnea should be assessed with pulse oximetry during activity, chest radiography, and electrocardiography. Patients who exhibit severe dyspnea with minimal activity may have pulmonary fibrosis. Definitive diagnosis may require computed tomographic scanning. Positron emission tomography may help distinguish fibrosis from cancer involving the lung parenchyma. Patients with cancer are at an elevated risk for venous thrombosis; therefore, a venous duplex study and possibly a ventilation-perfusion scan should be considered for persistent shortness of breath. Patients who have received doxorubicin (Adriamycin) or trastuzumab (Herceptin) should be evaluated with a multigated acquisition scan to rule out possible chemotherapy-related cardiac toxicity. Most patients will have undergone multigated acquisition screening before the administration of chemotherapy. The results of baseline tests can be compared with new evaluations for evidence of deterioration. Pericardial effusions may be a consequence of malignant spread or radiation-induced irritation or occur as a paraneoplastic phenomenon. An echocardiogram should be obtained for patients with a suggestive history and physical examination. Patients reporting insomnia or a failure to feel rested after a night’s sleep may benefit from a sleep study to rule out sleep apnea or related disorders.
Serologic evaluation may include thyroid-stimulating hormone concentration (to screen for thyroid myopathy in patients who have received irradiation to the anterior neck), calcium concentration, electrolyte values (Addison disease may occur with adrenal metastases or irradiation), hemoglobin concentration, and hematocrit. Hypercalcemia or persistent mechanical pain should be evaluated with a bone scan or plain films. Multiple myeloma and malignant neoplasms producing lytic metastases may fail to generate an abnormal bone scan despite diffuse skeletal involvement. Blood levels of centrally acting medications (e.g., tricyclic antidepressants, anticonvulsants) may warrant assessment in patients who describe fatigue with a significant cognitive dimension.
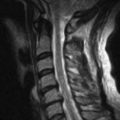
For patients with focal neurologic deficits, imaging of those portions of the neural axis implicated on physical examination should be performed. Magnetic resonance images should be obtained with gadolinium. Steroids administered in conjunction with chemotherapy may be of sufficient doses to cause myopathy. Electrodiagnostic studies can rule out alternative, treatable sources of neurologic compromise.
Patients complaining of generalized cognitive dysfunction may benefit from neuropsychological evaluation. Cognitive deficits have been detected after chemotherapy [49]. Multifocal brain metastases may be manifested with a global decrement in mental acuity and capacity to attend. Enhanced computed tomographic scanning of the head may be warranted when there is a high clinical probability of brain metastases (e.g., patients with melanoma, breast or lung cancers).
Screening for depression, anxiety, and other psychological distress is integral to the CRF evaluation. The Patient Health Questionnaire (PHQ-9) is a brief and valid screen for use in cancer populations. The PHQ-9 distinguishes both the presence and severity of depression [50]. The Generalized Anxiety Disorder (GAD-7) screen is another valid measure with low respondent burden that can be facilely integrated into routine history taking [51].
Treatment
Initial
It is important to address any remediable endocrine, hematologic, metabolic, or physical abnormalities before initiation of treatment (e.g., exercise that targets fatigue). Uncontrolled pain mandates the initiation or modification of analgesics. Opioid-based pharmacotherapy has emerged as the cornerstone of cancer pain management [52]. Secondary infections related to cancer therapy–induced neutropenia must be treated before aerobic conditioning can begin. The discovery of disease progression may warrant initiation or alteration of an antineoplastic regimen or the administration of radiation therapy. Cardiac toxicity may improve after the initiation of digoxin or medications to reduce afterload. Anemia generally responds to therapy with recombinant erythropoietin with associated improvements in the patient’s function and quality of life [53]. Patients with pulmonary fibrosis induced by radiation therapy or chemotherapy and those who have undergone lobectomy or pneumonectomy may require supplemental oxygen during rehabilitative efforts. Nutritional evaluation may be needed for cachectic or hypoproteinemic patients.
Rehabilitation
Well-powered, randomized, controlled trials have established that both strengthening and aerobic exercise can, regardless of the phase of the disease, effectively reduce the effects of CRF [54–63]. Modest exercise intensity levels (e.g., 60% of maximum oxygen consumption up to 5 days per week) suffice and are well tolerated by patients irrespective of treatment phase. A significant proportion of positive studies have used home-based exercise approaches [57–61], which work in noncancer populations suggests may be particularly effective in enhancing long-term adherence [64–66]. It is significant that whereas structured programs, particularly when they are augmented with supportive counseling [60,61], have proved highly efficacious, a more flexible program lacking specific exercise recommendations has not [67].
Exercise is not the only beneficial approach. Psychoeducational interventions emphasizing activity and stress management [68–71], mindfulness training [72], coping skills and problem-solving training [68,73], and cognitive-behavioral therapy [74] have also been shown to be capable of significantly reducing CRF. Although randomized, controlled trials of supportive and exercise therapies in isolation have yielded mixed results [75–79], supportive therapy alone may be effective when CRF is the principal therapeutic focus [79]. To date, no trial that has combined aerobic exercise with structured psychological support has failed to note significant improvements in fatigue [58,60,61]. However, treatments may be less effective in the advanced stages of cancer [80].
Unfortunately, the patient volumes, staff expertise, and infrastructure necessary to support these group and 1:1 interventions are available only at large tertiary medical centers. According to the National Cancer Institute estimates, only about 15% of U.S. cancer patients are diagnosed and treated at these centers, suggesting that the remaining 85% are being treated at facilities incapable of providing effective CRF management programs.
The literature supports interval training at 50% to 70% of the heart rate reserve or while working at an exertion of 11 to 14 (moderate intensity) on the 6 to 20 perceived rate of exertion scale. The intensity of the exercise program depends on baseline fitness levels, intensity of cancer treatment, and stage of cancer treatment. While the patient is undergoing treatment, most studies recommend decreasing the intensity to the lower end of the heart rate range. Once active chemotherapy or radiation therapy has been completed, the program may be progressed toward the higher end of the range. The intensity must also take into account daily laboratory values and patterns of fatigue associated with treatment. For example, fatigue peaks within the middle and end of the radiation therapy cycle, and the program should account for this pattern. Finally, duration and frequency should closely match the American College of Sports Medicine guidelines, which recommend that patients with cancer exercise for a total of 20 to 30 minutes three to five times per week [81].
Exercise precautions for cancer patients are seldom evidence based. They vary significantly between institutions and clinicians. The following limitations are conservative suggestions and should not be interpreted as absolute exercise contraindications. Aerobic and resistive exercise should be carefully reviewed if not discontinued when platelet levels fall below 10,000/μL. Contact and high-impact sports should be avoided when platelet levels fall below 50,000/μL or in patients with primary or metastatic bone disease. Light exercise is allowed when hemoglobin concentration is less than 8 g/dL, with patients closely monitored for symptoms. Therapeutic activities should be restricted to indoor exercise for patients at nadir with an absolute neutrophil count below 500/μL. Exercise should be deferred for febrile patients with temperatures above 101.5 °F.
In addition to aerobic conditioning, referral to occupational and physical therapy for training in energy conservation strategies, use of adaptive equipment, and progressive resistive exercise will benefit appropriate patients. Instruction in compensatory strategies for mobility and performance of activities of daily living can optimize autonomy within the constraints imposed by CRF. Adaptive equipment, such as canes, crutches, and walkers, may improve mobility; provision with adaptive devices, such as long-handled shoehorns and reachers, may facilitate independent self-care activities. Interventions for mobility and performance of activities of daily living benefit even end-stage cancer patients. For these patients, education and empowerment of caretakers may emerge as the primary therapeutic focus.
A psychiatric consultation may be indicated if depression emerges during evaluation. All nonessential centrally acting drugs should be eliminated. Pain medications should be chosen to minimize neuropsychological toxicity. Among the opioids, hydromorphone, fentanyl, and oxycodone have fewer active metabolites than morphine sulfate [82]. Their use may be associated with a more tolerable side effect profile in the elderly and patients with renal impairment. Pharmacologic approaches for cancer fatigue center predominantly around the administration of psychostimulants. The utility of these agents is equivocal since controlled trials of methylphenidate and pemoline have produced mixed results [83].
Procedures
Patients with pleural or pericardial effusions will benefit from percutaneous drainage of the fluid. Pleurocentesis or pericardiocentesis may be required to prevent the reaccumulation of effusions. Percutaneous stenting procedures have become commonplace when tumor compression narrows the lumen of ureters, bile ducts, bronchi, or blood vessels with adverse physiologic sequelae. When cancer pain cannot be adequately managed with systemic therapy or if side effects become untenable, neuraxial analgesic delivery may restore normal arousal, energy, and cognition [33]. Radiation therapy may be used palliatively to treat pain or to reduce tumor bulk that is compressing neurologic structures.
Surgery
Cancer patients with deconditioning and fatigue may benefit from surgical debulking of tumor or resection of isolated lung, liver, bone, or brain metastases. Fatigue, however, is not an independent indication for surgery. If focal sensory or motor deficits result from tumor compression of neural pathways, emergent resection may be required.
Potential Disease Complications
Patients commonly deteriorate functionally because of incremental tumor burden and morbidities as their cancers advance. Consequences of malignant progression may include new or worsening neurologic deficits, dyspnea, cognitive deterioration from intracranial metastases or radiation therapy–induced changes, pathologic fractures, visceral obstruction, and somatic pain syndromes.
Potential Treatment Complications
Potential complications of anticancer modalities are extensive. Radiation therapy can cause fibrosis, neurologic compromise, and worsening of CRF. Chemotherapy can similarly exacerbate CRF. Various chemotherapeutic agents have the capacity to impair cognitive, renal, pulmonary, cardiac, and neurologic function. The pharmacologic agents used to manage cancer-associated symptoms and pain can adversely affect neurologic, gastrointestinal, and urinary function as well as exacerbate peripheral edema and CRF.
Complications associated with rehabilitative interventions are few when strategies are used appropriately. Patients with bone-avid cancers (e.g., lung, prostate, breast, thyroid, multiple myeloma, and renal) are at risk for pathologic fractures, particularly those with lytic metastases. A recent bone scan or skeletal survey should be reviewed before initiating an exercise program.
Cancer patients should generally be considered more susceptible to common exercise-induced complications. Overly aggressive aerobic conditioning or strengthening programs may worsen CRF. Uncustomary exertion may aggravate chemotherapeutically induced electrolyte and fluid imbalances. Therapeutic regimens for cancer patients should be adapted and scrutinized accordingly.