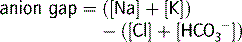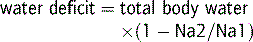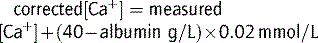Section 12 Metabolic
12.1 Acid–base disorders
Acidosis
Systemic acidosis is defined as the presence of an increased concentration of H+ ions in the blood. The physiological effects of acidosis are a decrease in the affinity of haemoglobin for oxygen and an increase in serum K+ of approximately 0.4–0.6 mmol/L for each decrease in pH of 0.1, although this does not appear to occur during anaesthesia.1 It is commonly believed that acidosis decreases myocardial contractility, but this is usually of little clinical significance at a pH of more than 7.1. Although the presence of acidosis is often associated with a poor prognosis, the presence of acidosis per se usually has few clinically significant effects, and it is the nature and severity of the underlying illness that determines its outcome. There is also evidence that acidosis may have a protective effect in tissues by decreasing phospholipase activity and inhibiting the development of the mitochondrial permeability transition defect that leads to apoptosis. In certain situations, such as in pregnant women and the neonate, increased acid production may result in a greater change in serum pH (and possibly greater adverse physiological effects) than expected, owing to the decreased buffering capacity of the plasma. A decrease in measured serum HCO3− of up to 5 mmol/L has also been reported as a result of underfilling of vacuum-type specimen tubes.2
Metabolic acidosis
Anion-gap metabolic acidosis
The normal value for the anion gap depends on the type of biochemical analyser used and whilst the upper limit of normal has been commonly quoted as 18, the mean range with some modern analysers is only 5–12.3 In the normal resting state the serum ionic proteins account for most of the anion gap, with a lesser contribution from other ‘unmeasured’ anions such as PO4− and SO4−. In pathological conditions where there is an increase in the concentration of unmeasured anions, an anion-gap metabolic acidosis results. The anions responsible for the increase in the anion gap depend on the cause of the acidosis. Lactic acid is the predominant anion in hypoxia and shock, PO4− and SO4− in renal failure, ketoacids in diabetic and alcoholic ketoacidosis, oxalic acid in ethylene glycol poisoning and formic acid in methanol poisoning.
Treatment of metabolic acidosis
The treatment of acidosis should usually be directed primarily towards correction of the underlying cause. Intravenous HCO3− is of use in the presence of acidosis and severe hyperkalaemia, severe sodium channel (e.g. tricyclic antidepressant), salicylate, methanol or ethylene glycol toxicity. It should be used to attempt to normalize the pH before Factor VIIa therapy is administered. It may be of use in rhabdomyolysis and cardiac arrest in young children or pregnant women or cardiac arrest of more than 15 min duration. The use of HCO3− in patients with diabetic ketoacidosis and lactic acidosis associated with sepsis or severe cardiorespiratory disease does not appear to improve outcome.4–6 The potential hazards of HCO3− therapy include a high solute load, hyperosmolarity, hypokalaemia, decreased ionized serum calcium, worsening of cerebrospinal fluid acidosis (which may precipitate hepatic encephalopathy in susceptible patients) and decreased metabolic degradation of citrate, lactate and ketone bodies in the liver. It also reduces oxygen off-loading by haemoglobin in the tissues and may inactivate calcium and adrenaline when administered through the same intravenous line.7
Respiratory acidosis
Respiratory acidosis is defined as an elevation of the arterial partial pressure of carbon dioxide (PCO2) and is due to alveolar hypoventilation. The effects of mild-to-moderate hypercarbia are usually confined to its effect on decreasing the alveolar partial pressure of oxygen (see alveolar gas equation). With more significant elevations, sweating, tachycardia, confusion and mydriasis occur. When the PCO2 is greater than 80 mmHg, the level of consciousness is usually depressed. There are many possible causes of alveolar hypoventilation. Central nervous system causes include severe hypotension, drugs with respiratory depressant effects (especially opioids and sedatives), cerebrovascular events, tumours, infections, neurotrauma and metabolic derangements. Ventilatory drive may also be reduced by high partial pressures of oxygen in patients with chronic obstructive airways disease (COAD) and chronic CO2 retention. Lesions of the spinal cord, such as tumours, infections, trauma or demyelination, may also result in alveolar hypoventilation if the lesion is above the level of C4. Lower in the afferent limb of respiratory muscle innervation, lesions of peripheral nerves such as Guillain–Barré syndrome or trauma to both phrenic nerves may also be causative. Neuromuscular junction dysfunction following postsynaptic destruction of acetylcholine receptors in myaesthenia gravis, inactivation of cholinesterase in organophosphate poisoning or the effects of spider or snake venoms and muscle relaxant drugs may also cause ventilatory failure. Aminoglycoside antibiotics may also precipitate ventilatory failure in susceptible patients. Muscular dystrophy, myopathies and severe electrolyte disorders may cause muscular weakness, and lesions of the chest wall, such as flail chest, severe kyphoscoliosis or arthritis, may also impair effective ventilation.
Alkalosis
Metabolic alkalosis
Alkali may be added to the body in the form of citrate by red cell transfusion, intravenous NaHCO3 administration or as urinary alkalinizers. The milk alkali syndrome may occur as a result of the chronic ingestion of more than 2 g of calcium salts each day (commonly in conjunction with vitamin D).8 The metabolic derangement known as post-hypercapnic alkalosis is caused by the decrease of a chronically elevated PCO2 to normal levels, when relative hyperventilation occurs. In such patients the HCO3− is usually elevated as a result of chronic hypercarbia, and when the PCO2 is acutely lowered to normal levels the appearance on blood gas analysis is that of a metabolic alkalosis, rather than that of a relative respiratory alkalosis. The most common example of this in the ED occurs in a patient who has chronic CO2 retention with an acute exacerbation of COAD. The ingestion of strong alkali is almost never a cause of systemic alkalosis.
Respiratory alkalosis
Common causes of respiratory alkalosis in the general population include exercise, altitude-elated hypoxia and stimulation of the medullary respiratory centre by progesterones during pregnancy. In the ED setting, causes such as hypoxia, early sepsis, cerebral oedema, hepatic cirrhosis, mechanical ventilation, anxiety and salicylate, theophylline and carbon monoxide toxicity should be considered.9 Treatment is directed towards correction of the underlying cause and the treatment of hypokalaemia or hypocalcaemia as required. Hydrochloric acid can be administered to correct the metabolic abnormality, but it is rarely required. A dose of 1–3 mmol/kg of hydrochloric acid can be given through a central venous line at a rate of no faster than 1 mEq/min.10
Whilst it was previously thought that, due to levels decreasing rapidly following sampling, serum lactate needed to be measured by immediate assay of arterial blood, more recent evidence has challenged this assumption with one study failing to demonstrate any significant difference in lactate levels measured at 15 min after sampling.11
1 Natalini G, Seramondi V, Fassini P, et al. Acute respiratory acidosis does not increase plasma potassium in normokalaemic anaesthetized patients. A controlled randomized trial. European Journal of Anaesthesiology. 2001;18(6):394-400.
2 Herr RD, Swanson T. Pseudometabolic acidosis caused by underfill of vacutainer tubes. Annals of Emergency Medicine. 1992;21(2):177-180.
3 Paulson WD, Roberts WL, Lurie AA, et al. Wide variation in serum anion gap measurements by chemistry analyzers. American Journal of Clinical Pathology. 1998;110(6):735-742.
4 Cooper DJ. Bicarbonate does not improve haemodynamics in critically ill patients who have lactic acidosis: a prospective controlled clinical study. Annals of International Medicine. 1990;112:492-498.
5 Mathieu D, Neviere R, Billard V, et al. Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study. Critical Care Medicine. 1991;19(11):1352-1356.
6 Okuda Y, Adrogue HJ, Field JB, et al. Counterproductive effects of sodium bicarbonate in diabetic ketoacidosis. Journal of Clinical Endocrinology and Metabolism. 1996;81(1):314-320.
7 Australian Resuscitation Council, Medications in cardiac arrest, February 2006.
8 Whiting SJ, Kim K, Wood R. Calcium supplementation. Journal of the American Academy of Nurse Practitioners. 1997;9(4):187-192.
9 Laffey JG, Kavanagh BP. Hypocapnia. New England Journal of Medicine. 2002;347(1):43-53.
10 Adrogue HJ, Madias NE. Management of life-threatening acid base disorders. New England Journal of Medicine. 1998;338(2):107-111.
11 Jones AE, Leonard MM, Hernandez-Nino J, et al. Determination of the effect of in vitro time, temperature, and tourniquet use on whole blood venous point-of-care lactate concentrations. Academic Emergency Medicine. 2007;14:587-591.
12.2 Electrolyte disturbances
Hyponatraemia
Introduction
Hyponatraemia, defined as serum sodium concentration of less than 130 mmol/L, is a common condition. The prevalence is estimated at 2.5% in hospitalized patients, of which two-thirds develop the condition whilst in hospital.1
Pathophysiology
Hyponatraemia causes cellular swelling as water moves down an osmotic gradient into the intracellular fluid. Most of the symptomatology of hyponatraemia is produced in the central nervous system (CNS) by the swelling of brain cells within the rigid calvarium, causing raised intracranial pressure (hyponatraemic encephalopathy). As intracranial pressure rises, adaptive responses come into play. Initially there is a reduction of the cerebral blood and cerebrospinal fluid (CSF) pools. Later, neuronal intracellular osmolality is reduced by extrusion of potassium, followed within hours to days by organic solutes such as amino acids, phosphocreatine and myoinositol. These processes return brain volume towards normal and restore cellular function.
Aetiology and classification
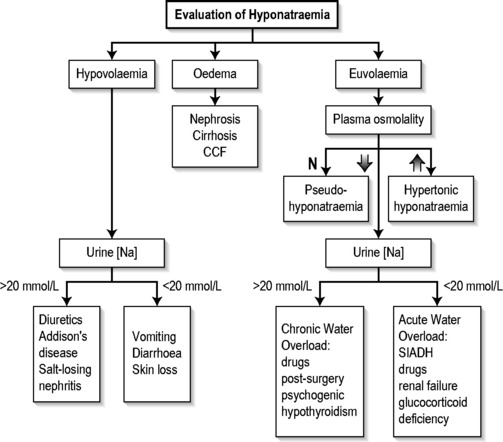
Hypovolaemic hyponatraemia
These patients have deficits in both total body sodium and total body water, but the sodium deficit exceeds the water deficit. Causes include renal and extra-renal fluid losses, and are listed in Table 12.2.1. Determination of the urinary sodium concentration can differentiate these two groups. Extrarenal losses are associated with low urinary sodium concentrations (<20 mmol/L) and hyperosmolar urine. The exception is with severe vomiting and metabolic alkalosis, where bicarbonaturia obligates renal sodium loss and urinary sodium is high (>20 mmol/L), despite volume depletion. However, urinary chloride, a better indicator of extracellular fluid (ECF) volume, is low.
TURP, transurethral resection of prostate; ADH, antidiuretic hormone; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; MAOI, monoamic oxidase inhibitor; SIAOH, syndrome of inappropriate antidiuretic hormone secretion.
Clinical features
In addition to the features of the underlying medical condition and alteration in extracellular volume, clinical manifestations of hyponatraemia per se usually develop when serum sodium is less than 130 mmol/L. The severity of symptoms depends partly on the absolute serum sodium concentration and partly on its rate of fall. At sodium concentrations from 125 to 130 mmol/L the symptoms are principally gastrointestinal, whereas at concentrations below 125 mmol/L the symptoms are predominantly neuropsychiatric. The principal signs and symptoms of hyponatraemia are listed in Table 12.2.3.
| Anorexia |
| Nausea |
| Vomiting |
| Lethargy |
| Muscle cramps |
| Muscle weakness |
| Headache |
| Confusion/agitation |
| Altered conscious state |
| Seizures |
| Coma |
Mild chronic ‘asymptomatic’ hyponatraemia in the elderly contributes to an increased rate of falls, probably due to impairment of attention, posture and gait mechanisms.2
Hyponatraemic encephalopathy carries a high mortality (50%) if left untreated.3 Population groups prone to hyponatraemic encephalopathy have been identified (Table 12.2.4).3–8
Table 12.2.4 Patient groups at risk of hyponatraemia
| Postoperative |
| Menstruating females |
| Elderly women on thiazide diuretics |
| Prepubescent children |
| Psychiatric polydipsic patients |
| Hypoxaemic patients |
| AIDS patients |
| Patients taking ‘Ecstasy’ (MDMA) |
| Endurance athletes |
Premenopausal women appear at risk of developing hyponatraemic encephalopathy because oestrogen and progesterone are thought to inhibit the brain Na-K-ATPase and increase circulating levels of antidiuretic hormone (ADH).5
Psychogenic polydipsia refers to a condition in which kidney function is normal and dilute urine is produced, but free water intake overwhelms the kidney’s capabilities and the serum sodium falls. It occurs primarily in patients which schizophrenia or bipolar disorder. These patients develop hyponatraemia with a far lower fluid intake than is usually necessary (over 20 L of water/day in a 60-kg man, in the absence of elevated levels of ADH)4,5 and it may arise through a combination of factors: antipsychotics, increased thirst perception, enhanced renal response to ADH and a mild defect in osmoregulation.
Exercise-associated hyponatraemia occurs in endurance athletes and mainly relates to the consumption of excessive fluid although non-osmotic release of vasopression and other mechanisms may be implicated.9,10
Hyponatraemia in AIDS is common and associated with a high mortality. It may be secondary to syndrome of inappropriate ADH (SIADH), adrenal insufficiency or volume deficiency with hypotonic fluid replacement.4
The use of ‘Ecstasy’ at ‘rave’ parties has been associated with acute hyponatraemia.6,7 This may be due to a combination of drug effect and drinking large quantities of water in an attempt to prevent dehydration.
Syndrome of inappropriate ADH secretion
This is a diagnosis of exclusion and is characterized by inappropriately concentrated urine in the setting of hypotonicity. It accounts for approximately 50% of all cases of hyponatraemia. These patients have elevated serum ADH levels without an obvious volume or osmotic stimulus. The diagnostic criteria for SIADH secretion are shown in Table 12.2.5 and conditions associated with the syndrome are listed in Table 12.2.6.
| Hypotonic hyponatraemia |
| Urine osmolality >100 mmol/kg (i.e. inappropriately concentrated) |
| Urine sodium >20 mmol/mL while on a normal salt and water intake |
| Absence of extracellular volume depletion |
| Normal thyroid and adrenal function |
| Normal cardiac, hepatic and renal function |
| No diuretic use |
Table 12.2.6 Conditions associated with SIADH
Clinical investigation
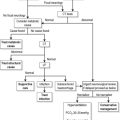
Measurement of serum and urine sodium concentrations and osmolalities, in addition to clinical assessment of volume status, are essential for the assessment of hyponatraemia (Fig. 12.2.1).
Treatment
Treatment should be carefully individualized and depends on the presence of symptoms, the duration of the hyponatraemia and the absolute value of sodium. Ideally correction of the serum sodium should be of a sufficient pace and magnitude to reverse the manifestations of hypotonicity but not be so rapid and large as to pose a risk of the development of osmotic demyelination.11 Treatment of the underlying cause is obviously essential and may correct the hyponatraemia. For hypovolaemic hyponatraemia, adequate volume replacement is essential.
Acute symptomatic hyponatraemia
Symptomatic hyponatraemia developing within 48 h is a medical emergency requiring prompt and aggressive treatment. The risks of developing osmotic demyelination are clearly outweighed by those of the encephalopathy.4 An immediate increase in serum sodium concentration by 8 mEq/L over 4–6 h is recommended.12 This can be achieved by infusing hypertonic saline (3% NaCl) at a rate of 1–2 mL/kg/h, which should raise the serum sodium by 1–2 mmol/L/h. Where neurological symptoms are severe, hypertonic saline can be infused at 4–6 mL/kg/h. Indications for ceasing rapid correction of hyponatraemia are cessation of life-threatening manifestations, moderation of other symptoms or the achievement of a serum sodium of 125–130 mEq/L.11 Other measures to reduce intracranial pressure, such as intubation and intermittent positive pressure ventilation (IPPV), may also be required.
Chronic symptomatic hyponatraemia
Hyponatraemia present for more than 48 h, or where the duration is unknown, presents the greatest dilemma. Care must be taken with correction of sodium as these patients are at the greatest risk of developing osmotic demyelination, yet the presence of encephalopathy mandates urgent treatment.4,5,13 Hypertonic saline can be infused so that a correction rate of no more than 1–1.5 mmol/L/h is maintained. Therapy with hypertonic saline should be discontinued when (a) the patient becomes asymptomatic, (b) the serum sodium has risen by 20 mmol/L or (c) the serum sodium reaches 120–125 mmol/L. Thereafter, slower correction with water restriction should follow. The serum sodium should never be acutely elevated to hypernatraemic or normonatraemic levels, and should not be elevated by more than 25 mmol/L during the first 48 h of therapy.
Chronic asymptomatic hyponatraemia
In this situation saline infusion is usually not required, and patients can be managed by treating the underlying disorder, discontinuing diuretic therapy or restricting fluids. Fluid restriction is inexpensive and effective but is often limited by patient non-compliance. Other treatment options include pharmacological inhibition of ADH with demeclocycline, which is limited by its neuro- and nephrotoxic side effects, or increasing solute with the use of furosemide or urea.4,14
Osmotic myelinolysis
This is an iatrogenic disorder which develops progressively over 3–5 days following the correction of hyponatraemia. It classically produces symmetrical lesions centred on the midline of the pons and was originally described as ‘central pontine myelinolysis’. However, about 10% of cases involve extrapontine lesions. It is reported as occurring in 25% of severely hyponatraemic patients following correction of serum sodium.15 Clinically, the disorder is initially manifested by dysarthria, mutism, lethargy and affective changes, which may be mistaken for psychiatric illness. Classically, pseudo-bulbar palsy and spastic quadriparesis are observed. Recovery is usually gradual and incomplete although both fatalities and complete recovery are reported.16 Demyelination in the central pons and extrapontine sites can be demonstrated on magnetic resonance imaging (MRI) scan or at autopsy.17
It appears that the risk of developing osmotic myelinolysis is associated with severity and chronicity of hyponatraemia. It rarely occurs if the serum sodium is >120 mmol/L or where hyponatraemia has been present for <48 h. Alcoholics, malnourished patients, hypokalaemic patients, burn victims and elderly patients on thiazides seem to be most at risk of developing osmotic demyelination.3,4
Both the rate and the magnitude of sodium correction appear important in the development of osmotic myelinolysis. Although there is as yet no agreed rate of correction that is regarded as completely safe, most authorities suggest that the serum sodium concentration should not rise by more than 10–14 mmol/L during any 24-h period.4,5,13
Hypernatraemia
Introduction
It is important to recognize hypernatraemia because it is usually associated with severe underlying medical illness. It is a condition of hospitalized patients, elderly and dependent people. The incidence of hypernatraemia in hospitalized patients ranges from 0.3 to 1%, with from 60 to 80% of these developing hypernatraemia after admission.14 In-hospital mortality is high (40–55%) and may be due to a combination of hypernatraemia and the severity of the underlying disease.14,18
Aetiology and classification
The clinical causes of hypernatraemia are listed in Table 12.2.7. Population groups at particular risk of developing hypernatraemia are listed in Table 12.2.8.4,14
| Altered perception of thirst |
| Osmoreceptor damage/destruction |
| Exogenous: trauma |
| Endogenous: vasculitis, carcinoma, granuloma |
| Idiopathic: psychogenic, head injury |
| Drugs |
| Normal perception of thirst |
| Poor intake |
| Confusion |
| Coma |
| Depression |
| Dysphagia |
| Odynophagia |
| Increased water loss and decreased intake |
| Diuresis |
| Renal loss |
| Diabetes insipidus |
| Chronic renal failure |
| Diuretic excess |
| GIT loss: fistulae, diarrhoea |
| Exogenous increase in salt intake |
Table 12.2.8 Groups at particular risk for hypernatraemia
| Elderly or disabled, unable to obtain oral fluids independently |
| Infants |
| Inpatients receiving: |
| hypertonic infusions |
| tube feedings |
| osmotic diuretics |
| lactulose |
| mechanical ventilation |
| Altered mental status |
| Uncontrolled diabetes mellitus |
| Underlying polyuric disorders |
Hypernatraemia is classified into these categories based on extracellular volume status: hypovolaemic, hypervolaemic and euvolaemic.4,14,19
Treatment
Treatment is based on clinical assessment of the patient’s volume status.
Hypovolaemic hypernatraemia
These patients require restoration of the volume deficit with isotonic saline, colloid or blood in the first instance, to prevent peripheral vascular collapse, and treatment of the underlying cause. Following this, the water deficit is corrected with 0.45% saline, 5% dextrose or oral water.4,14
Euvolaemic hypernatraemia
Calculate the water deficit as above and replace the deficit and ongoing losses with 5% dextrose, 0.45% saline or oral water.4,14 To avoid cerebral oedema, particularly in chronic hypernatraemia, 50% of the water deficit should be replaced over the first 6–12 h and the rest given slowly over 1–2 days. Serum sodium estimations should be repeated at regular intervals.
Hypokalaemia
Pathophysiology
Hypokalaemia may develop as a consequence of potassium depletion or a shift of potassium into cells. In either case there is an increase in the ratio of intracellular to extracellular potassium concentrations. This in turn produces hyperpolarization across excitable membranes, and is responsible for the effects of hypokalaemia on striated muscle and the cardiac conducting system.
Aetiology
| Inadequate dietary intake |
| Abnormal losses |
| Gastrointestinal |
| Vomiting, nasogastric aspiration |
| Diarrhoea, fistula loss |
| Villous adenoma of the colon |
| Laxative abuse |
| Renal |
| Mineralocorticoid excess |
| Conn syndrome |
| Bartter syndrome |
| Ectopic ACTH syndrome |
| Small cell carcinoma of the lung |
| Pancreatic carcinoma |
| Carcinoma of the thymus |
| Renal tubular acidosis |
| Magnesium deficiency |
| Drugs |
| Diuretics |
| Corticosteroids |
| Gentamicin, amphotericin B |
| Cisplatin |
| Compartmental shift |
| Alkalosis |
| Insulin |
| Na-K-ATPase stimulation |
| Sympathomimetic agents with β2 effect |
| Methylxanthines |
| Barium poisoning |
| Hypothermia |
| Toluene intoxication |
| Hypokalaemic periodic paralysis |
Clinical presentation
Hypokalaemia commonly produces no symptoms in otherwise healthy subjects.
Clinical features may include weakness, constipation, ileus and ventilatory failure. Myopathy may develop, with weakness of the extremities which characteristically worsens with exercise. If the hypokalaemia is severe and untreated, rhabdomyolysis may occur. Polyuria and polydipsia may result from the effect of hypokalaemia on the distal renal tubule (nephrogenic diabetes insipidus of hypokalaemia). Cardiac effects include ventricular tachycardias and atrial tachycardias, with or without block. Characteristic electrocardiogram (ECG) changes include PR prolongation, T-wave flattening and inversion and prominent U waves.20
Hyperkalaemia
Pathophysiology
Two homeostatic mechanisms are responsible for maintaining potassium balance. The renal system maintains external potassium balance by excreting 90–95% of the average daily potassium load (100 mmol/day); the gut excretes the remainder. This is a relatively slow process: only half the administered load of potassium will have been excreted in the urine after 3–6 h.21,22 The extra-renal system involves hormonal and acid–base mechanisms that rapidly translocate potassium intracellularly. This system is critical in the management of acute hyperkalaemia.
Aetiology
| Pseudohyperkalaemia |
| Delay in separating red cells |
| Specimen haemolysis during or after venesection |
| Severe leukocytosis/thrombocytosis |
| Excessive intake |
| Exogenous: i.v. or oral KCI, massive blood transfusion |
| Endogenous: tissue damage |
| Burns |
| Trauma |
| Rhabdomyolysis |
| Tumour lysis |
| Decrease in renal excretion |
| Drugs |
| Spironolactone, triamterene, amiloride |
| Indometacin |
| Captopril, enalapril |
| Renal failure |
| Addison’s disease |
| Hyporeninaemic hypoaldosteronism |
| Compartmental shift |
| Acidosis |
| Insulin deficiency |
| Digoxin overdose |
| Succinylcholine |
| Fluoride poisoning |
| Hyperkalaemic periodic paralysis |
Clinical features
The ECG changes (Table 12.2.11) are characteristic, but are an insensitive method of evaluating hyperkalaemia.
| Plasma potassium (mmol/L) | ECG characteristics |
|---|---|
| 6–7 | Tall peaked T waves (>5 mm) |
| 7–8 | QRS widening, small-amplitude P waves |
| 8–9 | Fusion of QRS complex with T wave producing sine wave |
| 9 | AV dissociation, ventricular tachycardia, ventricular fibrillation |
Serum biochemistry in almost all patients with hyperkalaemia shows some degree of renal impairment and metabolic acidosis. In dialysis patients, hyperkalaemia may develop without concomitant metabolic acidosis.
Treatment
Hyperkalaemia with ECG changes requires urgent management. The priorities are as follows:20,23
The use of insulin and glucose is well supported in the literature.19,22 A response is usually seen within 20–30 min, with lowering of plasma potassium by up to 1 mmol/L and reversal of ECG changes. Transient hypoglycaemia may be observed within 15 min of insulin administration. In some patients, particularly those with end-stage renal failure, late hypoglycaemia may develop. For this reason, a 10% dextrose infusion at 50 mL/h is recommended and the blood glucose should be monitored closely. The exact mechanism by which insulin translocates potassium is not known; it is thought to be stimulation of Na-K-ATPase independent of cAMP.
β2-Agonists significantly lower plasma potassium when given intravenously or via a nebulizer.21,22 Potassium levels are reduced by up to 1.00 mmol/L within 30 min following 10–20 mg of nebulized salbutamol. The effect is sustained for up to 2 h. Adverse effects of salbutamol administration include tachyarrhythmias and precipitation of angina in patients with coronary artery disease. Patients on non-selected β-blockers may not respond. Some patients with end-stage renal disease are also resistant to this therapy. The reason for this is unknown. Greater decreases in potassium have been observed when salbutamol treatment is combined with insulin and glucose. The additive effect is thought to be due to stimulation of Na-K-ATPase via different pathways. Transient hyperglycaemia may occur with combined therapy, but delayed hypoglycaemia does not occur.
HypocalCaemia
Introduction
A reduction in serum calcium concentration manifests principally as abnormal neuromuscular function.
Aetiology
Hypocalcaemia occurs when calcium is lost from the extracellular fluid at a rate greater than can be replaced by the intestine or bone. The major cause of severe hypocalcaemia is hypoparathyroidism, as a result of surgery for thyroid disease, autoimmune destruction or from developmental abnormalities of the parathyroid glands. Other causes are listed in Table 12.2.12.
| Factitious EDTA contamination |
| Hypoalbuminaemia |
| Decreased PTH activity |
| Hypoparathyroidism |
| Pseudohypoparathyroidism |
| Hypomagnesaemia |
| Decreased vitamin D activity |
| Acute pancreatitis |
| Hyperphosphataemia |
| Renal failure |
| Phosphate supplements |
| ‘Hungry bone’ syndrome |
| Drugs |
| Mithramycin |
| Diuretics: furosemide, ethacrynic acid |
Clinical features
Patients with acute hypocalcaemia are more likely to be symptomatic than those with chronic hypocalcaemia. Symptomatic hypocalcaemia is characterized by abnormal neuromuscular excitability and neurological sensations.24 Early signs are perioral numbness and paraesthesia of distal extremities. Hyperreflexia, muscle cramps and carpopedal spasm follow. Chvostek’s sign (ipsilateral contraction of the facial muscles elicited by tapping the facial nerve just anterior to the ear) and Trousseau’s sign (carpopedal spasm with inflation of a blood pressure cuff for 3–5 min) are signs of neuromuscular irritability. If muscle contractions become uncontrollable tetany results, and this can prove fatal if laryngospasm occurs. Seizures may occur when there is CNS instability. Cardiovascular manifestations include hypotension, bradycardia, impaired cardiac contractility and arrhythmias. ECG evidence of hypocalcaemia includes prolonged QT interval, and possibly ST prolongation and T-wave abnormalities.
Hypercalcaemia
Introduction
The normal total serum calcium concentration is 2.15–2.55 mmol/L. Hypercalcaemia is a relatively common condition with a frequency estimated at 1:1000–1:10 000.25 Although there are many causes, the most frequent are malignancy and hyperparathyroidism, with the former the most likely to cause hypercalcaemia requiring urgent attention.25
Pathophysiology
Three pathophysiological mechanisms may produce hypercalcaemia:25
Aetiology
The majority of cases of hypercalcaemia requiring urgent treatment are due to malignancy or, less commonly, primary hyperparathyroidism (parathyroid crisis). Malignant hypercalcaemia is most commonly seen with the solid tumours: lung and breast cancer, squamous cell carcinoma of the head and neck and cholangiocarcinoma and the haematological malignancies multiple myeloma and lymphoma.24 Other causes of hypercalcaemia are uncommon (Table 12.2.13).
| Factitious |
| Haemoconcentration |
| Postprandial |
| Malignancy |
| Primary hyperparathyroidism |
| Drugs |
| Thiazides |
| Vitamin D |
| Lithium |
| Vitamin A |
| Hormonal |
| Thyrotoxicosis |
| Acromegaly |
| Hypoadrenalism |
| Phaeochromocytoma |
| Granulomas |
| Tuberculosis |
| Sarcoidosis |
| Renal failure |
| Milk alkali syndrome |
| Immobilization |
Clinical features
Hypercalcaemia causes disturbances of the gastrointestinal, cardiovascular, renal and central nervous systems.24,26
Treatment
Irrespective of the cause, the management of hypercalcaemic crisis is the same. There are four primary treatment goals:26–29
Enhancement of renal excretion
Haemodialysis is the treatment of choice to rapidly decrease serum calcium in patients with heart failure or renal insufficiency.30
Hypomagnesaemia
Aetiology
From an emergency medicine perspective, hypomagnesaemia is most frequently encountered in the context of acute and chronic diarrhoea, acute pancreatitis, diuretic use, in alcoholics and in diabetic ketoacidosis, secondary to glycosuria and osmotic diuresis. Table 12.2.14 details causes of magnesium deficiency.
| Gastrointestinal losses |
| Acute and chronic diarrhoea |
| Acute pancreatitis |
| Severe malnutrition |
| Intestinal fistulae |
| Extensive bowel resection |
| Prolonged nasogastric suction |
| Renal losses |
| Osmotic diuresis – diabetes, urea, mannitol |
| Hypercalcaemia and hypercalciuria |
| Volume expanded states |
| Chronic parenteral fluid therapy |
| Drugs |
| ACE inhibitors |
| Alcohol |
| Aminoglycosides |
| Amphotericin B |
| Cisplatin |
| Ciclosporin |
| Diuretics – thiazide or loop |
| Other |
| Phosphate depletion |
Hypomagnesaemia has been found in 30% of alcoholics admitted to hospital and results from a combination of the direct effect of alcohol on the renal tubule, which increases magnesium excretion, and associated malnutrition, diarrhoea and metabolic acidosis.31
Early ECG changes of magnesium deficiency include prolongation of the PR and QT intervals, with progressive QRS widening and U-wave appearance as severity progresses. Changes in cardiac automaticity and conduction, atrial and ventricular arrhythmias, including torsades des pointes, can occur. Administration of a magnesium bolus can abolish torsades des pointes, even in the presence of normal serum magnesium levels.32 Magnesium is a co-factor in the Na-K-ATPase system and so magnesium deficiency enhances myocardial sensitivity to digitalis and may precipitate digitalis toxicity. Digitalis-toxic arrhythmias, in turn, can be terminated with intravenous magnesium.
Treatment
Oral replacement is the preferred option in asymptomatic patients, although this route takes longer.
Symptomatic moderate-to-severe magnesium deficiency should be treated with parenteral magnesium salts. The patient should be closely monitored and therapy discontinued if deep tendon reflexes disappear or serum magnesium exceeds 2.5 mmol/L.32 Suggested dosing regimes are outlined in Table 12.2.16.
| Emergency – i.v. route |
| 8–16 mmol statim |
| 40 mmol over next 5 h |
| Severely ill – i.m. route |
| 48 mmol on day 1 |
| 17–25 mmol on days 2–5 |
| Asymptomatic – oral route |
| 15 mmol/day |
Hypermagnesaemia
Hypermagnesaemia (serum magnesium above 0.95 mmol/L) is rare and usually iatrogenic.
1 Anderson RJ, Chung HM, Kluge R, Scrier RW. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Annals of Internal Medicine. 1985;102:164-168.
2 Decaux G. Is asymptomatic hyponatraemia really asymptomatic? American Journal of Medicine. 2006;119:S79-S82.
3 Berl T. Treating hyponatraemia: what is all the controversy about? Annals of Internal Medicine. 1990;113:417-419.
4 Kumar S, Berl T. Sodium-electrolyte quintet. Lancet. 1998;352:220-228.
5 Fraser C, Arieff A. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. American Journal of Medicine. 1997;102:67-77.
6 Maxwell D, Polkey M, Henry J. Hyponatraemia and catatonic stupor after taking ‘ecstasy’. British Medical Journal. 1993;307(6916):1399.
7 Box SA, Prescott LF, Freestone S. Hyponatraemia at a rave. Postgraduate Medical Journal. 1997;73(855):53-54.
8 Yeong-Hau HL, Shapiro JI. Hyponatremia: clinical diagnosis and management. American Journal of Medicine. 2007;120:653-658.
9 Almod CS, Shin AY, Fortescure EB, et al. Hyponatremia among runners in the Boston Marathon. New England Journal of Medicine. 2005;352:1550-1556.
10 Noakes TD, Sharwood K, Speedy D, et al. Three independent biological mechanisms cause exercise-associated hyponatraemia: evidence from 2,135 weighed competitive athletic performances. Proceedings of the National Academy of Science USA. 2005;102:18550-18555.
11 Androgue HJ, Madias NE. Hyponatremia. New England Journal of Medicine. 2000;342:1581-1589.
12 Kokko JP. Symptomatic hyponatraemia with hypoxia is a medical emergency. Kidney International. 2006;69:1291-1293.
13 Cluitmans F, Meinders A. Management of severe hyponatraemia: rapid or slow correction? American Journal of Medicine. 1990;88:161-166.
14 Fried L, Palevsky P. Myelinolysis after correction of hyponatraemia. Annals of Internal Medicine. 1997;3:585-689.
15 Sterns RH, Cappuccio JD, Silver SM, et al. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. Journal of the American Society of Nephrology. 1994;4:1522-1530.
16 Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurological disorder following rapid correction of hyponatraemia. Medicine (Baltimore). 1993;72:359-373.
17 Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Annals of Internal Medicine. 1997;126:57-62.
18 Long C, Marin P, Byer A, et al. Hypernatraemia in an adult in-patient population. Postgraduate Medical Journal. 1991;67:643-645.
19 DeVita M, Michelis M. Perturbations in sodium balance. Clinics in Laboratory Medicine. 1993;13(1):135-148.
20 Mandel A. Hypokalemia and hyperkalemia. Medical Clinics of North America. 1997;81(3):611-639.
21 Allon M. Treatment and prevention of hyperkalemia in end-stage renal disease. Kidney International. 1993;43:1197-1209.
22 Salem MM, Rosa RM, Battle DC. Extrarenal potassium tolerance in chronic renal failure: implications for the treatment of acute hyperkalemia. American Journal of Kidney Disease. 1991;18:421-440.
23 Halperin M, Kamel K. Potassium-electrolyte quintet. Lancet. 1998;352:135-140.
24 Bourke E, Delaney V. Assessment of hypocalcemia and hypercalcemia. Clinics in Laboratory Medicine. 1993;13(1):157-177.
25 Deftos L. Hypercalcemia. Postgraduate Medicine. 1996;100(6):119-126.
26 Bushinskey D, Monk R. Calcium-electrolyte quintet. Lancet. 1998;352:306-311.
27 Chisholm M, Mulloy A, Taylor T. Acute management of cancer-related hypercalcemia. Annals of Pharmacotherapy. 1996;30:507-513.
28 Bilezikian J. Management of acute hypercalcemia. New England Journal of Medicine. 1992;326(18):1196-1203.
29 Falk S, Fallon M. Emergencies—ABC of palliative care. British Medical Journal. 1997;315:1525-1528.
30 Edelson GW, Kleerekoper M. Hypercalcemic crisis. Medical Clinics of North America. 1995;79:79-92.
31 Weisinger JR, Bellorin-Font E. Magnesium and phosphorus-electrolyte quintet. Lancet. 1998;352:391-396.
32 Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology. British Journal of Anaesthesia. 1999;83(2):302-320.