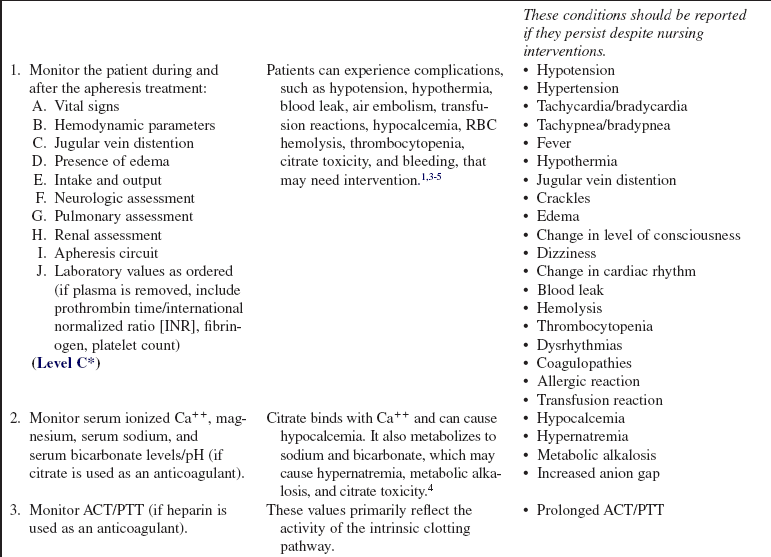
Apheresis and Therapeutic Plasma Exchange (Assist)
PREREQUISITE NURSING KNOWLEDGE
• Therapeutic apheresis (i.e., hemapheresis) is a technique for selective removal of cells, plasma, and substances from the patient’s circulation to promote clinical improvement. The different apheresis techniques vary according to the component of the blood removed or replaced or the substance removed.
 Plasma exchange is the process of replacing the plasma removed with an equal amount of either plasma or fluid (most commonly a combination of 5% albumin and normal saline solution).
Plasma exchange is the process of replacing the plasma removed with an equal amount of either plasma or fluid (most commonly a combination of 5% albumin and normal saline solution).
 Cytapheresis is the selective removal of the cellular components of blood. Blood is withdrawn from the patient and a specific cellular component is retained (i.e., white blood cell); the remainder of other cells and plasma is returned to the donor or patient.
Cytapheresis is the selective removal of the cellular components of blood. Blood is withdrawn from the patient and a specific cellular component is retained (i.e., white blood cell); the remainder of other cells and plasma is returned to the donor or patient.
 Leukocytapheresis is the removal of white blood cells from the blood; the remaining blood is returned to the patient. It is most commonly used as a therapeutic method for blast cell reduction in leukemias (leukocytosis).
Leukocytapheresis is the removal of white blood cells from the blood; the remaining blood is returned to the patient. It is most commonly used as a therapeutic method for blast cell reduction in leukemias (leukocytosis).
 Erythrocytapheresis is the process of removing erythrocytes (red blood cells) from whole blood.
Erythrocytapheresis is the process of removing erythrocytes (red blood cells) from whole blood.
 Thrombocytapheresis is the selective removal of platelets (thrombocytes).
Thrombocytapheresis is the selective removal of platelets (thrombocytes).
 Plasma adsorption/perfusion is the removal of plasma with a hollow fiber filter. Blood is returned to the patient, and the plasma is pumped over an adsorptive column that removes certain proteins or pathogens. The treated plasma is then returned to the patient.
Plasma adsorption/perfusion is the removal of plasma with a hollow fiber filter. Blood is returned to the patient, and the plasma is pumped over an adsorptive column that removes certain proteins or pathogens. The treated plasma is then returned to the patient.
 Lymphoplasmaphersis is the separation and removal of lymphocytes and plasma from the withdrawn blood, with the remainder of the blood retransfused into the donor.
Lymphoplasmaphersis is the separation and removal of lymphocytes and plasma from the withdrawn blood, with the remainder of the blood retransfused into the donor.
 Immunoadsorption is the removal of an antigen in the blood by a specific antibody lining the surface of a filter or cartridge.
Immunoadsorption is the removal of an antigen in the blood by a specific antibody lining the surface of a filter or cartridge.
 Photopheresis uses apheresis techniques to remove and return blood to the patient. Photosensitizing drugs are given to the patient, and white blood cells are removed and exposed to an ultraviolet light, then returned to the patient. Photopheresis induces cellular changes that have been shown to be effective in certain diseases such as cutaneous T-cell, graft-versus-host disease, post–bone marrow transplant, and solid organ transplant rejection. Apheresis techniques are also used for the procurement of peripheral stem cells for bone marrow transplantation.
Photopheresis uses apheresis techniques to remove and return blood to the patient. Photosensitizing drugs are given to the patient, and white blood cells are removed and exposed to an ultraviolet light, then returned to the patient. Photopheresis induces cellular changes that have been shown to be effective in certain diseases such as cutaneous T-cell, graft-versus-host disease, post–bone marrow transplant, and solid organ transplant rejection. Apheresis techniques are also used for the procurement of peripheral stem cells for bone marrow transplantation.
• During plasma exchange procedures, the plasma removed must be replaced; the most common replacement fluids are albumin, fresh frozen plasma (FFP), thawed plasma (derived from thawed FFP and maintained at low temperatures for use within 1 to 5 days), and normal saline.1 Because clotting factors are transiently reduced by plasma exchange, FFP can also be used as a fluid replacement in patients when bleeding is an issue.
• Plasma volume is an estimate of the patient’s total volume based on gender, height, weight, and hematocrit value. Exchange volume is the ratio of the patient’s plasma volume to be removed and replaced; this is usually 1:1 or 1.5:1 of the patient’s estimated plasma volume.4
• In plasma exchange, an average of 3 to 5 L of plasma is removed and replaced.4
• Treatments can be done with two different machines.4
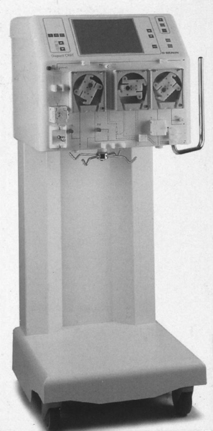
 Centrifugal apheresis machine: Separates plasma and other blood components with use of a centrifuge (Fig. 116-1).
Centrifugal apheresis machine: Separates plasma and other blood components with use of a centrifuge (Fig. 116-1).
 Filtration: A hollow-fiber cell separator, permeable to plasma proteins, is used to remove the patient’s plasma via an apheresis machine or continuous renal replacement machines adapted for pheresis (Fig. 116-2).
Filtration: A hollow-fiber cell separator, permeable to plasma proteins, is used to remove the patient’s plasma via an apheresis machine or continuous renal replacement machines adapted for pheresis (Fig. 116-2).
• Treatment length and frequency vary according to the disease being treated, rate of production of the substance being removed, and the patient’s response to treatment. Acute conditions, such as thrombotic thrombocytopenia purpura or graft-versus-host disease, usually require daily treatments for 5 to 7 days.6,7 Other conditions usually require plasma exchanges two or three times weekly for up to 6 weeks.2,5–7 The total amount of plasma to be exchanged is used as a guide for treatment. A single treatment, referred to as a plasma exchange, usually takes 2 to 3 hours with a centrifugal machine and 2 to 6 hours with filtration methods.4
• Apheresis procedures are performed by healthcare professionals such as registered nurses or blood bank personnel, with special knowledge and skills in apheresis. These procedures are commonly performed both in critical care units and on an outpatient basis, depending on the type of disease being treated and on the patient’s condition.
• The most commonly used apheresis systems use two large-bore peripheral venous catheters, a double-lumen vascular access catheter (VAC), or a dialysis graft or fistula to access the vascular system. Peripherally inserted central venous catheters or implantable venous access ports do not provide for adequate blood flow and are not acceptable for use.4
• The system should be primed with an anticoagulant (e.g., heparin or citrate) to prevent clotting. If citrate is used, the patient must be monitored closely for hypocalcemia. Citrate works as an anticoagulant by binding calcium (Ca++), therefore decreasing the amount of Ca++ available for normal clotting.4
• Plasma exchange is used to treat antibody-mediated disorders because the pathogenic antibodies are contained in the plasma. Removal of these antibodies through plasma exchange reduces the number of circulating antibodies, temporarily decreasing the patient’s symptoms.
• Conditions treated by plasma exchange may include the following2,5–7:
 Solid organ transplantation for ABO incompatibility and rejection
Solid organ transplantation for ABO incompatibility and rejection
 Cytokine-mediated injury, such as sepsis, burns, and multisystem organ dysfunction syndrome (MODS); experimental use
Cytokine-mediated injury, such as sepsis, burns, and multisystem organ dysfunction syndrome (MODS); experimental use
• Current indication categories for therapeutic apheresis, as endorsed by the American Association of Blood Banks (AABB) and the American Society for Apheresis (ASFA), are listed in Table 116-1.
Table 116-1
Indication Categories for Therapeutic Apheresis
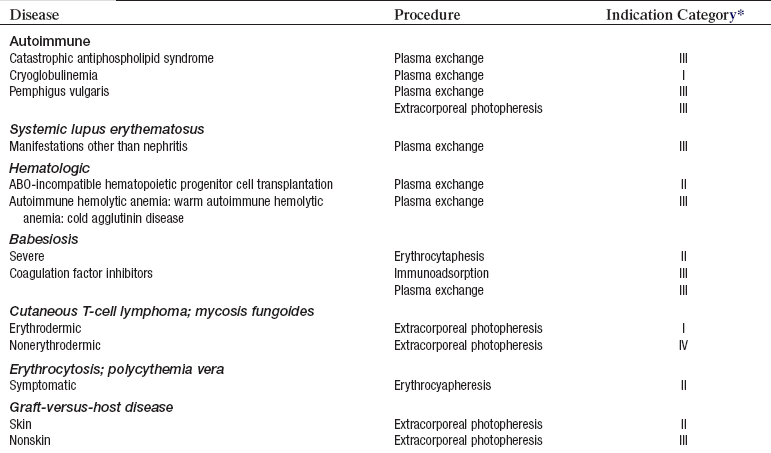
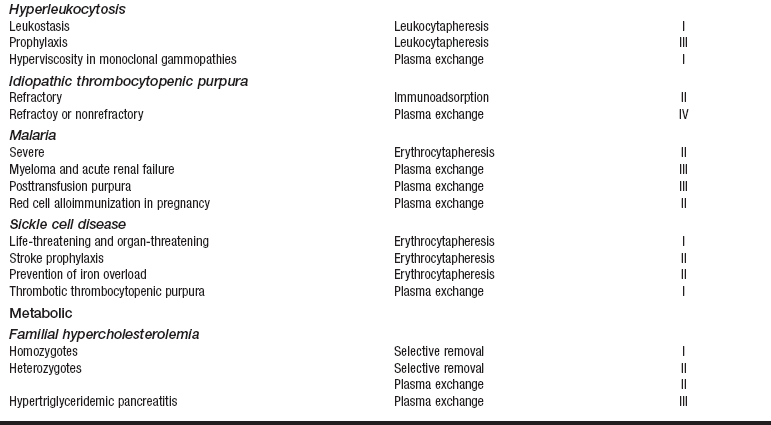
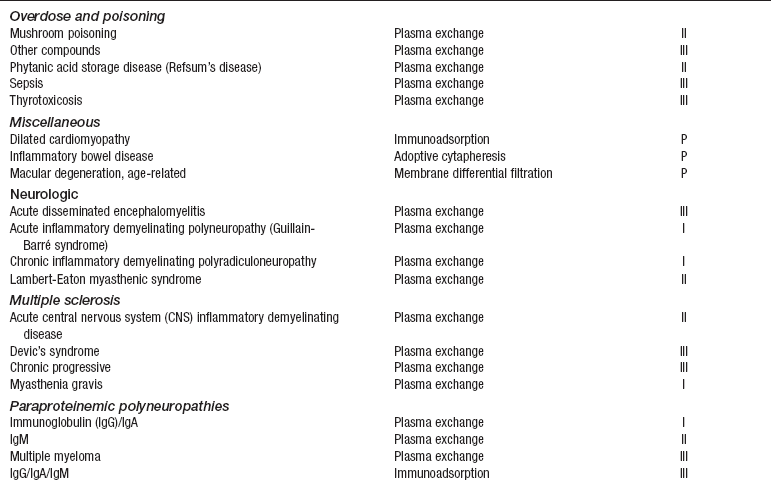
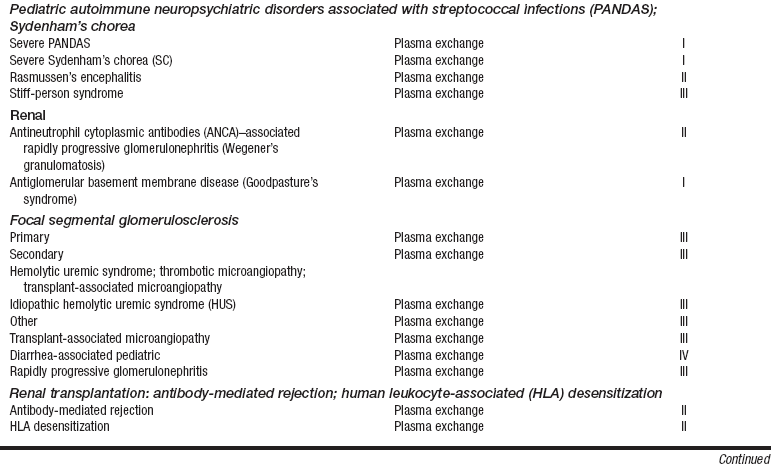
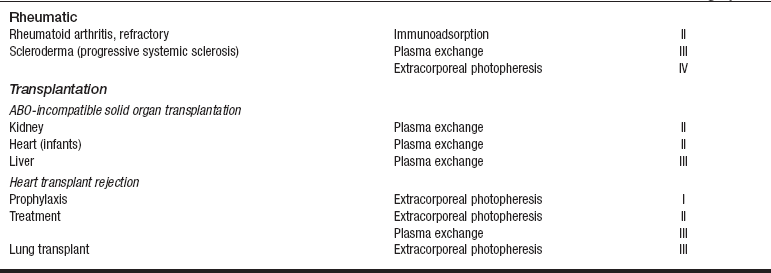
*Indication categories as established by the American Medical Association:
From Szczepiorkowski ZM, Shaz BH, Bandarenko N, et al: The new approach to assignment of ASFA categories: introduction to the fourth special issue: clinical applications of therapeutic apheresis, J Clin Apherisis 22:96-105, 2007.
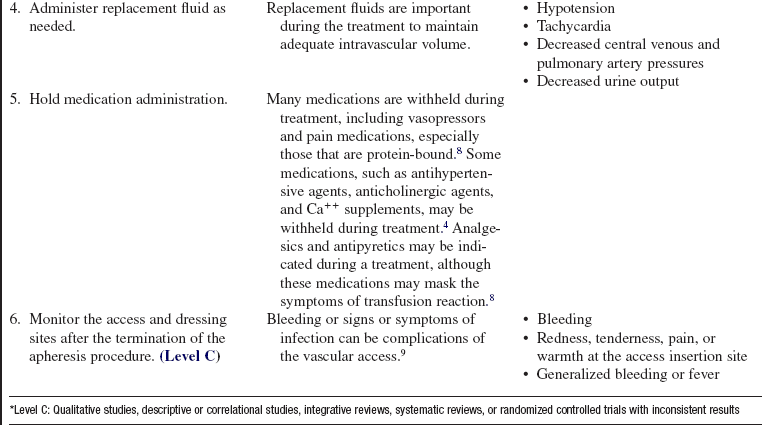
• If the patient is taking angiotensin-converting enzyme (ACE) inhibitors, contact with certain filters or membranes in the apheresis system can cause an anaphylactic reaction and severe hypotension as a result of increased levels of bradykinin, a potent vasodilator. ACE inhibitors are recommended to be withheld for 48 to 72 hours before treatment.
• Invasive procedures should be delayed until after the treatment, unless FFP is used as a replacement fluid.
• Potential complications of apheresis techniques include the following:
PATIENT AND FAMILY EDUCATION
• Explain the procedure, including risks, length of treatment, and patient positioning, and answer any questions the patient may have.  Rationale: Explanation provides information and may decrease patient anxiety.
Rationale: Explanation provides information and may decrease patient anxiety.
• Explain the purpose of the apheresis procedure, why this treatment is being performed, and the expected clinical outcomes.  Rationale: Plasmapheresis is used to treat antibody-mediated disorders.
Rationale: Plasmapheresis is used to treat antibody-mediated disorders.
• Explain the need for careful sterile technique for the duration of treatment.  Rationale: Sterile technique is important to decrease the chance of systemic infection because pathogens can be transported throughout the entire body via the circulation.
Rationale: Sterile technique is important to decrease the chance of systemic infection because pathogens can be transported throughout the entire body via the circulation.
• Explain the need for careful monitoring of the patient for complications.  Rationale: Hypocalcemia, hypotension, bleeding, and hypothermia are all potential complications of apheresis.
Rationale: Hypocalcemia, hypotension, bleeding, and hypothermia are all potential complications of apheresis.
• Explain the importance of the patient informing the nurse how he or she is feeling during the treatment.  Rationale: Patient symptoms can be important signs of complications related to the procedure. Examples include lightheadedness as a sign of hypotension and numbness and tingling as a sign of hypocalcemia.
Rationale: Patient symptoms can be important signs of complications related to the procedure. Examples include lightheadedness as a sign of hypotension and numbness and tingling as a sign of hypocalcemia.
• Explain the importance of preventing bleeding complications: pressure dressings at vascular sites, avoiding shaving, and care of access catheter.  Rationale: Alterations in blood composition and anticoagulation can put the patient at risk for bleeding.
Rationale: Alterations in blood composition and anticoagulation can put the patient at risk for bleeding.
• Explain the apheresis circuit setup to the patient and family.  Rationale: Blood will be removed from the patient’s body and will be visible during the plasmapheresis treatment.
Rationale: Blood will be removed from the patient’s body and will be visible during the plasmapheresis treatment.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Obtain baseline vital signs, body system assessment, hemodynamic parameters (if appropriate), weight, and pretreatment fluid balance.  Rationale: Total body assessment should be based specifically on the patient’s diagnosis and reason for treatment. Pretreatment assessment provides a baseline for comparison once the treatment is started, allowing for appropriate modification of intervention as needed. Changes in weight during and after treatment are an indicator of fluid balance.
Rationale: Total body assessment should be based specifically on the patient’s diagnosis and reason for treatment. Pretreatment assessment provides a baseline for comparison once the treatment is started, allowing for appropriate modification of intervention as needed. Changes in weight during and after treatment are an indicator of fluid balance.
• Review medications and ensure that the patient has not taken an ACE inhibiter within 48 hours.  Rationale: Contact with certain fibers or membranes in the apheresis system can cause an anaphylactic reaction and severe hypotension.
Rationale: Contact with certain fibers or membranes in the apheresis system can cause an anaphylactic reaction and severe hypotension.
• Assess pretreatment laboratory values.  Rationale: Baseline values are needed of the complete blood count (CBC) with differential, platelet count, and electrolytes before these are altered by treatment. Coagulation parameters are particularly important: fibrinogen, prothrombin time (PT), activated clotting time (ACT), and partial thromboplastin time (PTT), if heparin is used; and ACT and ionized Ca++, if citrate is used. Serum sodium and serum bicarbonate levels/pH also should be evaluated in patients when citrate is used as the anticoagulant. Disease-specific tests should also be obtained pretreatment as needed.
Rationale: Baseline values are needed of the complete blood count (CBC) with differential, platelet count, and electrolytes before these are altered by treatment. Coagulation parameters are particularly important: fibrinogen, prothrombin time (PT), activated clotting time (ACT), and partial thromboplastin time (PTT), if heparin is used; and ACT and ionized Ca++, if citrate is used. Serum sodium and serum bicarbonate levels/pH also should be evaluated in patients when citrate is used as the anticoagulant. Disease-specific tests should also be obtained pretreatment as needed.
• Obtain vascular access.  Rationale: A properly functioning vascular access is necessary to perform plasmapheresis.
Rationale: A properly functioning vascular access is necessary to perform plasmapheresis.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that informed consent has been obtained.  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient.
• Ensure that patient understands preprocedural teachings. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Assist the patient to a position of comfort that also facilitates optimal blood flow through the vascular access.  Rationale: Facilitating patient comfort helps to minimize the amount of patient movement during treatment. Movement can change the blood flow through the access site. Different access sites may require different patient positions to facilitate optimal blood flow.
Rationale: Facilitating patient comfort helps to minimize the amount of patient movement during treatment. Movement can change the blood flow through the access site. Different access sites may require different patient positions to facilitate optimal blood flow.
References
1. Garner, Sf, et al, Apheresis donors and platelet function. inherent platelet responsiveness influences platelet quality. Transfusion 2008; 48:673–680.
2. McLeod, BC. Evidence based therapeutic apheresis in -autoimmune and other haemolytic anemias. Curr Opin Hematol. 2007; 14:647–654.
3. Rahman, T, Harper, L, Plasmapheresis in nephrology. an update. Curr Opin Nephrol Hypertens 2006; 15:603–609.
4. Rohe, RM. Therapeutic plasma exchange. In: Counts C, et al, eds. Core curriculum for nephrology nursing. ed 5. Pitman, NJ: American Nephrology Nurses’ Association; 2008:277–308.
5. Roth, SH. Role of apheresis in rheumatoid arthritis. Drugs. 2006; 66:1903–1908.
6. Szczepiorkowski, ZM, et al, The new approach to assignment of ASFA categories. introduction to the fourth special issueclinical applications of therapeutic apheresis. J Clin Apher 2007; 22:96–105.
7. Szczepiorkowski, ZM, et al, Guidelines on the use of -therapeutic apheresis in clinical practice. evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apheresis 2007; 22:106–175.
8. Tobian, AA, et al, Transfusion premedications. a growing practice not based on evidence. Transfusion 2007; 47:1089–1096.
Young, EJ, et al. Incidence and influencing factors -associated with exit site infections in temporary catheters for hemodialysis and apheresis. Nephrol Nurs J. 2005; 32:41–50.
Heddle, NM, et al, Comparing the efficacy and safety of apheresis and whole blood-derived platelet transfusions. a systematic review. Transfusion 2008; 48:1447–1458.
George, JN. Evaluation and management of patients with thrombotic thrombocytopenic purpura. J Intensive Care Med. 2007; 22:82–91.
Greenberg, BM, Idiopathic transverse myelitis. corticosteroids, plasma exchange, or cyclophosphamide. Neurology 2007; 68:1614–1617.
Thompson, GR, et al. Recommendations for the use of LDL apheresis. Atherosclerosis. 2008; 198:247–255.
Van de Watering L. The intention-to-treat principle in clinical trails and meta-analyses of leukoreduced blood transfusions in surgical patients. Transfusion. 2007; 47:573–581.