CHAPTER 111
Radiation Fibrosis Syndrome
Katarzyna Ibanez, MD; Michael D. Stubblefield, MD
Definition
Radiation-induced toxicity after cancer treatment may result in significant long-term disability. Radiation fibrosis describes the insidious pathologic fibrotic tissue sclerosis that occurs in response to radiation exposure. Radiation fibrosis syndrome (RFS) is the term used to describe the myriad clinical manifestations of progressive fibrotic tissue sclerosis that can result from radiation treatment. It is estimated that about half of the approximately 14 million cancer survivors in the United States will receive radiation treatment during the course of their disease [1]. The incidence of RFS is unknown, and its severity is affected by multiple factors (see later). Radiotherapy is typically combined with surgery or chemotherapy; therefore the toxicities of these modalities may be cumulative and difficult to separate clinically.
The therapeutic goal of radiation therapy is to kill rapidly proliferating tumor cells by inducing apoptosis or mitotic cell death through free radical–mediated DNA damage [2]. Various dose-sculpting techniques have been developed to minimize exposure to normal tissues; however, radiation exposure to normal body cells cannot be completely eliminated [3]. The effects of radiation can be acute (occurring during or immediately after treatment), early-delayed (up to 3 months after completion of treatment), or late-delayed (occurring more than 3 months after completion of treatment) [4]. Radiation fibrosis is generally a late complication of radiation therapy and may become clinically apparent many years after treatment. Its progression can be insidious or rapid, but it is invariably irreversible [5,6]. It can damage any tissue type, including skin, muscle, ligament, tendon, nerve, viscera, and bone [7]. The underlying mechanism of radiation fibrosis is complex and not completely understood. It has been postulated that radiation-induced vascular endothelial injury causes thrombomodulin deficiency, resulting in its inability to scavenge locally formed thrombin, which in turn leads to abnormal accumulation of proliferative fibrin in the intravascular, perivascular, and extravascular compartments [2].
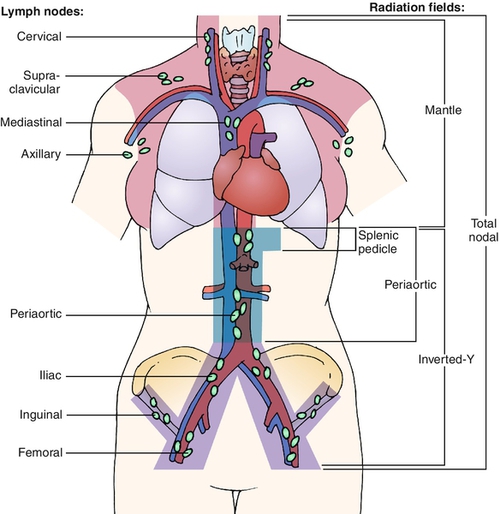
The long-term morbidity due to RFS is largely determined by the size of the radiation field, the type and susceptibility of underlying tissues to radiation, and the patient’s individual resistance to the effects of radiation. Other factors include the patient’s age, overall health, and medical and degenerative disorders, particularly degenerative spine disease; cancer status; exposure to neurotoxic, cardiotoxic, and other chemotherapy types; and time since radiation was administered [8]. For signs or symptoms to be considered referable to RFS, either the structures generating them must be within the radiation field or the neural, vascular, lymphatic, muscular, tendinous, or other structures important in their genesis must traverse the field. It is therefore necessary to understand which type of radiation field was used to treat a given patient to determine whether the signs, symptoms, or functional deficits can be attributable to RFS. The common radiation fields used in Hodgkin lymphoma (HL) are depicted in Figure 111.1. Extensive radiation fields, such as mantle field used to treat HL, can result in widespread sequelae of RFS. Patients with head and neck cancer (HNC) treated with radiation are also likely to develop RFS because of the high dose of radiation required for tumor control as well as the proximity of many vital tissues to the radiation field [9].
Symptoms
Patients with RFS can present with a variety of symptoms as virtually every organ system can be affected. Symptoms should be anatomically congruent to the radiation field and involved tissues. HL survivors frequently present with neck extensor weakness (dropped head syndrome), pain and limited range of motion of the neck and shoulders, weakness, fatigue, gait and dexterity problems, neuropathic symptoms, and difficulty in performing activities of daily living. Spasms are frequently described as tight, pulling, or cramping sensations. Neuropathic pain is usually described as burning, stabbing, or searing. HNC patients commonly have trismus, cervical dystonia, facial lymphedema, dysphagia, and dysarthria. Radiation-induced trigeminal neuralgia (commonly in the V2-V3 distribution on the affected side, but bilateral involvement is possible) and anterior cervical neuralgia are also common complications. Neuropathic pain in patients with RFS can be severe and markedly out of proportion to the perceived pathologic process. If the spinal cord was in the radiation field, patients may present with spastic paraparesis or quadriparesis, depending on the level of the spinal cord affected by radiation. Early-delayed radiation-induced myelopathy is almost always reversible, whereas late-delayed is almost always progressive and permanent [10]. If autonomic nerves are affected, patients can present with orthostatic hypotension, baroreceptor failure, bowel and bladder dysfunction, and sexual dysfunction. Shortness of breath in HL patients treated with mantle field radiation may be due to pulmonary insufficiency from bilateral phrenic nerve dysfunction [11].
Physical Examination
Comprehensive examination, including detailed neuromuscular and musculoskeletal evaluation, is of paramount importance. Physical examination findings will vary greatly from patient to patient; however, a full account of physical examination findings is beyond the scope of this chapter.
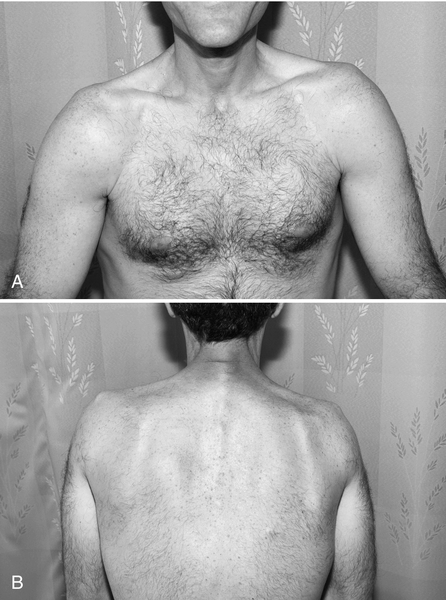
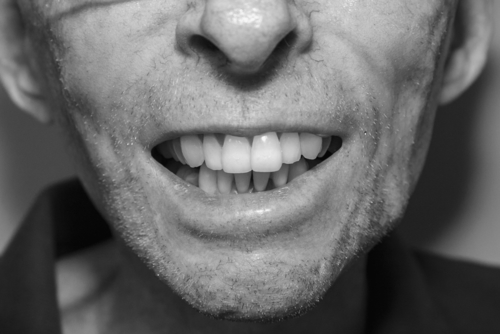
Examination of patients treated for HL commonly demonstrates cervicothoracic paraspinal, shoulder girdle, and rhomboid muscle atrophy and often a C-shaped posture due to forwardly positioned neck and shoulders secondary to relatively strong pectoral muscles (Fig. 111.2). HNC patients commonly present with asymmetric positioning of the head and neck due to severe neck tightness, pain, and spasms of trapezius, sternocleidomastoid, and scalene muscles, among others. This radiation-induced cervical dystonia may progress to fixed contracture of the anterior cervical musculature [12]. Trismus seen in HNC patients is commonly associated with spasms in the masseter and pterygoid muscles (Fig. 111.3). Marked loss of range of motion and function may be seen if joints were involved in the radiation field. Rotator cuff tendinitis and adhesive capsulitis may develop because of perturbation of normal shoulder motion secondary to C5 and C6 radiculopathy or upper brachial plexopathy [13]. Neurologic testing may reveal sensory loss, including light touch, pain, temperature, vibration, and proprioception. Weakness and gait dysfunction may be secondary to damage of neural tissue (spinal cord, plexus, cauda equina, peripheral nerves) or muscle itself. Not infrequently, the clinical picture fits that of myelo-radiculo-plexo-neuro-myopathy, with some or all components of the neuromuscular axis affected to varying degrees. The upper brachial plexus, frequently included in the radiation field of head and neck radiation ports, may be more susceptible to radiation injury because of its apical location in the neck and the long course traversed by its fibers relative to the middle and lower trunk. The pyramidal shape of the thorax and the clavicle may provide less protective tissue around the upper plexus, but the clinical validity of this phenomenon is unclear [14].
Functional Limitations
Sensory loss, pain, and weakness may have profound effects on gait and ability to perform activities of daily living. Loss of sensation also renders the patient more susceptible to secondary musculoskeletal injuries. Patients with severe trismus may have deficits including but not limited to difficulty in speaking, eating, drinking, and performing oral hygiene. Inability to position the head due to progressive fibrosis and contracture of the anterior cervical musculature can also affect swallowing, phonation, and work-related tasks. Patients with neck extensor weakness frequently have to stop driving and limit their work and leisure activities. Disability may also be related to bowel, bladder, and sexual dysfunction.
Diagnostic Studies
Imaging is often useful in the evaluation of RFS. A baseline magnetic resonance imaging (MRI) study of the entire spine for HL patients with RFS and of the cervical spine for HNC patients is often indicated to exclude degenerative processes of the spine or secondary malignant neoplasms. The addition of gadolinium contrast is required to exclude brain metastasis, intramedullary spinal tumor, or leptomeningeal disease and to differentiate tumor from scarring or fibrotic tissue in patients with a history of prior surgery. In addition, MRI of the spine may demonstrate overt demyelinating lesions or nodular enhancement of the cauda equina mimicking leptomeningeal disease in some patients treated for HL with high-dose radiation (Fig. 111.4). Computed tomography (CT) with contrast enhancement is used to evaluate the viscera of the chest, abdomen, and pelvis for metastatic or progressive disease. CT may also be used if MRI is contraindicated (because of a pacemaker, aneurysm clips, or breast tissue expanders, for example). CT myelography is indicated when metallic hardware causes excessive artifact, precluding adequate visualization of the spinal canal. Radiography is useful to evaluate suspected loosening or failure of spinal stabilization hardware or joint replacement prostheses.
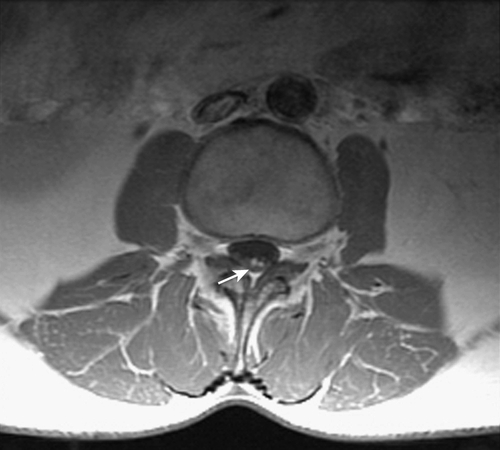
Electrodiagnostic evaluation may provide invaluable information by identifying, localizing, confirming, or differentiating radiculopathy, plexopathy, neuropathy, or myopathy in patients with RFS. Needle electromyography can confirm muscle denervation and may identify myokymia, fasciculations, and muscle spasms. Given the high likelihood of multiple overlapping disorders present on electrodiagnostic testing, these studies should be interpreted by electromyographers with considerable experience in the evaluation of complex neuromuscular disorders.
Treatment
Initial
Whereas there is no cure for or way to stop progression of fibrotic tissue sclerosis leading to RFS, the patient’s symptoms and function are likely to improve with appropriate treatment. Treatment is geared specifically toward the patient’s complaints and functional deficits. Specialized care and a multidisciplinary approach are critical to successful rehabilitation. Patient education with an emphasis on the benefits of a lifelong home exercise program is of paramount importance. Many patients, especially HL survivors, require physical, occupational, and lymphedema therapy. The goals of physical therapy are to restore muscle balance and to correct the posture and body mechanics by stretching tight structures, strengthening weak musculature (core and neck extensors), and improving proprioception and endurance. Physical and occupational therapy are critical elements to rehabilitation of HNC patients with trismus and cervical dystonia. Patients with trismus also benefit from speech and swallowing therapy.
Rehabilitation
Orthotic devices are prescribed according to the principles used in other neuromuscular and musculoskeletal disorders, regardless of the underlying cause. Patients with weak neck extensors may benefit from a cervical collar, such as the Headmaster Collar (Symmetric Designs, Salt Spring Island, British Columbia, Canada). It is intended only for intermittent use and serves as an energy conservation device. Upper extremity orthotic devices may be appropriate in select patients with severe radiculopathy or plexopathy but are infrequently used. Lower extremity orthotic devices, such as ankle-foot orthoses, may be used for patients with a footdrop from any cause, including myelopathy, radiculopathy (usually L4-L5), plexopathy (lumbosacral trunk), and neuropathy (sciatic, common peroneal [fibular], deep peroneal [fibular] nerves). A posterior leaf spring ankle-foot orthosis is the most commonly used as it is lighter and less bulky than the alternatives. Patients with excessive spasticity (rarely seen in RFS unless myelopathy predominates) or significant mediolateral ankle instability require a custom molded ankle-foot orthosis. Control of the knee to prevent buckling is sometimes indicated. Patients with weak quadriceps may develop compensatory genu recurvatum, which may result in knee pain and degeneration. Older patients and those with weakness affecting multiple joints may benefit from a knee immobilizer. If weakness of both knee extension and ankle dorsiflexion is present, a knee immobilizer can often be fabricated to fit in a modular fashion over an ankle-foot orthosis and tends to be easier to manipulate than a knee-ankle-foot-orthosis.
Medications are often needed to control pain and muscle spasms in RFS. For the pain to be effectively managed, one must determine whether the pain is neuropathic or musculoskeletal (somatic) in nature. In many patients, both neuropathic and somatic pain may coexist. Neuropathic pain and, to some extent, muscle spasms associated with RFS may respond to nerve stabilizing agents. Pregabalin (Lyrica) is preferred to gabapentin (Neurontin), given its favorable pharmacokinetics, efficacy, and low potential for drug-drug interaction [15]. Duloxetine (Cymbalta) is also a suitable agent for neuropathic pain in RFS and can be used in conjunction with pregabalin. Duloxetine, as a serotonin and norepinephrine reuptake inhibitor, may adversely interact with other serotonin reuptake inhibitors and should generally be avoided with tricyclic antidepressants, tramadol, and triptans. Opioids are often added to pregabalin or duloxetine when needed but should be considered second-line agents. Tricyclic antidepressants might be helpful in neuropathic pain, but their unfavorable side effect profile (anticholinergic effects resulting in dry mouth, blurred vision, constipation, urinary retention, arrhythmia, drowsiness, orthostatic hypotension, sexual dysfunction) limits their use. A short course of a nonsteroidal anti-inflammatory drug (NSAID), especially during acute exacerbation of symptoms, may be used to control somatic pain from inflammation of the rotator cuff, adhesive capsulitis, and other underlying degenerative changes. Long-term use of NSAIDs is relatively contraindicated, given their known gastrointestinal and cardiovascular side effects, particularly in the HL survivor population, who are at increased risk. Opioids are often used to control somatic pain when NSAIDs are ineffective or contraindicated. Muscle relaxants, including baclofen, tizanidine (Zanaflex), and benzodiazepines, may help relieve pain associated with muscle spasms but should be discontinued if deemed ineffective after a short trial.
Patients with trismus should be evaluated for a jaw stretching device. In the first 6 months of treatment, the TheraBite Jaw Motion Rehabilitation System (Atos Medical Inc., West Allis, Wisconsin) may be used. The Dynasplint Trismus System (Dynasplint, Severna Park, Maryland) is generally preferred in patients with chronic trismus because it is customizable and uses the principle of low-torque, prolonged duration stretch, which may be more effective at improving range of motion in an experimental model [16].
Procedures
Trigger point injections and botulinum toxin injections are of particular benefit in specific conditions associated with RFS [17]. Trigger point injections may provide significant pain relief for up to 1 month and should be offered to patients with painful muscle spasms in the trapezius, levator scapulae, rhomboids, cervical paraspinal, and other muscles. Usually, 2 to 5 mL of 0.25% bupivacaine or a similar anesthetic is used per location, up to a maximum of 20 mL divided between one and ten sites. Botulinum toxin injections may be used as both primary and adjunctive treatment for musculoskeletal pain, muscle spasms, spasticity, migraines, neuropathic pain, and a variety of other disorders [18]. Some studied cancer-related indications for botulinum toxin injections include chronic and neuropathic pain after neck dissection, muscle spasms after radiation therapy to the head and neck, and radiation-induced trismus [12,19,20].
Patients with RFS-related neuromuscular and musculoskeletal pain who fail to respond to conservative management may also benefit from interventional procedures, including epidural steroid injections, peripheral nerve blocks, and intra-articular joint injections. Placement of implantable epidural drug pumps is usually discouraged because it would preclude patients from undergoing MRI in the future, which might be needed for disease surveillance.
Surgery
In general, conservative measures are the mainstay of treatment because surgery is unlikely to improve the marked and progressive neuromuscular disorders due to RFS. Severe dysfunction and atrophy of the underlying neuromuscular structures confer poor surgical outcomes. Coronoidectomy may be considered in a patient with severe trismus for whom conservative management failed, although clinical evidence for its efficacy is limited [21].
Potential Disease Complications
The clinical complications of RFS vary greatly and depend on multiple factors, as discussed earlier. Multiple non-neuromuscular complications (e.g., fatigue, alopecia, lymphedema, esophagitis, pericarditis, volvulus) as well as neuromuscular and musculoskeletal complications of RFS (e.g., myelo-radiculo-plexo-neuro-myopathy, osteoporosis, osteoradionecrosis, adhesive capsulitis) are well recognized. Patients with a history of HL treated with mantle or other types of radiation are at a greatly increased risk for development of secondary cancers (including thyroid, breast, lung, and sarcoma) and accelerated cardiovascular disease (including atherosclerosis, valvular heart disease, pericardial disease, cardiomyopathy, arrhythmias, and subclavian and carotid artery stenosis) [22]. Childhood cancer survivors are at the greatest risk for complications, including radiation-induced neoplasms, osteonecrosis, and abnormal bone growth either from cranial radiation affecting the endocrine system or from abnormal maturation of growth plates affected by radiation.
Potential Treatment Complications
Medications used to treat muscle spasms as well as neuropathic and musculoskeletal pain can result in significant side effects, in some cases leading to medication discontinuation by the patient. This can frequently be avoided by slow dose titration with the goal of finding the lowest effective dose of medications. Trigger point and botulinum toxin injections carry a low risk of adverse effects if they are given by an experienced practitioner. Altered anatomy from radiation and surgery should be taken into consideration. Some of the possible side effects include pneumothorax, bleeding, and infection. Botulinum toxin injections can cause focal muscle weakness ranging from a droopy eyelid or neck extension weakness to a severe dysphagia necessitating feeding tube placement; fortunately, these effects are transient. Starting doses of botulinum toxin should be low, and only relatively strong muscles should be targeted for injections.