CASE 105



History: A 45-year-old African-American woman presents with a palpable area of concern in the upper inner right breast found on self-breast examination. She has no family history of breast cancer.
1. What should be included in the differential diagnosis? (Choose all that apply.)
2. Ultrasound-guided biopsy was performed and showed polygonal cells in bundles and cords, with abundant granular eosinophilic cytoplasm and stromal fibrosis. What is the next step in management?
A. Repeat of ultrasound-guided biopsy
B. Follow-up mammogram, ultrasound, and MRI in 6 months
D. Lumpectomy and radiation therapy
3. Granular cell tumors may appear as any of the following except:
B. Partially circumscribed mass with calcifications
C. Partially circumscribed mass without calcifications
D. Partially ill-defined hypoechoic mass with posterior acoustic shadowing
4. Which of the following statements regarding granular cell tumors is true?
A. They are typically found in the breast.
B. They may mimic breast cancer both clinically and on imaging.
C. They usually occur as an incidental finding on screening mammogram.
D. They are a subtype of ductal breast carcinoma.
ANSWERS
CASE 105
Granular Cell Tumor
1. A and D
2. C
3. B
4. B
References
Adeniran A, Al-Ahmadie H, Mahoney M, et al. Granular cell tumor of the breast: a series of 17 cases and review of the literature. Breast J. 2004;10(6):528–531.
Porter GJ, Evans AJ, Lee AH, et al. Unusual benign breast lesions. Clin Radiol. 2006;61(7):562–569.
Comment
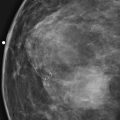
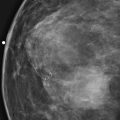
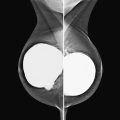
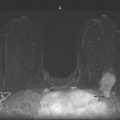
Granular cell tumors are rare benign lesions, thought to derive from Schwann cells. They can be located in almost any tissue, but the most common site involved is the tongue. Approximately 5% to 8% of cases occur in the breast, and they are more common in premenopausal African-American women.
Granular cell tumors mimic breast cancer both clinically and on imaging. Clinically, they usually manifest as a firm, painless, palpable mass, usually fixed to the pectoral fascia, which may cause retraction of the skin. However, in contrast to breast carcinomas, which usually occur in the upper outer quadrant, granular cell tumors are predominantly found in the upper inner quadrant of the breast (area of cutaneous innervation by the supraclavicular nerve).
The appearance on mammography is more frequently a poorly defined or spiculated mass, without calcifications. On ultrasound, they may appear as an irregular or spiculated mass with posterior acoustic shadowing and less frequently as a circumscribed mass. Reported MRI findings of granular cell tumors in sites other than the breast show variable T2 signal intensity (from low or intermediate to high compared with the adjacent muscle) and low to intermediate signal on T1-weighted images; both homogeneous and peripheral enhancement have been described after contrast agent administration (see the figures).
Biopsy should be performed to obtain a definitive diagnosis. Although these are benign lesions, treatment is wide local excision, owing to their potential local recurrence. Rare malignant cases have been reported.
CASE 106


History: A 54-year-old woman presents for screening mammogram.
1. What should be included in the differential diagnosis for the calcifications in the far posterior upper outer right breast? (Choose all that apply.)
B. Atypical ductal hyperplasia
2. Which of the following situations does not make stereotactic biopsy difficult (requiring special positioning of the patient) or technically impossible?
B. Central cluster of microcalcifications measuring 2 to 3 mm
C. Microcalcifications located close to the chest wall
D. Microcalcifications located in the axillary tail of the breast
3. Which of the following is not a special maneuver in patient and breast positioning during stereotactic biopsy to improve visualization of posterior or axillary tail microcalcifications?
A. Pull the breast, gently but firmly, through the opening of the stereotactic table.
B. Encourage the patient to relax the chest muscles as much as possible.
C. Place the patient’s arm through the breast opening of the table.
D. Roll the patient into a supine position.
4. Which of the following is not a limitation of stereotactic biopsy compared with ultrasound-guided biopsy?
A. Compression of the breast is a necessity.
B. The patient must remain still during the biopsy procedure.
C. The patient must lie on her stomach.
D. The patient is typically lying supine.
ANSWERS
CASE 106
Difficult Stereotactic Biopsy—Calcifications in the Axillary Tail of the Breast
1. A, B, and C
2. B
3. D
4. D
References
Jackman RJ, Marzoni FA Jr. Stereotactic histologic biopsy with patients prone: technical feasibility in 98% of mammographically detected lesions. AJR Am J Roentgenol. 2003;180(3):785–794.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:215.
Comment
Stereotactic biopsy is the method used when indeterminate or suspicious calcifications are seen on a mammogram (see the figures). If there is no associated mass, these lesions may be seen only on the mammogram. To obtain a biopsy specimen of these lesions, stereotactic biopsy, a mammographically derived procedure, is performed. This procedure may be performed with a special add-on unit that is attached to the regular mammogram device, and the procedure is completed with the patient sitting in a chair. A specially designed table may also be used. On this table, the patient lies prone, and the breast is pulled through a round opening in the table. The biopsy is performed below the patient, by a radiologist sitting on a chair.
In 2003, nearly 2000 stereotactic biopsies and reasons for incomplete and canceled studies were reviewed. When stereotactic biopsies were canceled for technical reasons, most were canceled because the lesion was too close to the chest wall. Lesions close to the chest wall and in the axillary tail of the breast are difficult to position in the stereotactic window for biopsy. However, maneuvers can be used to visualize posterior lesions better so that they can be sampled stereotactically.
The breast should be pulled, gently but firmly, as completely as possible down through the opening in the stereotactic table. The patient should be encouraged to relax the chest muscles as much as possible. If the lesion cannot be seen, the patient’s arm can be placed through the breast opening of the table, which drops more of the axillary area into position for biopsy. The patient also can be rolled into a slightly decubitus position, with the side of interest down.
Biopsy specimens of calcifications in the axillary tail may be easier to obtain from the mediolateral oblique (MLO) or mediolateral approach rather than the craniocaudal (CC) approach. From the lateral approach, the calcifications are closer to the skin (see the figures). A biopsy clip should be placed at the site of biopsy after the procedure, so that if excision is needed, the area can be easily localized. Placement of a clip is especially important in difficult biopsies and if the cluster of microcalcifications is too small because it may be completely removed on the core biopsy. Specimen radiograph should be performed prior to clip placement, to ensure that the target has been sampled.
The mammogram obtained after biopsy in this case shows the clip at the site where the microcalcifications had been present. Histopathology of the calcifications on biopsy was atypical ductal hyperplasia, which should be surgically excised.
CASE 107




History: Two patients undergo routine screening mammograms. New calcifications are seen in the subareolar breast in each patient. Magnification views are shown.
1. What should be included in the differential diagnosis for both patients? (Choose all that apply.)
B. Ductal carcinoma in situ (DCIS)
2. What is the next step in management?
A. Follow-up diagnostic mammogram in 1 year with magnification views
B. Short-interval follow-up magnification views in 6 months
D. Image-guided vacuum-assisted needle biopsy
3. The needle biopsy result for both patients showed intraductal papilloma. Which of the following is not recommended?
B. Short-interval follow-up magnification views in 6 months
D. Follow-up ultrasound examination in 6 months
4. What symptom is commonly associated with subareolar intraductal papilloma?
ANSWERS
CASE 107
Calcified Subareolar Masses
1. B, C, and D
2. D
3. A
4. D
References
Al Sarakbi W, Worku D, Escobar PF, et al. Breast papillomas: current management with a focus on a new diagnostic and therapeutic modality. Int Semin Surg Oncol. 2006;3:1.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:120.
Comment
A papilloma is a tumor that arises from ductal epithelium. The term papilloma includes solitary papilloma (as in this case, see the figures), multiple papillomas, and juvenile papillomatosis. Intraductal papilloma is classified as a benign tumor of the breast, although the condition of multiple peripheral papillomas confers an increased risk of malignancy. Solitary papillomas, as in this case, are typically found in large ducts in the retroareolar breast and are less likely to be associated with DCIS. A solitary papilloma in a large duct is the most common presentation of all papillary lesions and affects an older age group compared with multiple peripheral papillomas.
Surgical consultation is recommended for women who have a biopsy result showing papilloma because there is an increased incidence of DCIS within the papilloma, which may not be found on a needle biopsy sample. This risk is reported to be 20%. There is some controversy surrounding this issue if a benign papilloma is found at core biopsy. However, if atypia or malignancy is present in the core specimen, surgical excision is universally recommended. There seems to be no statistical difference between 11-gauge and 14-gauge needle in the cancer yield in excisional biopsy in benign papillomas.
Papillomas may twist on the fibrovascular stalk and can cause bloody or clear spontaneous nipple discharge. Papilloma is the most common cause of pathologic nipple discharge (in 40% to 70% of women). However, most women with an intraductal papilloma are asymptomatic. Detection on mammogram is limited because these masses are contained within a duct that appears outwardly normal. When calcifications are present, mammographic detection increases (see the figures). A papilloma may also appear on mammogram as a well-defined mass.
CASE 108


History: A 56-year-old woman has a 1-year history of bilateral bloody nipple discharge. Mammogram and ultrasound are both negative. Galactography was unsuccessful. Clinical breast examination is positive only for easily elicited bloody discharge from both nipples; no masses are palpated.
1. What should be included in the differential diagnosis of the bilateral, post–contrast subtraction MRI images shown? (Choose all that apply.)
A. Bilateral intraductal carcinoma
C. Bilateral duct ectasia with debris
2. What is the management of this imaging finding?
A. Targeted ultrasound with biopsy
C. Follow-up in 6 months because these masses are likely benign
3. Is MRI useful in this clinical setting?
A. No, because approximately 25% of papillomas do not enhance
B. No, because galactography can provide the same information
C. Yes, because MRI can provide the location and number of enhancing masses
D. No, because the surgeon can perform central duct excision
4. Which of the following is not pathologic nipple discharge?
A. Blackish and greenish discharge from multiple orifices
B. Spontaneous watery discharge
D. Dark maroon and spontaneous
ANSWERS
CASE 108
MRI of Nipple Discharge
1. A, B, and D
2. B
3. C
4. A
References
An HY, Kim KS, Yu IK, et al. Image presentation. The nipple-areolar complex: a pictorial review of common and uncommon conditions. J Ultrasound Med. 2010;29(6):949–962.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:383.
Comment
Nipple discharge should be clinically evaluated because it may be a symptom of breast cancer. However, most causes of nipple discharge are benign. Pathologic nipple discharge includes spontaneous discharge that is unilateral, persists, and can range from clear (watery) to serous to bloody. Nonpathologic discharge is typically elicited only on squeezing the nipple; benign discharge can be milky, green, or gray to black. Nonpathologic discharge typically occurs in more than one orifice of the nipple and may be bilateral.
Masses that can cause nipple discharge include papillary masses arising in the lactiferous ducts, ranging from benign papilloma to papillary DCIS to invasive papillary cancer. Other masses include nipple adenoma, which is a proliferation of tubules in the duct, and DCIS and invasive ductal carcinoma.
MRI can be useful in the diagnosis of pathologic nipple discharge when standard diagnostic imaging fails. Intraductal masses may be the cause of discharge, and if the mass is not calcified, it is unlikely to be seen on mammography. Ultrasound is useful to evaluate the cause of discharge, but debris in the duct can look like a mass. With ultrasound, it is helpful to orient the transducer along the long axis of the duct. Galactography is the standard interventional method of evaluating discharge. It may be technically difficult to cannulate the duct, and distal nipple masses, such as in this patient (see the figures), can block the placement of the cannula.
Papillomas do not consistently enhance on MRI. Approximately 75% are seen after contrast injection, and kinetics range from slow, persistent enhancement to a malignant profile. Other findings on MRI include an enlarged duct, with fluid hyperintense on T2 images, with an intraductal hypointense mass.
Biopsy may be performed with imaging guidance if the lesion is seen on ultrasound or contains calcifications that can be targeted for stereotactic biopsy. Biopsy of masses in the nipple is technically difficult using any imaging modality, and these masses should be surgically excised.
CASE 109



History: A 44-year-old woman is scheduled for surgery to remove a mass in the left breast after core biopsy.
1. What should be included in the differential diagnosis for the ultrasound image of the mass in the left breast? (Choose all that apply.)
2. Which of the following statements regarding the best selection of imaging modality to guide needle localization is false?
A. Masses are generally localized under ultrasound guidance.
B. Calcifications are generally localized under mammographic guidance.
C. Areas of non-masslike enhancement are generally localized under MRI guidance.
D. Needle localization can be performed only under mammographic guidance.
3. Which of the following are the correct steps, in order, to perform ultrasound-guided needle localization?
4. Which of the following statements regarding ultrasound-guided needle localization is true?
A. It may not be necessary to send mammogram films to the operating room.
B. Obtaining a radiograph of the specimen is unnecessary.
C. The specimen radiograph is evaluated by the surgeon in the operating room.
D. The surgeon does not need to be notified of the specimen radiograph findings.
ANSWERS
CASE 109
Ultrasound-Guided Needle Localization
1. A, C, and D
2. D
3. B
4. A
References
DePalo AJ. Surgical considerations in needle localization procedures. Semin Surg Oncol. 1991;7(5):253–256.
Klimberg VS. Advances in the diagnosis and excision of breast cancer. Am Surg. 2003;69(1):11–14.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:200.
Comment
Needle localization procedures are less frequently performed at the present time because of the wide acceptance of percutaneous core biopsies. The most common indication for needle localization is excision of nonpalpable high-risk lesions or carcinoma diagnosed at core biopsy. Another indication is to aid in the excision of a nonpalpable lesion when biopsy with imaging guidance cannot be performed.
Needle localization may be performed under mammographic, ultrasound, or MRI guidance. The selection of the guiding modality should be based on the ease of seeing the lesion. Generally, calcifications are localized under mammographic guidance, masses are localized under ultrasound guidance, and areas of non-masslike enhancement are localized under MRI guidance.
For ultrasound-guided needle localization, the patient is positioned so that the skin entry site for placement of the localizing needle and hook wire is as close to the lesion as possible. The needle length is chosen by measuring the distance from the closest skin surface to the site of the lesion on the ultrasound screen. After the lesion is identified and the skin entry site has been selected, the skin is cleansed and anesthetized, and the needle is placed into the breast, parallel to the chest wall (see the figures). Needle placement into the lesion is confirmed by ultrasound in orthogonal planes (needle parallel and perpendicular to the transducer) in real time during the procedure. The needle is adjusted so that the tip is at or just beyond the lesion. The hook wire is placed through the needle and secured in the breast tissue. Some surgeons prefer to keep the needle in the breast to aid in localizing the lesion in the operating room, whereas other surgeons prefer to remove the needle and keep only the wire in the breast. Some surgeons prefer blue dye to be injected into the lesion to aid removal.
The ultrasound images are sent to the operating room, with the lesion circled. Some surgeons prefer that mammographic films also be sent to the operating room to serve as additional guidance. A specimen radiograph is obtained in every case (see the figures), but the lesion may not be evident radiographically, and ultrasound of the specimen may be necessary to confirm that the lesion has been removed. To aid the surgeon, a metal BB may be placed on the skin at the needle insertion site before the radiograph is taken. An “X” may be marked on the patient’s skin overlying the mass because the needle is not placed through the skin directly over the mass.
The radiologist must review the specimen to ensure that the lesion has been removed (or sampled, depending on the case). The surgeon is notified of the findings, and if the lesion has not been removed or adequately sampled, the surgeon should be directed to obtain more tissue.
CASE 110



History: A 44-year-old woman had invasive lobular carcinoma (ILC) diagnosed 3 years previously, was treated with breast conservation therapy, and is asymptomatic. She now presents for her annual mammogram.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
A. Normal postoperative mammogram and MRI
B. Recurrent carcinoma at the lumpectomy site
C. Fat necrosis at the lumpectomy site
D. Residual disease at the lumpectomy site
2. Is ILC more likely to have recurrence compared with invasive ductal carcinoma not otherwise specified (IDC NOS)?
A. Yes, because this tumor is more likely multifocal and multicentric
B. Yes, because ILC is typically larger at diagnosis compared with IDC
C. No, the recurrence rates are similar
D. Yes, because of the tumor biology of cells infiltrating in single file in ILC
3. What is the management of recurrent disease?
D. Surgery and additional radiation therapy
4. What is the most common presentation of recurrent disease after breast conservation?
A. Mass and/or calcifications away from the lumpectomy site, within 3 years of treatment
B. Mass and/or calcifications at the lumpectomy site, on the first mammogram after treatment
C. Mass and/or calcifications in the opposite breast, 3 years or more after treatment
D. Mass and/or calcifications at the lumpectomy site, 18 months or more after treatment
ANSWERS
CASE 110
Invasive Lobular Carcinoma with Recurrence
1. B, C, and D
2. C
3. A
4. D
References
Arpino G, Bardou VJ, Clark GM, et al. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149–R156.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:314. 324
Comment
ILC is more difficult to detect on mammography, ultrasound, and MRI compared with IDC. It is more likely to spread through the breast in single files of cells, inciting little desmoplastic reaction by the host. For this reason, ILC is larger at diagnosis compared with IDC NOS, more likely to need reoperation because of positive margins at surgery, and more likely to necessitate mastectomy at the time of surgery for lumpectomy because of positive margins. It is also more commonly multifocal and multicentric and more likely to have contralateral breast involvement.
The above-mentioned characteristics do not affect the recurrence rate or the disease-free survival. In one large series reviewing more than 50,000 cases of early breast cancer, the recurrence rate of ILC was slightly lower than the recurrence rate of IDC NOS, although this was not clinically significant. The two breast cancer types should be considered to have essentially the same clinical outcome; the failure rate of breast cancer treatment for both types is approximately 1% per year.
The patient in this case was 41 years old at the time of diagnosis, making her younger than the average patient with ILC (average age is 65 years). She had a normal screening mammogram and felt a palpable mass in her left breast 2 months later (false-negative mammogram is common with ILC). At the time of the work-up for the palpable mass, the diagnostic mammogram was negative, and ultrasound showed an 8-mm mass. Ultrasound is more sensitive than mammography in ILC but tends to underestimate the size of the tumor, as in this patient. MRI was negative; no enhancement was seen at the tumor site. No enhancement is rare for infiltrating cancers but more common with ILC than with IDC. When MRI shows ILC as an enhancing mass, it more accurately shows tumor size than mammography and ultrasound. At surgery, this patient required two reexcisions to obtain clear margins, which is more common with ILC than with IDC.
At 3 years after surgery, mammogram showed increased density at the lumpectomy site (see the figures); follow-up imaging had been unremarkable at 1 year after lumpectomy. MRI (see the figures) showed a new area of non-masslike enhancement at the lumpectomy site. Ultrasound and ultrasound-guided core biopsy were performed and showed ILC at the lumpectomy site, consistent with recurrence. The patient underwent mastectomy.
CASE 111



History: A 37-year-old woman who is BRCA-positive has a tiny irregular mass in the outer left breast found on screening MRI. The mass showed avid enhancement with gadolinium and was new compared with prior MRI studies. It was visible only on MRI.
1. What should be included in the differential diagnosis for the mass in the left breast? (Choose all that apply.)
2. Indications for MRI-guided needle localization include all of the following except:
A. Areas of non-masslike enhancement
B. Cluster of amorphous microcalcifications
C. Lesions not visible on mammography or ultrasound
D. Lesions visible on MRI, with equivocal results on mammogram and ultrasound
3. Which of the following statements regarding MRI-guided needle localization procedures is true?
A. The patient is positioned supine within a dedicated breast coil.
B. No special positioning of the breast is required.
C. The needle should be placed at the site of the lesion.
D. Adequate placement of the hook wire is confirmed after the patient is released from compression.
4. Which of the following statements is false?
A. A two-view mammogram is performed after MRI-guided localization.
B. Obtaining a radiograph of the specimen is unnecessary.
C. The specimen radiograph is evaluated by the radiologist.
D. The surgeon is notified of the specimen radiograph findings.
ANSWERS
CASE 111
MRI-Guided Needle Localization
1. A, C, and D
2. B
3. C
4. B
References
Klimberg VS. Advances in the diagnosis and excision of breast cancer. Am Surg. 2003;69(1):11–14.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:275.
Comment
Needle localization procedures generally are less frequently performed because of the wide acceptance of percutaneous core biopsies. MRI-guided needle biopsy and needle localization procedures are performed in lesions not clearly visible on either mammogram or ultrasound, such as areas of non-masslike enhancement. MRI-guided needle biopsy could be performed with a clip marker placed in the breast after biopsy. The clip can be localized mammographically if excision is needed.
In this case, the lesion was only seen on MRI, had irregular margins, showed avid enhancement with gadolinium with rapid washout, and was new compared with prior MRI examinations (see the figures). MRI-guided biopsy could have been performed, but the patient requested excision.
For MRI-guided needle localization, the patient is positioned prone within a dedicated breast coil, and the breast is gently compressed with a special compression paddle that includes a grid and a needle guide. Compression minimizes motion and allows consistent lesion location, but overly tight compression should be avoided because tight compression may hinder or delay enhancement of the lesion. After positioning, the patient is scanned, and when the lesion is identified, it is triangulated in a similar way as on mammographic guided localization (this can be done manually or with computer-assisted detection [CAD]). The needle is placed at the site of the lesion, and the hook wire is released (see the figures). Adequate placement of the hook wire is confirmed with MRI before the patient is released from compression.
The needle localization procedure can be done from a lateral or medial approach. Some breast coils allow medial access, whereas others offer only a lateral approach, which can be problematic when the lesion is located in the medial aspect of the breast because the distance from the skin would not be the shortest, and the available needle localization devices may not reach the lesion.
A two-view mammogram is performed to show the location of the wire in the breast, even if the lesion was not previously visible mammographically. Surgeons may need this mammogram in the operating room to help plan the surgical procedure. The mammogram may reveal an abnormality that was not seen before localization. Also, these images provide a baseline for mammograms after biopsy.
A specimen image cannot be obtained with MRI. However, a mammographic specimen radiograph is performed. The radiologist reviews the specimen and notifies the surgeon of the findings. In this case, a small spiculated mass was visualized within the specimen (see the figures). Histologically, this mass was a 5-mm invasive ductal carcinoma.
CASE 112




History: A 60-year-old woman has a history of right breast cancer, diagnosed as ductal carcinoma in situ (DCIS), 11 years before. She has extremely dense breasts on mammogram and is followed annually with mammogram and MRI. Previous MRI examinations have been negative.
1. What should be included in the differential diagnosis for the left breast MRI post–contrast subtraction images shown? (Choose all that apply.)
A. Infiltrating ductal carcinoma
2. What is the next step in management for this developing lesion?
A. Because the mass is tiny, repeat MRI in 6 months.
B. Because the morphology of the mass is benign, return the patient to routine evaluation.
3. Which of the following statements about MRI-guided biopsy is not true?
B. The breast is in compression.
C. A grid is used to help localize the mass.
D. A vacuum-assisted biopsy apparatus is preferred for sampling.
4. Which of the following is not an appearance of DCIS on MRI?
D. Large mass with spiculated margins
ANSWERS
CASE 112
MRI-Guided Biopsy
1. A, B, and C
2. D
3. A
4. D
References
Han BK, Schnall MD, Orel SG, et al. Outcome of MR-guided breast biopsy. AJR Am J Roentgenol. 2008;191(6):1798–1804.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:265. 277
Comment
MRI is a well-established modality for evaluation of the breast in selected patients. The postmenopausal patient in the present case has a personal history of right breast cancer and has dense breasts, which limit mammographic interpretation. She has been evaluated with mammogram and supplementary MRI for several years (see the figures). On the most recent MRI, a new 5-mm mass was noted in the subareolar left breast (see the figures).
Targeted ultrasound, or “second-look” ultrasound, is a useful examination after MRI shows a suspicious mass. If the lesion is found on ultrasound, ultrasound-guided biopsy is faster to perform and more easily tolerated by the patient. However, the use of targeted ultrasound should be tailored to the patient and to the MRI finding. Very small masses, non-masslike enhancement, and ductal enhancement can be difficult to identify on ultrasound. If a mass is not seen on targeted ultrasound, the finding cannot be presumed benign; in one large series, 14% of such lesions were malignant.
MRI-guided biopsy can be performed relatively easily and is similar to stereotactic biopsy with certain important differences. The contrast medium washes out of the breast lesion quickly, so once the location of the mass is noted, the patient may not move because the mass cannot be retargeted without administering more contrast medium. As in stereotactic biopsy, the patient is placed in prone position. With MRI-guided biopsy, the breast is only slightly compressed (see the figures). The compression cannot be too tight because the contrast medium may be inhibited from entering the breast blood vessels. The compression paddle has a grid to facilitate the localization of the biopsy site within the breast. A marker is placed on the skin, in one of the grid openings (see the figures), and the contrast-enhanced study is performed. The x, y, and z coordinates of the lesion are noted on the image, and then the location is compared with the location of the marker (called a fiducial). The appropriate x and y lesion location is noted on the skin, and the distance to the lesion from the skin (the z axis) is calculated. This localization of the mass can be accomplished by a computer-assisted detection (CAD) system or can be done by hand.
The skin is cleansed, local anesthesia is given, and the biopsy trocar is placed, similar to a stereotactic biopsy. When the desired location is reached, the metal trocar is replaced by an MRI-compatible stylet, and images are obtained to check the location of the stylet (see the figures). If the location is accurate, the stylet is removed; the biopsy device is placed through the sheath, and the samples are taken. With a vacuum-assisted device, multiple samples can be taken easily without removing the device from the breast. A clip is placed after the samples are taken, and additional images are obtained to assess the biopsy cavity and location of the clip, relative to the location of the mass as initially noted at the beginning of the examination. A mammogram is obtained after the biopsy to document the clip location.
The malignancy rate of MRI-detected areas of abnormal enhancement is approximately 20% to 60%. Lesions that are found to be benign by MRI-guided biopsy should be followed in 6 months with repeat MRI to ensure that the finding is smaller or resolved. If the lesion is unchanged or larger on follow-up, biopsy of the mass should be repeated.
In this patient, the small mass was DCIS. DCIS can have multiple features on MRI, ranging from a small mass to clumped enhancement in a ductal distribution to segmental, non-masslike enhancement. Kinetics is variable, with a slow or a rapid initial increase, with persistence or plateau. In the older literature, 25% to 40% of DCIS that was seen on mammography was not recognized on MRI. However, newer studies show DCIS detection on MRI to be greater than 90%.
CASE 113


History: A 43-year-old woman with juvenile-onset diabetes presents for a routine screening mammogram. A palpable mass is noted in the right subareolar breast by the technologist when positioning her for the mammogram.
1. What should be included in the differential diagnosis? (Choose all that apply.)
2. Which of the following statements regarding diabetic mastopathy is true?
A. It typically occurs in patients with type 2, non–insulin-dependent diabetes.
B. Incidence is similar in women and men.
D. The most common clinical presentation is an incidental mammographic finding.
3. Which of the following is not an imaging finding in diabetic mastopathy?
A. Extensive microcalcifications on mammogram
B. Poorly defined mass or dense focal asymmetry on mammogram
D. Poorly defined mass or areas of intense acoustic shadowing on ultrasound
4. Which of the following statements regarding diagnosis and management of diabetic mastopathy is false?
A. Tissue diagnosis is necessary to exclude malignancy.
B. Fine-needle aspiration is very accurate in providing a diagnosis of diabetic mastopathy.
C. Core or excisional biopsies are often necessary for conclusive diagnosis of diabetic mastopathy.
D. No specific treatment is necessary when diabetic mastopathy is diagnosed on biopsy.
ANSWERS
CASE 113
Diabetic Mastopathy
1. A, B, and D
2. C
3. A
4. B
References
Camuto PM, Zetrenne E, Ponn T. Diabetic mastopathy: a report of 5 cases and a review of the literature. Arch Surg. 2000;135(10):1190–1193.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:400.
Comment
Diabetic mastopathy is an uncommon condition that occurs in patients with long-standing insulin-dependent diabetes. It is most often diagnosed in premenopausal women and has been reported only rarely in men. The most common presentation is a firm, palpable mass that may mimic carcinoma on breast examination, as in the patient in this case. Patients frequently have associated complications from diabetes, such as renal disease, retinopathy, and cardiac disease.
The mammographic findings in diabetic mastopathy are usually a poorly defined mass or a dense focal asymmetry (see the figures). Patients also may have mammographically detected vascular calcifications as a complication of long-standing diabetes. However, because many of these patients are young, the palpable mass may not be mammographically visible owing to surrounding dense breast tissue (see the figures). Ultrasound may show a poorly defined mass or areas of intense acoustic shadowing (see the figures).
The diagnosis of diabetic mastopathy may be suggested with the appropriate clinical history and ultrasound features, but tissue diagnosis is necessary to exclude malignancy. Fine-needle aspiration cytology has been reported to be nondiagnostic in half of lesions because diabetic fibrous tissue contains little cellular material. Core or excisional biopsies are often necessary for conclusive diagnosis of diabetic mastopathy. Core biopsy reveals thick bundles of collagen and periductal, lobular, and vascular inflammatory infiltrates.
An autoimmune etiology has been postulated for this condition. No association has been described between diabetic mastopathy and carcinoma. No specific treatment is necessary for this entity.
CASE 114


History: A 43-year-old woman presents with left bloody nipple discharge.
1. What should be included in the differential diagnosis? (Choose all that apply.)
B. Ductal carcinoma in situ (DCIS)
2. Which of the following statements regarding imaging of DCIS is false?
A. The mammographic appearance consists of microcalcifications.
B. The ultrasound appearance is characteristic and consists of an intraductal solid filling defect.
C. The MRI appearance consists of non-masslike enhancement.
D. DCIS can appear on mammography as a mass.
3. Signs of microinvasion or invasion in high-grade DCIS include all of the following except:
A. Palpable mass on clinical examination
B. Extensive linear, branching, pleomorphic, or casting calcifications
C. Irregular hypoechoic area or mass on ultrasound
D. Focal area of clumped non-masslike enhancement in a ductal distribution on MRI
4. Which of the following statements regarding management or prognosis of DCIS is true?
B. The best imaging modality to guide percutaneous biopsy is ultrasound.
D. After adequate treatment, the recurrence rate of high-grade DCIS (comedocarcinoma) is low.
ANSWERS
CASE 114
High-Grade Ductal Carcinoma In Situ
1. B and D
2. B
3. D
4. C
References
Estevez L, Alvarez I, Segui MA, et al. Current perspectives of treatment of ductal carcinoma in situ. Cancer Treat Rev. 2010;36(7):507–517.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:65. 170
Comment
DCIS is a type of noninvasive cancer in which the cancer cells have not extended beyond the basal membrane of a duct. DCIS accounts for approximately 20% to 30% of the cancers detected on screening mammogram.
DCIS is classified according to its nuclear grade as low, intermediate, and high grade and morphologically as cribriform, micropapillary, solid, and comedo subtypes. The presence or absence of necrosis is also important in classification of DCIS. High-grade DCIS (comedocarcinoma) is a poorly differentiated form of DCIS that tends to have continuous growth along the ductal system (other subtypes of DCIS have a higher incidence of discontinuous or skip-type growth patterns).
High-grade DCIS appears on mammography as linear, branching, pleomorphic, or casting microcalcifications (see the figures). Because of its tendency to have a continuous growth pattern, the linear branching calcifications seen on mammography may provide an accurate estimate of extent of disease.
DCIS is usually not seen on ultrasound. Occasionally—especially with newer equipment—microcalcifications can be visualized on ultrasound as hyperechoic dots, but the main contribution of ultrasound is to assess for possible additional invasive components (hypoechoic areas or mass or both).
On MRI, DCIS appears as non-masslike enhancement (usually clumped) in a linear, ductal, segmental, or regional distribution. MRI is useful to evaluate extent of disease, multifocality or multicentricity, and presence of contralateral disease. It may detect masses not seen on mammography, and it may show areas of noncalcified DCIS (see the figures).
To establish a diagnosis, stereotactic biopsy of microcalcifications is the best initial approach. When there are multifocal or multicentric areas of microcalcifications, biopsy specimens of any suspicious areas may be obtained to document extent of disease or at least the two most distant sites of microcalcifications. Ultrasound and MRI can be used to guide biopsy of a possible invasive component not detected with mammography.
As the volume of DCIS increases, the chance of microinvasion increases as well. When the extent of disease seen on mammography is 5 cm or greater, the likelihood of microinvasion is high enough that many surgeons perform a sentinel lymph node biopsy even though no invasion may have been documented histologically. Other symptoms and signs of microinvasion or invasion include palpable abnormality on self-examination or clinical examination, hypoechoic areas or mass on ultrasound, and enhancing mass on MRI.
Management of DCIS is the same as management of invasive carcinoma. Treatment includes surgery (lumpectomy or mastectomy), radiation therapy, and chemotherapy or hormonal therapy. Comedocarcinoma is associated with a higher rate of recurrence than other subtypes of DCIS because of the high nuclear grade and the radioresistance of the tumor.
CASE 115


History: A 48-year-old woman presents for screening mammogram.
1. What should be included in the differential diagnosis for the calcifications in the lower outer right breast? (Choose all that apply.)
B. Atypical ductal hyperplasia (ADH)
C. Ductal carcinoma in situ (DCIS)
D. Lobular carcinoma in situ (LCIS)
2. Stereotactic biopsy revealed ADH, fibrocystic change, and columnar cell change. ADH is usually found associated with which type of calcifications?
B. Pleomorphic microcalcifications
C. Regional amorphous microcalcifications
D. Fine, linear, branching microcalcifications
3. Which of the following statements regarding management of ADH is true?
A. ADH does not require further management.
4. What is the role of biopsy clip placement after stereotactic biopsy?
A. Biopsy clip placement after stereotactic biopsy of calcifications is unnecessary.
D. A biopsy clip placed after stereotactic biopsy is used as a guide for radiotherapy.
ANSWERS
CASE 115
Atypical Ductal Hyperplasia
1. A, B, and C
2. C
3. C
4. B
References
Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision?. Radiology. 2002;224(2):548–554.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:230.
Comment
ADH is in the spectrum of hyperplastic changes of the breast that range from usual ductal hyperplasia to DCIS. ADH refers to the proliferation of monomorphic epithelial cells within the duct. ADH is found in approximately 5% of all biopsy specimens obtained for any type of calcifications but is frequently found associated with regional amorphous calcifications (approximately 20%). In this case, the patient had a focal area of clustered heterogeneous microcalcifications (see the figures) and was recalled for additional magnification views. On the additional views, some of the calcifications layer (suggestive of milk of calcium), but other calcifications are more irregular and raise concern for atypia or DCIS (see the figures). When a cluster of microcalcifications exhibits both benign and suspicious features, management should be based on the most suspicious features.
A patient with ADH diagnosed by core biopsy should always undergo excisional biopsy because of the high incidence of histologic underestimation of DCIS. When an 11-gauge vacuum-assisted core needle is used, there is an underestimation of malignancy of approximately 25% when ADH is the core needle diagnosis. Lesions on which core biopsies are performed and that are interpreted as ADH show various histology on excision, including fibrocystic change, ADH, DCIS, and invasive ductal carcinoma.
A biopsy clip should be placed at the site of biopsy so that the area can be easily localized if excision is needed. A clip is especially important if the cluster of microcalcifications is small because the microcalcifications may be completely removed on the core biopsy. Complete removal of all mammographic evidence of the microcalcifications at core biopsy does not obviate the need for excision. The goal of stereotactic biopsy is not complete removal, but rather adequate sampling of the lesion. In this patient, after excisional biopsy with complete removal of the lesion, the histopathology was low-grade DCIS. The vacuum-assisted core biopsy result was an underestimation of the disease present. Approximately 75% of underestimation of ADH is DCIS. The patient went on to definitive management of stage 0 breast cancer.
CASE 116

Initial presentation.

Six months later.

Six months later.
History: A 38-year-old woman in her third trimester of pregnancy presents with a palpable mass. Her family history is significant for breast cancer in her mother.
1. What should be included in the differential diagnosis for the initial presentation? (Choose all that apply.)
2. Why did the patient undergo ultrasound evaluation only at initial presentation?
A. Because ultrasound is always the first step in evaluating a palpable mass
B. Because of the patient’s age
C. Because the patient is pregnant
D. Because of her family history
3. Which of the following is not a reasonable option for further evaluation?
4. The patient underwent needle core biopsy. She complained of a persistent lump at the biopsy site 6 months later, larger than the original mass (mammogram and ultrasound are shown). The differential diagnosis includes all of the following except:
ANSWERS
CASE 116
Milk Fistula and Galactocele: Complication of Core Biopsy during Lactation
1. C and D
2. C
3. C
4. D
References
Kim MJ, Kim EK, Park SY, et al. Galactoceles mimicking suspicious solid masses on sonography. J Ultrasound Med. 2006;25(2):145–151.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:379. 380
Comment
This patient presented with a palpable mass in the left breast. Ultrasound was performed as the initial examination because she was in the third trimester of pregnancy at the time of detection of the mass (see the figures). A needle core biopsy of the 0.7-cm palpable mass in the left upper inner breast was performed, and histology of the mass showed a lactating adenoma. No immediate complications were seen. Several weeks after biopsy, the patient returned with a palpable mass, larger than the original palpable finding. Ultrasound of the new finding showed a well-circumscribed, oval mass consistent with a complex cyst, likely a galactocele resulting from milk fistula, 2.4 cm in diameter (see the figures). The patient was reassured that this should resolve spontaneously, and she was followed by ultrasound. The mass decreased in size and resolved completely after several months.
The most common palpable masses in pregnant and lactating women are lactating adenomas and fibroadenomas; lactating adenomas account for 70% of masses undergoing biopsy in this population. A lactating adenoma is most often seen in ultrasound as a well-circumscribed, oval, hypoechoic mass, parallel to the chest wall (see the figures). However, a lactating adenoma can have a more suspicious appearance, with shadowing and irregular margins, making malignancy more difficult to exclude.
Although the mass had benign features on ultrasound examination, this patient was concerned about the possibility of malignancy and requested a biopsy. This is an acceptable approach in a probably benign lesion, BI-RADS (Breast Imaging Reporting and Data System) 3. Any intervention in the breast carries a possibility of complications, although complications of needle core biopsy are unusual (approximately 1% to 2% in many published series). The most common complications are hematoma and infection. An additional complication that can occur in the third trimester of pregnancy or during lactation is milk fistula (see the figures). Damage to the ducts by the needle causes leaking of duct contents (milk) into the biopsy cavity, which may result in the formation of a galactocele, which appears as a mixed-density, partially lucent mass on mammogram (see the figures). The ultrasound appearance is commonly a mass with thin echogenic walls and homogeneous hypoechoic internal echoes (see the figures).
CASE 117
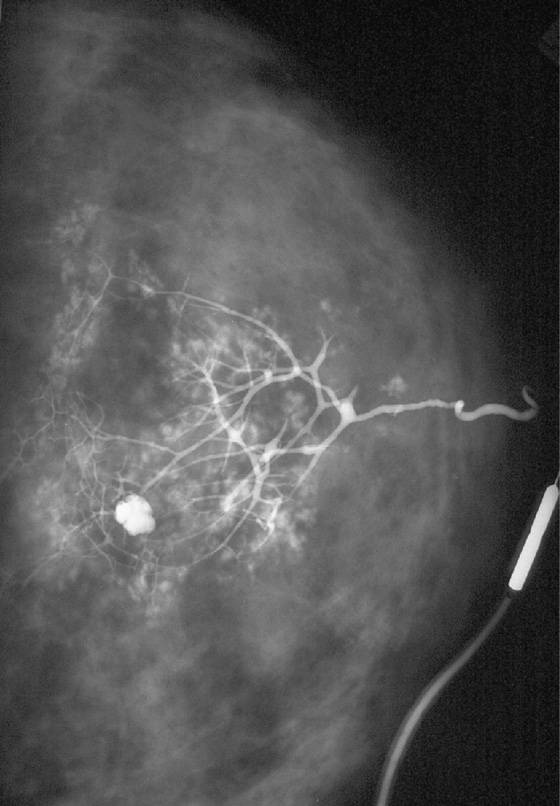
History: A 51-year-old woman presents with spontaneous clear yellow discharge from one opening of the left nipple. Her galactogram is shown.
1. What is the differential diagnosis from this single image of a galactogram? (Choose all that apply.)
A. Malignant mass communicating with duct
B. Multiple small intraductal papillomas
D. Normal ducts, forceful injection during galactogram
2. Why is contrast seen in the faint rounded areas adjacent to the ducts?
A. Contrast has entered lobules.
B. Contrast has extravasated from the ducts into the adjacent stroma.
C. There is malignancy in the lobules (ILC).
3. Does this exam answer the question of the etiology of the discharge?
A. Yes, the duct is normal, so the discharge must be normal.
B. No, because the duct is normal, it is probably not the discharging duct.
C. Yes, a normal galactogram allows you to follow the discharge expectantly.
4. What is the best next step in managing this discharge?
B. Perform MRI without IV contrast.
C. Perform a mammogram to evaluate for intraductal mass.
D. Recommend that the patient return in 6 months for re-evaluation.
ANSWERS
CASE 117
Normal Galactogram
1. C and D
2. A
3. B
4. A
References
Cardenosa G, Doudna C, Eklund GW. Ductography of the breast: technique and findings. Am J Roentgenol. 1994;162:1081–1087.
Slawson SH, Johnson BA. Ductography: how to and what if?. Radiographics. 2001;21(1):133–150.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:383–389.
Comment
This is an example of a galactogram of a normal duct. There are no suspicious features. In this normal exam, shown in the figure, the ducts are thin, with smooth walls, regular branching, and no filling defects. Abnormal ducts are enlarged and can have irregular duct walls, abrupt termination of ducts, and filling defects.
This example (see the figure) shows the effect of maximal pressure exerted during the galactogram. The contrast has entered the lobules. The rounded areas of contrast outside the ducts in this patient represent contrast that has entered the lobules, called lobular blush. This is not extravasation, which occurs when the cannula perforates the side wall of the duct and a pool of contrast occurs outside the duct lumen and which is to be avoided. The rounded area of contrast is likely a cyst in communication with the duct.
Galactography is performed when there is a clinically suspicious discharge, which is a unilateral, spontaneous, clear, yellow, pink, or bloody discharge. Patients with this type of discharge often have an underlying cancer (up to 33%). Benign causes of discharge, such as papilloma, are more common than malignancy. It is not necessary to perform galactography for nipple discharge that is bilateral, nonspontaneous, and occurring in multiple duct orifices and that is green, gray, amber, or milky.
CASE 118
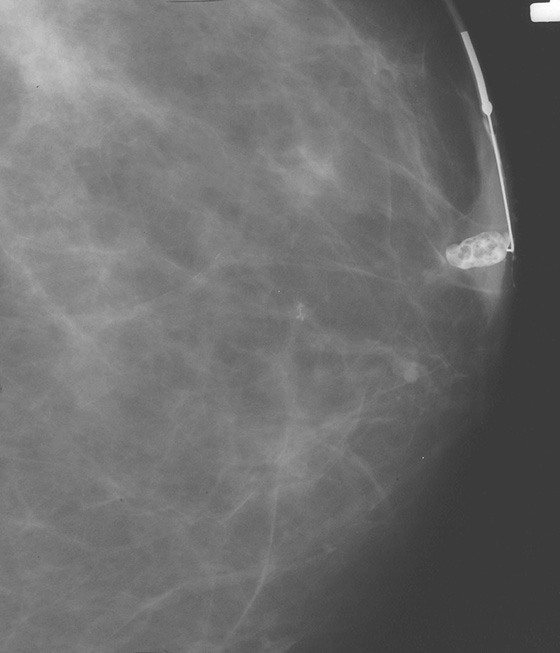
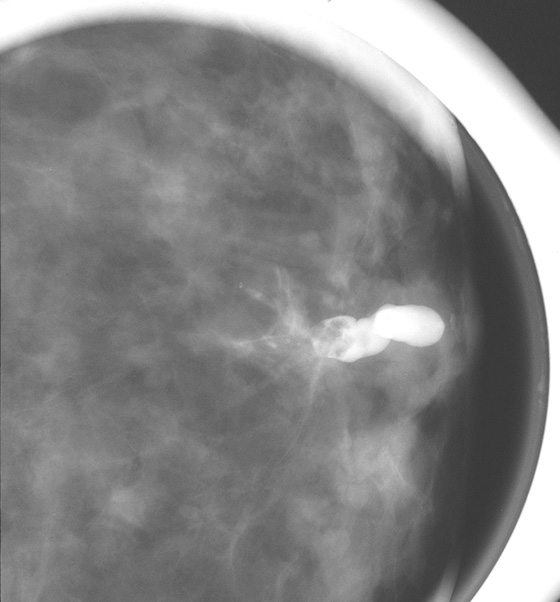
History: Two different patients have a spontaneous, nonbloody unilateral nipple discharge emanating from one nipple orifice.
1. What is the differential diagnosis for these two different patients? (Choose all that apply.)
B. Apocrine metaplasia within ducts
D. Air bubbles in the contrast column
2. What is the purpose of the galactogram?
A. To identify whether intraductal masses are malignant
C. To identify the presence of abnormal cells in duct fluid
D. To depict the extent of intraductal masses
3. What other modalities can be used to evaluate nipple discharge?
D. Mammography alone is the best imaging modality for breast ducts
4. What type of discharge is evaluated with galactography?
A. Nonspontaneous clear yellow discharge from multiple openings of one nipple
B. Milky spontaneous discharge from both breasts
C. Bloody discharge from multiple openings in both breasts in a woman who has just delivered a baby
D. Spontaneous discharge that is not milky from one opening of one nipple
ANSWERS
CASE 118
Intraductal Mass
1. A and C
2. D
3. B
4. D
References
Cardenosa G, Doudna C, Eklund GW. Ductography of the breast: technique and findings. AJR Am J Roentgenol. 1994;162:1081–1087.
Slawson SH, Johnson BA. Ductography: how to and what if?. Radiographics. 2001;21:133.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:383.
Comment
The steps in evaluating patients with spontaneous nipple discharge are controversial. First, the type of discharge and its true spontaneous nature must be determined. Many women can express discharge from their nipples on manipulation, but true spontaneous discharge is uncommon and requires a work-up regardless of the nature of the discharge. If the discharge is bilateral and milky, prolactin levels are usually checked. However, if the discharge is bloody, clear, or serous and is from a single duct and truly spontaneous, further evaluation is necessary. This may be carried out with surgical exploration of the duct or radiologically, with ultrasound, MRI, and/or galactography.
The purpose of a galactogram is to locate any intraductal lesions and map out the extent of the disease in the discharging duct. The procedure is generally simple. The discharging duct must be localized. Often, this requires a hot compress or heating pad to be placed on the nipple to relax the musculature of the nipple. Adequate lighting and magnification are commonly necessary to identify the opening of the duct. Often, the patient is able to demonstrate a trigger point in the breast that initiates the discharge. Once the discharging duct is localized, a 30-gauge blunt-tip sialogram cannula is gently placed into the duct orifice. The cannula is attached to tubing and a Luer lock syringe is filled with water-soluble contrast. The tubing must be checked carefully so that no air bubbles are present because bubbles injected into the ductal system can mimic intraductal lesions. The Luer lock apparatus will help prevent additional air from entering the tubing system once it has been filled and the initial bubbles have cleared. The contrast is instilled into the duct until the patient feels fullness in the breast or the contrast is seen spilling retrograde from the duct orifice. The procedure should not be painful. If the patient experiences any pain or burning when the contrast is injected, the injection should be stopped because the contrast might have extravasated outside of the ductal system into the surrounding parenchyma. Once a lesion or lesions have been mapped, the areas may be localized for surgical excision.
There is a wide spectrum of findings on galactography, but most significant are intraductal filling defects (see the figures), obstruction with blunt termination of the contrast (see the figures), or wall irregularity or distortion (see the figures). There is a significant overlap in the galactogram findings seen with papillomas and carcinomas. However, the greater the irregularity of the ductal architecture, the higher the likelihood that there is a malignancy. Any of these findings are suspicious and a biopsy is recommended. The first figure shows a lobulated distal intraductal mass that was shown to be a papilloma at excision. The second figure was a small intraductal carcinoma.
This case is courtesy of Emily F. Conant, M.D., Professor and Chief, Division of Breast Imaging, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania.
CASE 119
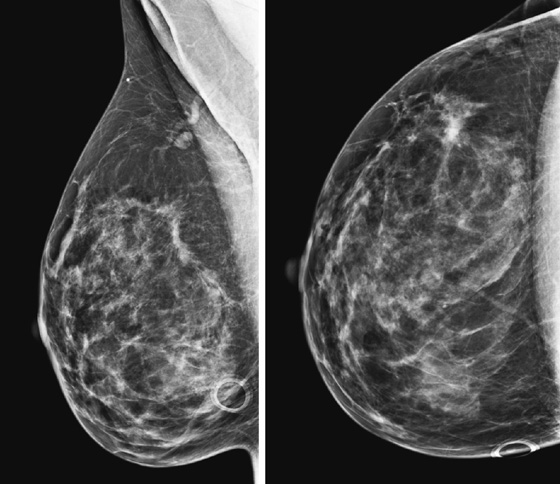
Contralateral breast.
History: A 40-year-old woman had an area of distortion seen in the right upper outer breast on routine baseline screening mammogram. Biopsy was performed, and bilateral MRI was performed.
1. What should be included in the differential diagnosis of the right breast abnormality? (Choose all that apply.)
A. Infiltrating ductal carcinoma
D. Infiltrating lobular carcinoma
2. Which indication for MRI after a needle biopsy of carcinoma is incorrect?
A. MRI may reveal multifocal disease not seen on mammogram.
B. MRI may reveal multicentric disease not seen on mammogram.
C. MRI is used to image the axilla, which is poorly seen on mammogram.
D. MRI may reveal contralateral malignancy not seen on mammogram.
3. What is the most likely histology causing the enhancement in the contralateral left breast?
C. Invasive lobular carcinoma (ILC)
4. What is the next step in management after MRI in this patient?
A. Follow-up MRI of the left breast in 6 months
C. MRI-guided biopsy of the left breast
D. Positron emission mammography
ANSWERS
CASE 119
Invasive Lobular Carcinoma, Contralateral Disease
1. A, B, and D
2. C
3. A
4. C
References
Dixon JM, Anderson TJ, Page DL, et al. Infiltrating lobular carcinoma of the breast: an evaluation of the incidence and consequence of bilateral disease. Br J Surg. 1983;70(9):513–516.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:265. 305
Comment
ILC accounts for 8% to 14% of breast cancers. However, it is more difficult to detect—and is more frequently multicentric, multifocal, and bilateral—compared with the more common infiltrating ductal carcinoma.
When ILC is found on a mammogram (see the figures), the next step in management after needle biopsy is MRI. This study helps diagnose the extent of disease. Extent of disease includes assessment of the size of the known tumor, which may be larger than expected based on the mammogram. It has been shown that positive margins after surgery are much more common in ILC compared with infiltrating ductal carcinoma, exceeding 50%, likely owing to the pattern of spread of this insidious tumor. If tumor size is measured on MRI before surgery, this can aid the surgeon in surgical planning.
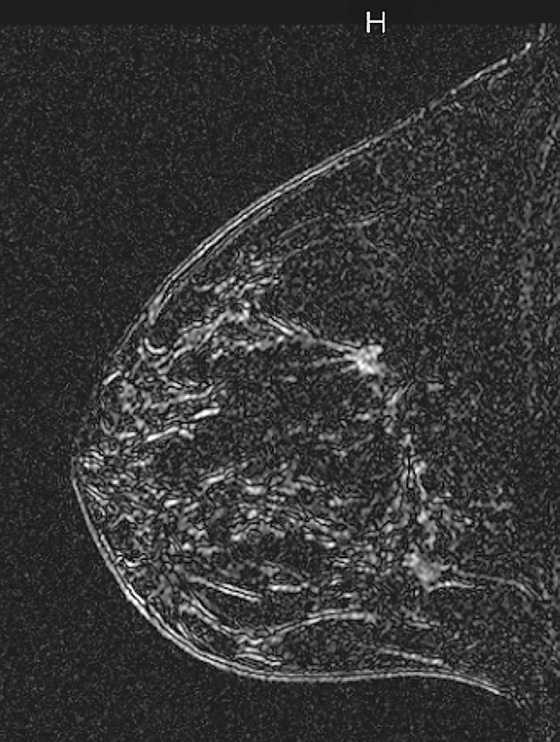
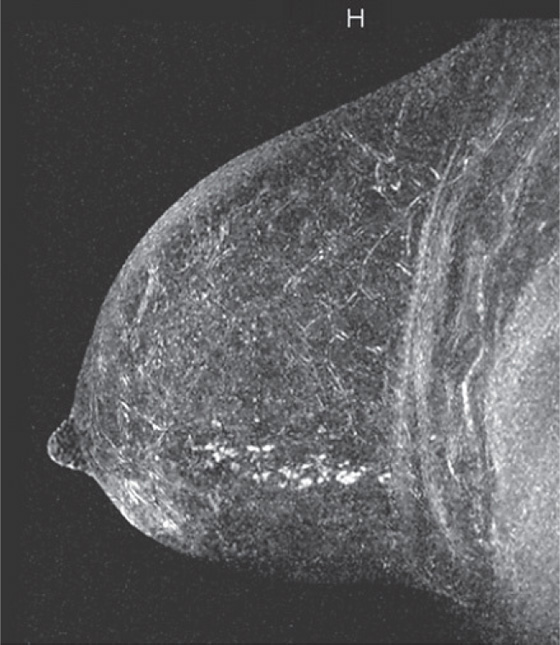
Extent of disease also includes whether there is additional tumor in that quadrant or elsewhere in the same breast (multifocal or multicentric disease). In a meta-analysis, MRI showed additional ipsilateral disease in 32% of patients. MRI of the opposite breast should also be performed. The same meta-analysis showed contralateral disease seen only on MRI in 7%. MRI may show a different enhancement pattern for ILC than for infiltrating ductal carcinoma because ILC may be slower to enhance and fail to have wash-out kinetics. This enhancement pattern is similar to background fibroglandular tissue and may be difficult to detect. The most common enhancement pattern seen in ILC is the irregular or spiculated mass, seen in approximately 30% to 40% (see the figures). It may also manifest as a mass surrounded by multiple smaller masses or foci or multiple enhancing foci with enhancing interconnecting strands.
In the patient in this case, the ipsilateral disease is unifocal (see the figures). The enhancement pattern in the ipsilateral breast seen in the second figure mimics the mammographic appearance. There is enhancement in the contralateral breast, which is of some concern for malignancy. The enhancement pattern in the contralateral breast is different, consisting of a stippled area of non-masslike enhancement that extends from anterior to posterior in a linear distribution (see the figures). This pattern suggests a ductal process, possibly ductal carcinoma in situ. The next step in management of the contralateral breast is MRI-guided needle biopsy.
CASE 120
History: A 71-year-old patient presents for routine mammography. She gives a history of having silicone implants and experiencing rupture of the left implant. Her implants were removed 8 years ago. This is her first mammogram after the surgery.
1. What is the differential diagnosis for this mammogram? (Choose all that apply.)
A. High-density irregular masses in the left breast, possible malignancy
B. Pleomorphic calcifications in both breasts, suspicious for ductal carcinoma in situ (DCIS)
C. Dense masses in the left breast, possible metastatic disease
D. Dense material in the left breast, consistent with silicone granulomas
2. What additional work-up is needed?
A. Magnification views of the calcifications bilaterally
B. Ultrasound of the left breast dense masses
3. What is the etiology of silicone granulomas?
A. Silicone gel that has leaked from the implant is walled off by inflammation
B. Occurs in patients with intact silicone implants and TB or sarcoid
C. Caused by fat necrosis, related to silicone implant surgery
D. Seen in women with intact silicone implants and multiple fibroadenomas
4. Would MRI help in this case?
A. No, because the masses will enhance whether they are silicone granuloma or malignant masses.
B. Yes, because the masses will not enhance if they are silicone granulomas.
C. Yes, but contrast enhancement is not needed for silicone evaluation in this patient.
D. Yes, but mammography and ultrasound are diagnostic, so MRI is not needed.
ANSWERS
CASE 120
Explanted Ruptured Silicone Implants
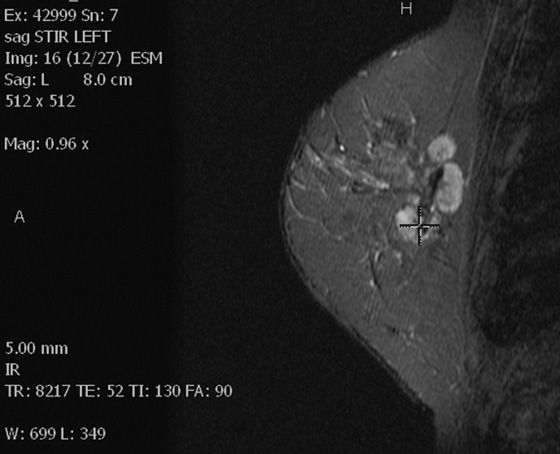
Left breast STIR image.
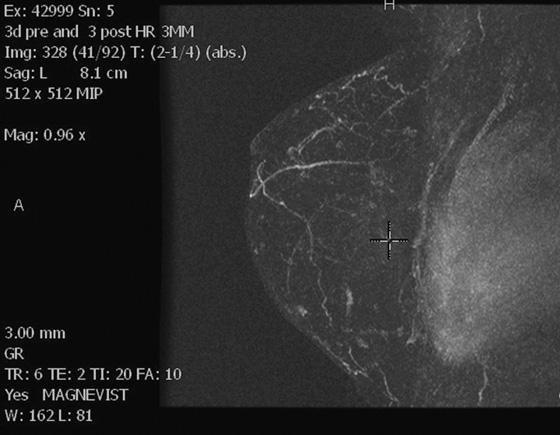
Left breast post–contrast MIP image.
1. A, C, and D
2. D
3. A
4. B
References
Berg WA, Nguyen TK, Middleton MS, et al. MR imaging of extracapsular silicone from breast implants: diagnostic pitfalls. AJR Am J Roentgenol. 2002;178:465–472.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:346.
Comment
Silicone implants consist of a silicone gel within a shell made of a polymer that might also contain silicone. The implants may be placed in front of (subglandular) or behind (subpectoral) the pectoralis muscle.
The body makes a fibrous capsule that surrounds the silicone implant. Silicone implants can rupture, and two broad types of rupture are recognized: intracapsular and extracapsular. These two designations describe whether the silicone that has escaped from the polymer shell is contained by the patient’s fibrous capsule (intracapsular rupture) or escapes beyond the fibrous capsule and into the breast (extracapsular rupture). It can be difficult to recognize rupture on mammographic images, particularly intracapsular rupture. Ultrasound is more sensitive than mammography for the presence of rupture, and MRI is the most sensitive for the diagnosis of rupture, intracapsular or extracapsular.
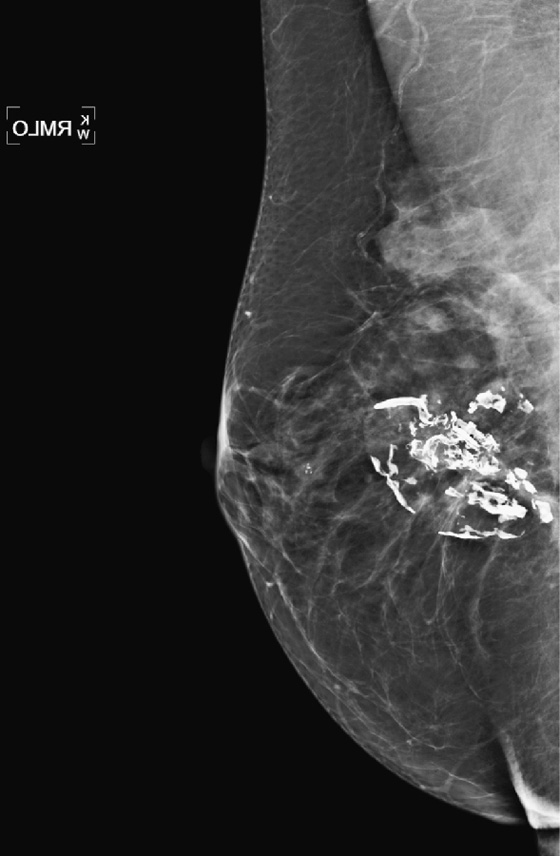
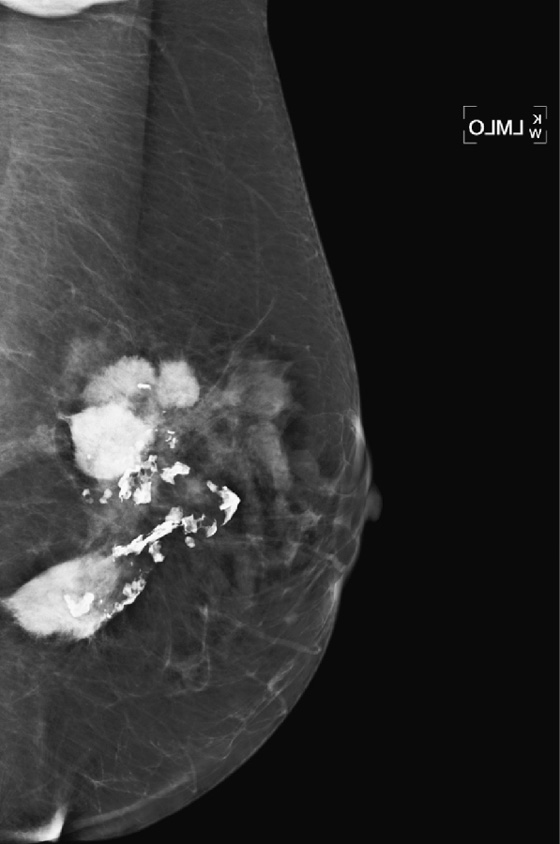
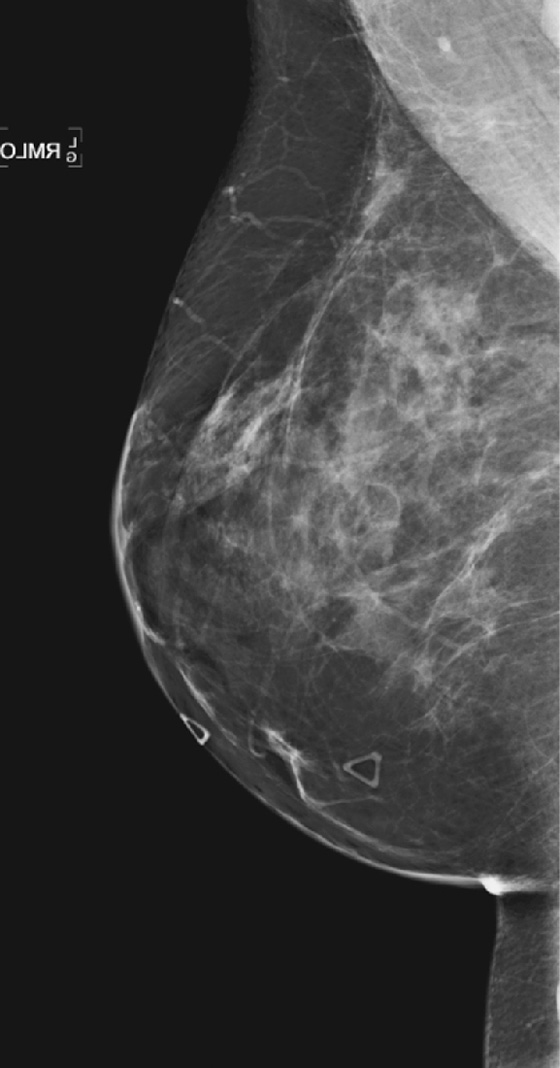
Once a silicone implant has undergone extracapsular rupture, there is free silicone in the breast, which may be taken up by the lymphatics and deposited in the axillary lymph nodes. This can be seen as dense particles in the nodes, similar to calcium. The free silicone in the breast is walled off as granulomas, which can calcify, and in this patient, the granulomas are seen as dense masses in the left breast (see the figures). The silicone granuloma is also termed siliconoma. The implant capsule can also calcify, likely a reaction to gel bleed, and can be seen in mammograms with and without implant rupture.
When implants are removed, free silicone that is formed into granulomas might not be excised, as in this patient (see the figures). The capsule might or might not be removed. In this patient, the capsules, which have calcified, were not removed. The calcifications in the capsule are typically coarse and benign, as in this patient (see the figures), but the calcifications can be difficult to interpret if they are faint.
In this patient, the diagnosis of the left breast masses was not certain, and MRI was recommended. MRI demonstrates nonenhancing masses in the left breast (see the figures) that have increased signal on T1- and water-suppressed inversion recovery (STIR) T2-weighted images (see the figures), consistent with silicone granulomas. Biopsy is not indicated. This patient can be followed with annual mammography.
CASE 121
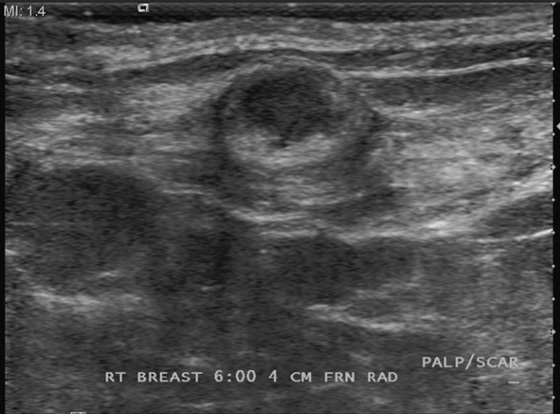
History: A 46-year-old woman had bilateral reduction surgery 2 years ago and now feels a palpable lump in her right lower breast at the 6 o’clock position.
1. What is the differential diagnosis for the mammographic and ultrasound images? (Choose all that apply.)
2. Is this location typical for fat necrosis after breast reduction surgery?
A. No, the location is usually in the upper outer quadrant.
B. Yes, this is a typical location.
C. No, this can occur anywhere in the breast; there is no typical location.
D. No, the location is usually along the inframammary fold.
3. Why does fat necrosis occur after breast reduction surgery?
A. Ischemia due to surgical disruption of blood supply
B. Poor postoperative wound care
D. The breast has been made too small
4. What ultrasound appearance is seen in fat necrosis?
A. The appearance is typically a complex cyst.
B. There is always shadowing seen in oil cysts.
C. Complex cystic mass with mural nodules is one appearance.
D. The border of fat necrosis is typically smooth.
ANSWERS
CASE 121
Palpable Mass after Reduction Surgery
1. B, C, and D
2. B
3. A
4. C
References
Miller CL, Feig SA, Fox JW. Mammographic changes after reduction mammoplasty. AJR Am J Roentgenol. 1987;149:35–38.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:360.
Comment
Breast reduction surgery is cosmetic surgery performed to reduce the size of the breasts. Commonly, patients who have this surgery have large, fatty, and pendulous breasts. The surgical incisions include a transverse incision along the inferior aspect of the breast, near the inframammary fold, and a vertical incision at the 6 o’clock position. The inferior breast tissue is removed, decreasing the volume of the breast. A keyhole incision around the edge of the areola may also be made to move the nipple superiorly on the breast mound.
Fat necrosis is often seen at the scar lines, particularly along the 6 o’clock vertical scar and the circumareolar scar. The patient might feel a palpable mass. Fat necrosis is caused by the trauma to the breast by the surgery: ischemia of the relatively poorly vascularized fatty tissue, interruption of blood vessels, and bleeding into the site. The mammographic features are more typical than the ultrasound features because of the appearance of fat lucency. Rim calcifications and thick, noncalcified fibrous walls can be seen surrounding the fat (see the figures). The encapsulated fat may be present as a palpable mass. The ultrasound features are more variable, and the fat necrosis can appear echogenic, can shadow, and can be a suspicious-appearing complex cyst with mural nodules (see the figures).
In this patient, the location and mammographic appearance are typical of fat necrosis, and the size of the ultrasound complex cyst matches the size of the lucent mass on mammogram. No further work-up is required. The findings can be given a Breast Imaging Reporting and Data System (BI-RADS) score of 2.
CASE 122
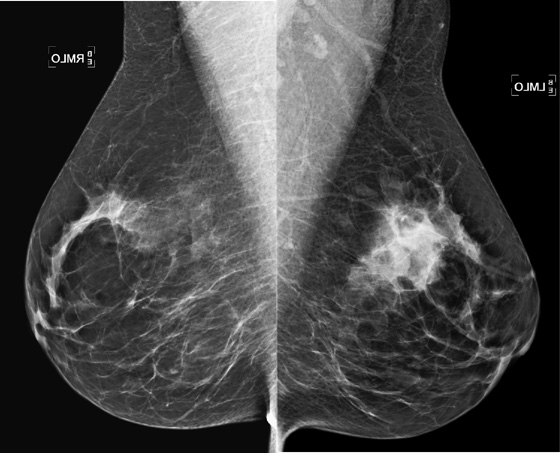
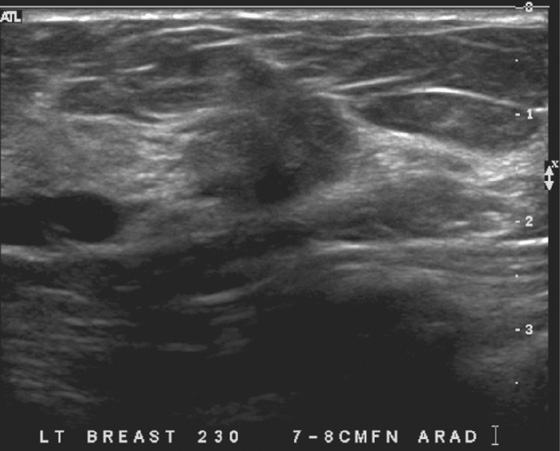
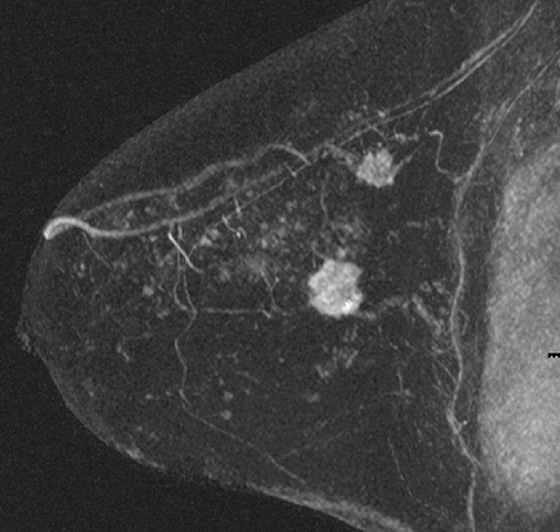
History: A 51-year-old woman is recalled for additional views and ultrasound after a routine screening mammogram shows a subtle change in the left breast asymmetry compared with previous mammogram.
1. What should be included in the differential diagnosis of the screening mammogram? (Choose all that apply.)
A. Asymmetric gland tissue in the left upper outer breast
B. Cyst in the left outer breast
C. Malignant mass in the left outer breast
2. What is the next step in management after mammogram and ultrasound?
B. Short-term follow-up for probably benign findings
C. Fine-needle aspiration biopsy
3. Which of the following is not a reason to perform MRI?
A. MRI should be performed before needle biopsy to ensure there is an enhancing mass.
B. MRI can give information about the contralateral breast.
C. MRI can establish the presence of multicentric disease.
D. MRI can establish the presence of multifocal disease.
4. How is multifocal disease defined?
A. Multifocal disease is additional cancer in the opposite breast.
B. Multifocal disease is additional cancer in the same quadrant.
C. Multifocal disease is additional disease in a separate quadrant.
D. Multifocal disease refers to cancer that has spread to the ipsilateral lymph nodes.
ANSWERS
CASE 122
Multifocal Disease Seen on MRI
1. A, B, and C
2. D
3. A
4. B
References
Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26(19):3248–3258.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:300. 305
Comment
MRI is performed for evaluation of both breasts before surgery in patients with a diagnosis of breast cancer; this is also termed preoperative MRI. In a meta-analysis, proponents of preoperative MRI showed that an additional lesion is seen in 16% of patients. The most common secondary lesion found is in the ipsilateral breast and may be in the same quadrant, a multifocal lesion, as in the patient in this case (see the figures). Additional lesions may also be seen in a different quadrant; this is termed multicentric disease. Less commonly (approximately 2% to 3%), additional lesions are found in the contralateral breast.
The location of the additional disease relative to the primary tumor is very important for surgical planning. Disease in the same quadrant may still be considered for breast conservation therapy, whereas disease in different quadrants may be treated with mastectomy. Patients who are being considered for partial breast irradiation may undergo MRI before treatment to determine if they qualify for this procedure. Partial breast irradiation is performed only in select patients.
Biopsy is indicated for additional lesions that are identified on MRI, just as biopsy was performed on the initial lesion before excision. Although MRI is very sensitive, its specificity is lower, and many lesions seen on MRI are benign and need no excision.
The need for additional time before surgery causing delay in treatment, additional biopsy procedures, and cost are several of the reasons cited against the use of preoperative MRI. Opponents also state that the detection of additional disease and the subsequent change in surgical management do not alter outcome; trials have not substantiated that patients who undergo preoperative MRI have a decrease in local recurrence. Preoperative MRI is still widely performed in patients with new diagnoses, in particular, patients with invasive lobular carcinoma, patients with dense breasts, patients with implants limiting the mammogram, and patients with a posterior lesion that may involve the chest wall.
CASE 123
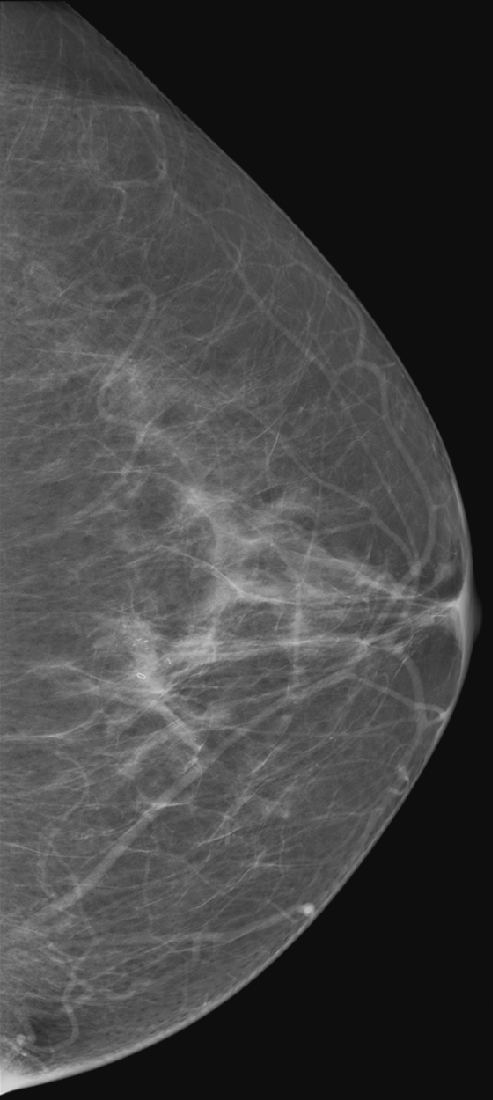
History: A 63-year-old woman is scheduled for surgery for removal of microcalcifications in the left lower breast after stereotactic guided biopsy.
1. What should be included in the differential diagnosis for the calcifications in the lower left breast? (Choose all that apply.)
B. Atypical ductal hyperplasia
2. Which of the following statements regarding the best selection of imaging modality to guide needle localization is false?
A. Needle localization can be performed only under mammographic guidance.
B. Calcifications are generally localized under mammographic guidance.
C. Masses are generally localized under ultrasound guidance.
D. Areas of non-masslike enhancement are generally localized under MRI guidance.
3. What are the correct steps, in order, to perform mammography-guided needle localization?
4. Which of the following statements regarding mammography-guided needle localization is true?
A. It is unnecessary to send mammogram films to the operating room.
B. A radiograph of the specimen should always be obtained.
C. The specimen radiograph is evaluated by the surgeon in the operating room.
D. The surgeon does not need to be notified of the specimen radiograph findings.
ANSWERS
CASE 123
Mammography-Guided Needle Localization
1. B, C, and D
2. A
3. C
4. B
References
DePalo AJ. Surgical considerations in needle localization procedures. Semin Surg Oncol. 1991;7(5):253–256.
Klimberg VS. Advances in the diagnosis and excision of breast cancer. Am Surg. 2003;69(1):11–14.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:195.
Comment
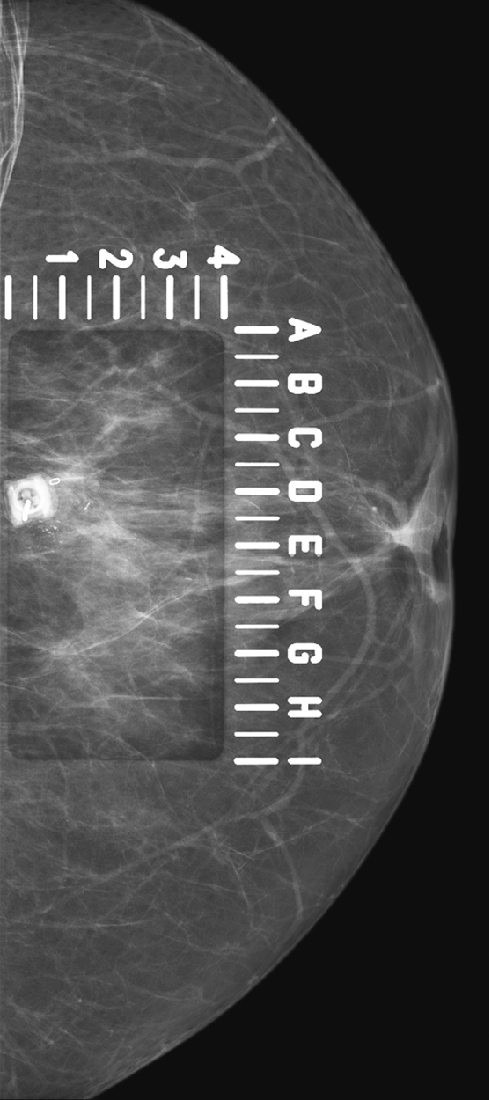
Needle localization under mammographic guidance can be guided by mediolateral and craniocaudal (CC) images from the standard imaging equipment (see the figures) or can be guided stereotactically. This case illustrates the standard-imaging approach. Needle localizations are necessary to guide the surgeon accurately to a lesion that is nonpalpable and requires excision.
To expedite the localization procedure on the day of the surgery, a complete work-up of the lesions should have been performed in advance (e.g., magnification views, if needed, to establish the extent of microcalcifications) and triangulated on orthogonal imaging. The closest skin surface to the lesion is determined. Needle localization may be performed under mammographic, ultrasound, or MRI guidance. The selection of the guiding modality should be based on the ease of seeing the lesion. Generally, calcifications are localized under mammographic guidance, masses are localized under ultrasound guidance, and areas of non-masslike enhancement are localized under MRI guidance.
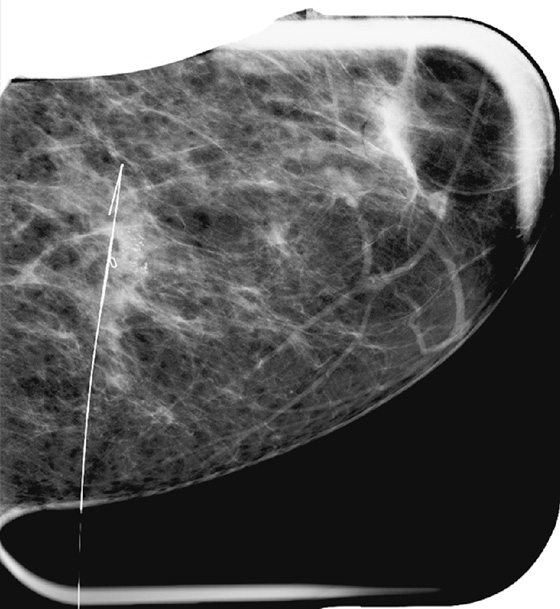
For mammography-guided needle localization, the most important step is positioning the patient so that the skin entry site for placement of the localizing needle and hook wire is from the closest skin surface. The needle length is chosen by measuring the distance from the closest skin surface to the site of the lesion. The patient is placed in compression with the alphanumeric grid open over the appropriate skin surface. The patient is imaged, and the coordinates at the center of the lesion are chosen (in this case, ½ and D½; see the figures). The skin is cleansed and anesthetized, and the needle is placed into the breast. To ensure that the needle is placed into the lesion, an image of the needle hub superimposed over the shaft of the needle is obtained (see the figures).
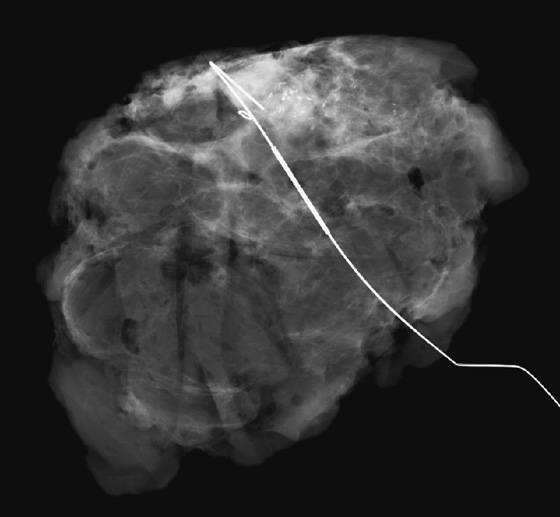
The patient is removed from compression, and an orthogonal view is obtained showing the position of the tip of the needle relative to the lesion (see the figures). The needle depth is adjusted so that the tip of the needle is at or just beyond the lesion (in this case, the needle was placed slightly deep to the lesion and had to be withdrawn slightly). The hook wire is placed through the needle and secured in the breast tissue. Some surgeons prefer to keep the needle in the breast to aid in localizing the lesion in the operating room, whereas other surgeons prefer to remove the needle and keep only the wire in the breast. Two orthogonal films (usually a CC view and mediolateral or lateromedial view) showing the wire and its relationship to the lesion, with the lesion circled, are sent to the operating room to serve as a “map” for the surgeon.
The specimen radiograph is an essential component of mammography-guided needle localization (see the figures). The radiologist must review the specimen to ensure that the lesion has been removed (or sampled, depending on the case). The surgeon is notified of the specimen radiograph findings, and if the lesion has not been removed or adequately sampled, the surgeon should be directed to obtain more tissue. In this case, the surgeon had intended to remove the entire area of microcalcifications and biopsy clip and obtain clear margins for a lumpectomy. The specimen radiograph revealed that the microcalcifications are at the edge of the tissue, and the surgeon was directed to obtain more tissue. Communication from the radiologist assists the surgeon in obtaining clean margins and reducing the likelihood of reexcision.
CASE 124
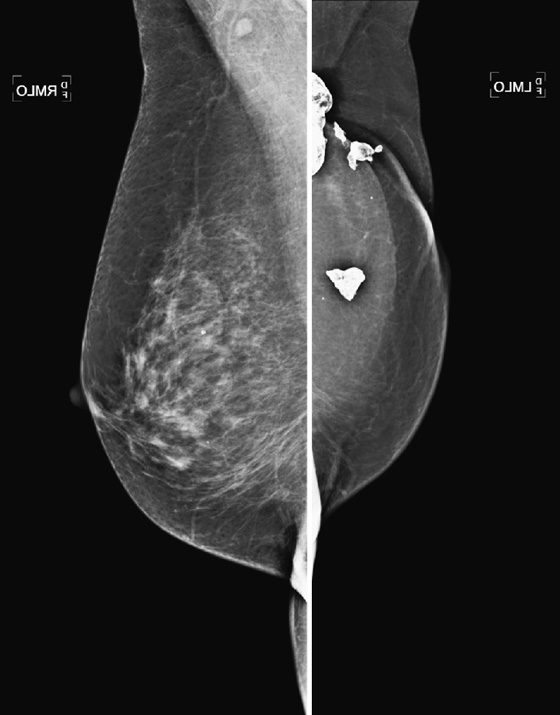
History: A 71-year-old woman who had a mastectomy for cancer with TRAM reconstruction 10 years previously now feels a mass in her reconstructed left breast.
1. What is the differential diagnosis for a palpable mass in a reconstructed breast? (Choose all that apply.)
B. Calcified fat necrosis, Breast Imaging Reporting and Data System (BI-RADS) 2
2. What is the first imaging study in a patient who has a personal history of breast cancer and has a new lump?
C. Positron emission tomography (PET)
3. Is the mammogram alone sufficient for diagnosis in a patient with palpable mass in a reconstructed breast?
A. Yes, mammogram is sufficient if the palpable finding is an oil cyst.
B. Yes, mammogram is sufficient if the mammogram is completely normal.
C. No, ultrasound is always needed for a palpable finding.
D. No, MRI is necessary to further evaluate the palpable mass.
4. Which description best fits fat necrosis calcifications?
B. Layering on 90-degree true lateral view
C. Large, coarse, and irregular
ANSWERS
CASE 124
Palpable Mass in Transverse Rectus Abdominis Myocutaneous (TRAM) Flap
1. C and D
2. C
3. C
4. D
References
Helvie MA, Bailey JE, Roubidoux MA, et al: Mammographic screening of TRAM flap breast reconstructions for detection of nonpalpable recurrent cancer. Radiology 1002;224:211
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:75. 355
Comment
One common method of breast reconstruction after mastectomy is the transverse rectus abdominis myocutaneous (TRAM) flap. Skin, fat, and rectus muscle are brought up from the abdomen to construct a breast mound. This procedure uses living tissue from the mastectomy patient (autogenous transplant).
Fat necrosis after a surgical procedure is not uncommon because areas of fat can lose their blood supply during the procedure. The area of fatty tissue death can calcify, and the calcifications can initially appear as rim types or as pleomorphic shapes that, in time, become coarse, often with associated fat lucency. On mammogram, fat necrosis is most commonly seen as oil cyst associated with coarse calcification (see the figures) but may also present with a focal asymmetry, spiculated density, or pleomorphic microcalcifications. The many appearances result from the cytologic makeup of the process: Fat cells degenerate and hemorrhage, which results in an oil cyst. Fibrosis can surround the oil cyst. It is the varying degree of fibrosis that causes the different appearances on the mammogram. In the cases with the least amount of fibrosis, the palpable mass is lucent (oil cyst), with developing peripheral calcifications forming early and coarse central calcifications forming later. With this appearance of a calcified oil cyst, the appearance is pathognomonic of fat necrosis, and no additional imaging or biopsy is needed. Fibrosis may be the only finding, with an irregular or spiculated density, and the calcifications can be pleomorphic. In these cases, the mammographic appearance overlaps with malignancy, and biopsy may be needed.
The mechanism of malignancy developing in the TRAM reconstruction includes residual cancer tissue, tumor seeding at the time of mastectomy, and tumor sequestered in lymphatics. It can also develop in remnants of native breast tissue not removed entirely at mastectomy. The patient who has undergone a mastectomy for malignant disease is at increased risk for developing a new breast cancer or a recurrence of the known cancer.
The patient in this example presented with a palpable finding, and a diagnostic mammogram of the TRAM flap was performed. Although it is controversial, it may be useful to perform routine screening mammography of the TRAM reconstruction at the time of the mammogram of the remaining breast. Helvie and colleagues noted that malignancy was found as nonpalpable lesions in the TRAM flap in 2% of cases, approximately the same rate of malignancy found after breast conservation therapy.
CASE 125
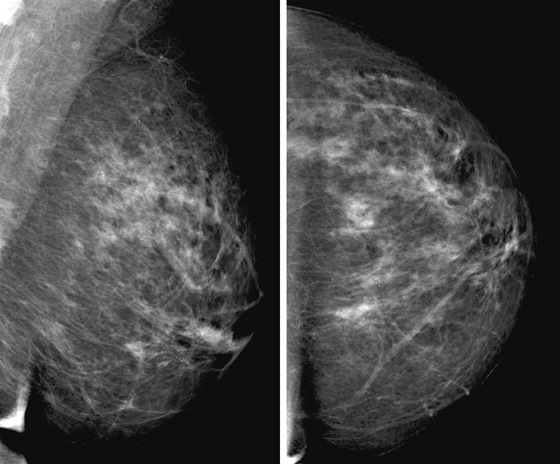
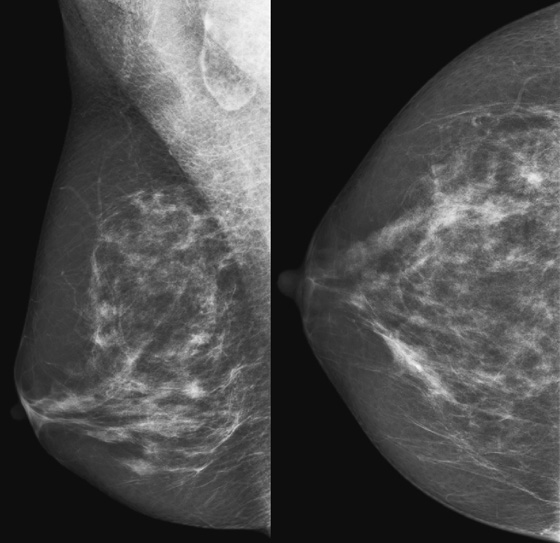
History: A 53-year-old asymptomatic woman presents for screening mammogram.
1. What should be included in the differential diagnosis for the calcifications in the upper inner left breast? (Choose all that apply.)
B. Atypical lobular hyperplasia
C. Lobular carcinoma in situ (LCIS)
2. Stereotactic biopsy revealed benign calcifications associated with fibrocystic change and multiple adjacent foci of LCIS. Which of the following statements regarding LCIS and microcalcifications is true?
A. Microcalcifications are a predisposing factor for the development of LCIS.
B. Microcalcifications exclude the diagnosis of LCIS.
C. Microcalcifications are pathognomonic of LCIS.
D. Microcalcifications may or may not be associated with LCIS.
3. All of the following statements regarding LCIS and cancer are true except:
A. LCIS is an important risk factor for future development of breast cancer.
C. Patients with LCIS are at increased risk for developing invasive lobular carcinoma only.
D. Future cancer can develop in either breast.
4. Which of the following statements regarding management of LCIS is true?
A. LCIS does not require further management.
ANSWERS
CASE 125
Management of Lobular Carcinoma In Situ
1. A, B, and C
2. D
3. C
4. C
References
Anderson BO, Calhoun KE, Rosen EL. Evolving concepts in the management of lobular neoplasia. J Natl Compr Canc Netw. 2006;4(5):511–522.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:26.
Comment
LCIS is a high-risk lesion and an important risk factor for future development of breast cancer. The increased risk is for both breasts, not specifically at the site where the LCIS was found. In the patient in this case, biopsy specimens of the microcalcifications present in the left breast were obtained and shown to be fibrocystic change and multiple foci of LCIS (see the figures). The LCIS was not associated with the calcifications and was an incidental finding, as is often the case. A new spiculated mass was seen 3 years later in the contralateral breast (see the figures). Needle core biopsy showed invasive ductal carcinoma.
Patients with LCIS have an approximately 8 to 12 times increased risk of developing breast cancer. LCIS is detected on imaging-guided biopsy and at excisional biopsy for calcifications and masses, and it is usually an incidental finding. It has no imaging features of its own. The risk of subsequent cancer increases when multiple lobules of LCIS are seen on histopathology. LCIS is multicentric in 85% of cases and bilateral in 30% to 67% of cases. The pathology of the future cancer can be either ductal or lobular.
In the past in women diagnosed with LCIS, recommendations for surveillance included bilateral prophylactic mastectomy. That is no longer recommended, and annual screening mammogram is now recommended. Some recommendations include ultrasound as a screening tool, in addition to mammogram, particularly in women with dense breasts, but this is not universally accepted. MRI can be used as an adjunct to mammography but is not recommended, either for or against, in the 2007 American Cancer Society guidelines for screening MRI. Some patients with LCIS are given selective estrogen receptor modulator therapy, such as tamoxifen. Tamoxifen has been shown to reduce the risk of developing breast cancer by 50%.
Excisional biopsy is recommended in patients who have LCIS found at needle core biopsy because there is a 10% to 20% chance that DCIS or invasive cancer is near the site of LCIS, and the core biopsy did not represent the entire lesion. The same is true for core biopsy specimens showing atypical lobular hyperplasia and atypical ductal hyperplasia.
CASE 126
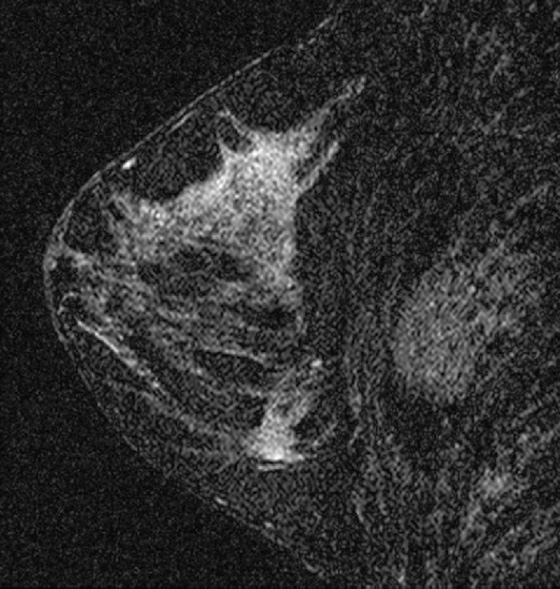
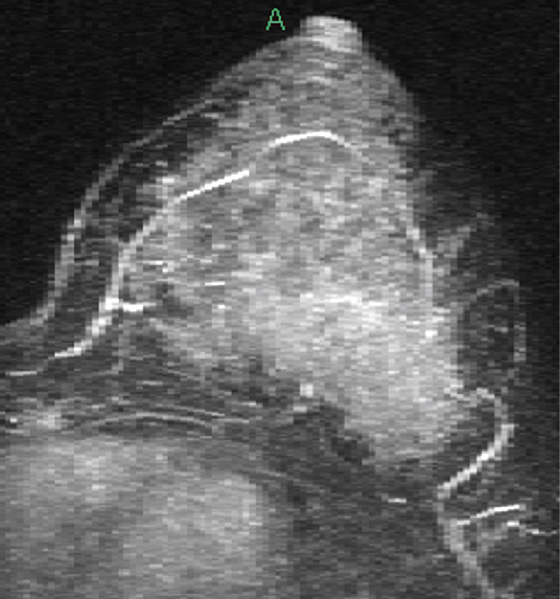
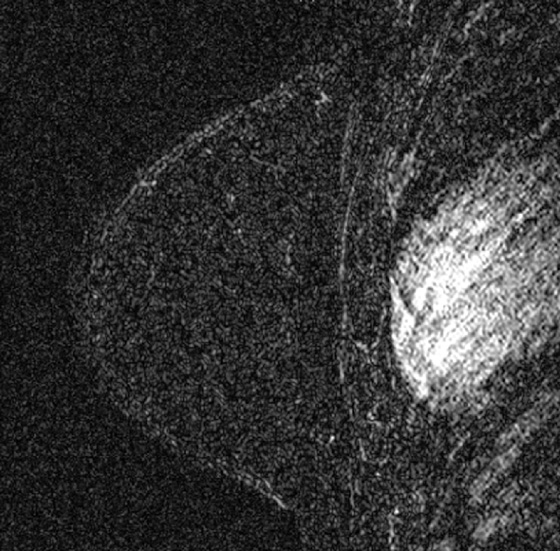
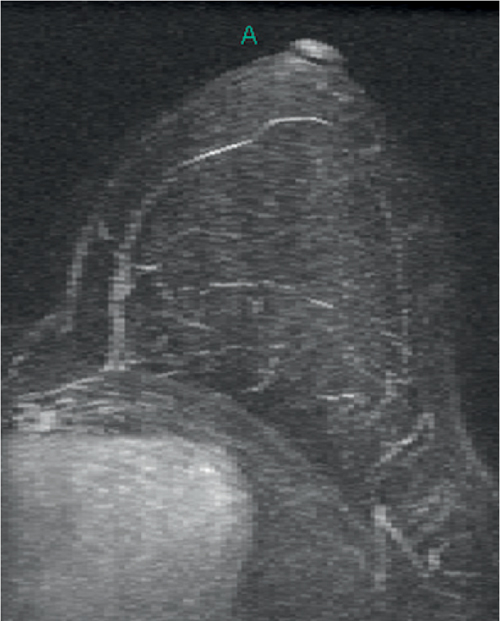
History: A 46-year-old woman with a greater than 20% lifetime risk of breast cancer based on personal history and family history of the disease undergoes screening MRI and follow-up MRI 6 months later.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
A. A woman scanned before and after tamoxifen therapy
B. A woman scanned at two different times of the normal menstrual cycle
C. Two different patients have been scanned
D. Two scans from a postmenopausal patient
2. Which of the following is not an indication for screening MRI?
B. A woman with a 30% lifetime risk of breast cancer
C. A woman whose sister carries the BRCA1 gene mutation
D. A woman with Cowden syndrome
3. Why is the timing of the MRI examination during the menses important?
B. The patient is more comfortable if scanned during the latter half of the cycle.
D. The sensitivity of detecting small masses is greater during the follicular phase.
4. What is the next step in imaging management for this patient?
A. Because MRI is normal, no further MRI should be performed.
B. Annual screening with MRI should be performed; no mammogram is necessary.
C. Annual MRI and mammography screening are needed.
D. The patient needs no further screening.
ANSWERS
CASE 126
MRI in Follicular and Luteal Menstrual Phase
1. A, B, and C
2. A
3. D
4. C
References
Chan S, Su MY, Lei FJ, et al. Menstrual cycle-related fluctuations in breast density measured by using three-dimensional MR imaging. Radiology. 2011;261(3):744–751.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:268.
Comment
Screening MRI is recommended as an adjunct to screening mammogram for women who have BRCA gene mutation, who have a lifetime breast cancer risk based on risk assessment models of 20% or greater, or who have certain high-risk syndromes, such as Cowden syndrome and Li-Fraumeni syndrome. The patient in this case was diagnosed at age 40 with right breast cancer. Her grandmother and two maternal aunts were diagnosed with breast cancer in their thirties and forties. The patient has heterogeneously dense breasts on mammogram. Her lifetime risk is greater than 20%.
Our practice is to ask women younger than 55 about their last menstrual period before scheduling a breast MRI. This patient had not had a menses in several months and so was scheduled at the next available opening. MRI showed bilateral areas of non-masslike enhancement (see the figures). The enhancement has benign characteristics on time-intensity curve, with persistence over time, coded in blue on computer-assisted detection (CAD). This enhancement was believed to be due to the increased estrogen and progesterone levels of the luteal phase of the menstrual cycle, and follow-up was recommended.
The patient returned after her next menses; breast MRI was scheduled during days 7 to 14 of the menstrual cycle. The change in the appearance of the enhancement pattern is remarkable; there is no glandular enhancement (see the figures). This case illustrates the benefit of performing breast MRI during the follicular phase of the cycle if possible. It is much easier to detect a small enhancing mass against the background of nonenhancing glandular tissue compared with during the luteal phase against the background of areas of non-masslike enhancement.
CASE 127
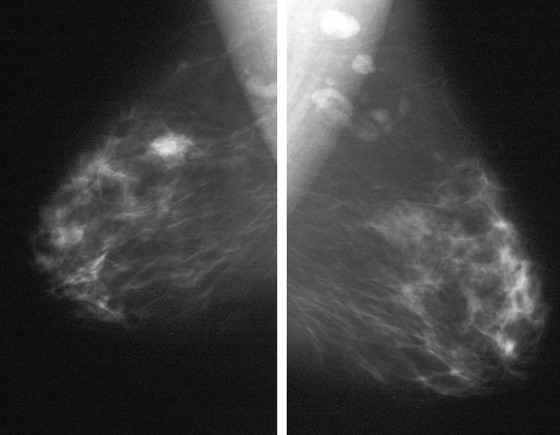
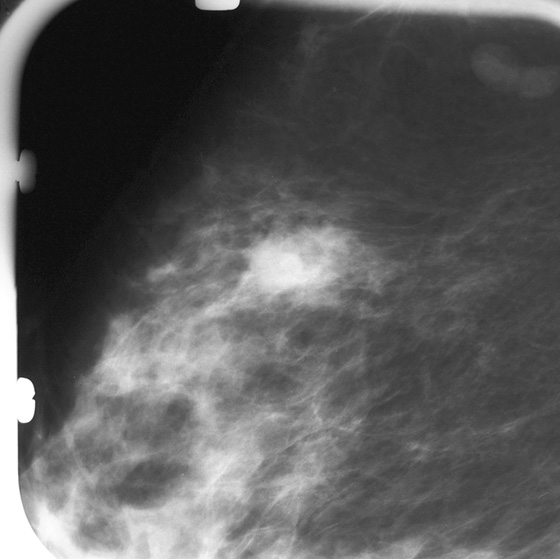
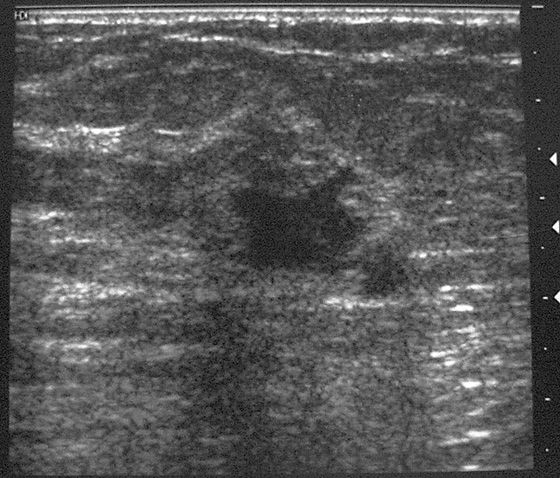
History: A 63-year-old woman presents for a screening mammogram.
1. What should be included in the differential diagnosis for the initial presentation? (Choose all that apply.)
2. Which of the following is not a rare type of invasive breast cancer?
D. Ductal carcinoma not otherwise specified
3. Why is it useful to know the pathologic subtype of a breast cancer?
B. The pathologic subtype often predicts the prognosis of the cancer.
C. Treatment may be unnecessary.
D. Knowing the subtype of the breast cancer is of no use.
4. Which of the following is not characteristic of adenoid cystic carcinoma?
A. It cannot be distinguished from other subtypes of breast cancer by imaging only.
C. The prognosis is excellent.
ANSWERS
CASE 127
Adenoid Cystic Carcinoma
1. A and C
2. D
3. B
4. D
References
Glazebrook KN, Reynolds C, Smith RL, et al. Adenoid cystic carcinoma of the breast. AJR Am J Roentgenol. 2010;194(5):1391–1396.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:131.
Comment
Adenoid cystic carcinoma is an unusual subtype of breast cancer, which accounts for less than 1% of all breast cancers. This subtype rarely spreads beyond the breast to the lymph nodes or to distant sites. The appearance on mammography and ultrasound is similar to other breast cancers, most often a lobulated mass (see the figures). It is important to know the cell type of breast cancers because the cell type often determines the clinical outcome. Adenoid cystic carcinoma has a better prognosis than other types. In a study of 28 patients with adenoid cystic carcinoma, the 5-year disease-free survival was 100%. This excellent prognosis affects treatment options. Axillary dissection and chemotherapy are unnecessary. However, the mass must be completely excised, or recurrence may occur in the breast. The average age of diagnosis is postmenopausal, age 65 years.
In the patient in this case, a developing irregular, high-density mass with microlobulated margins was seen in the upper outer quadrant of the right breast on routine screening mammogram (see the figures). The patient was 63 years old when the diagnosis was made; on pathology, this was a 2-cm mass that was predominantly in situ adenoid cystic cancer, with rare areas of invasion.
CASE 128
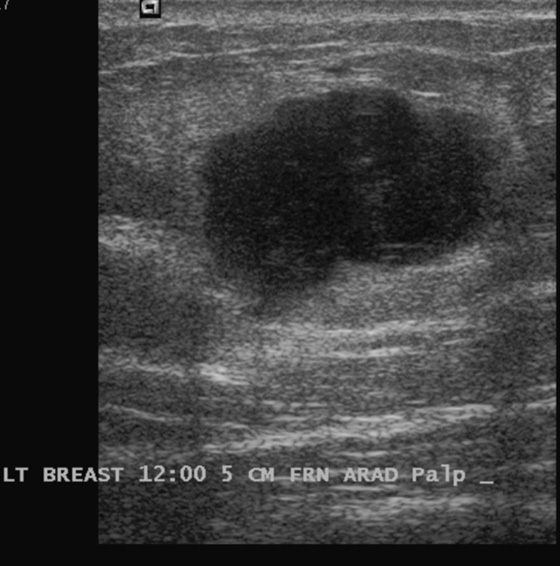
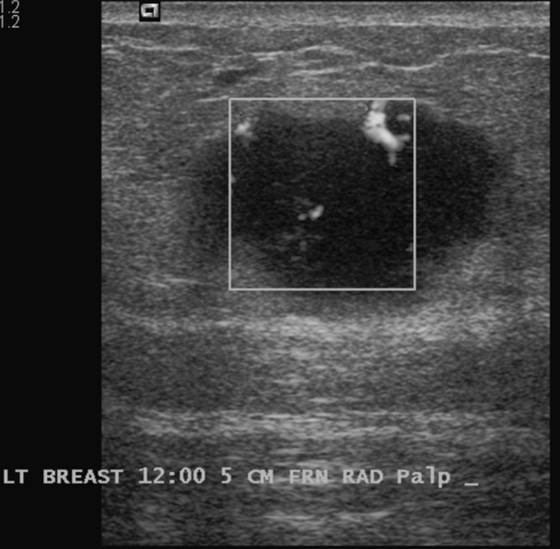
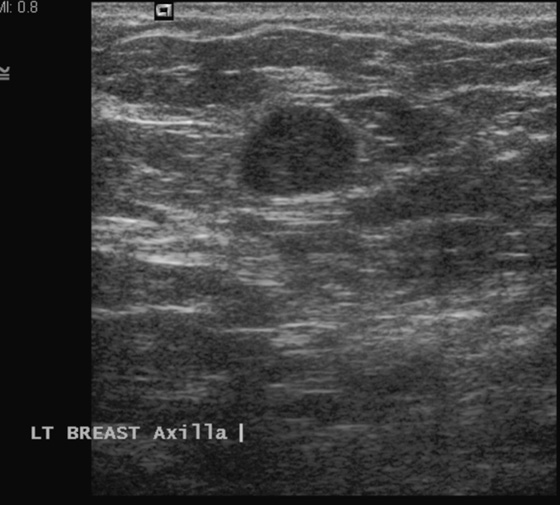
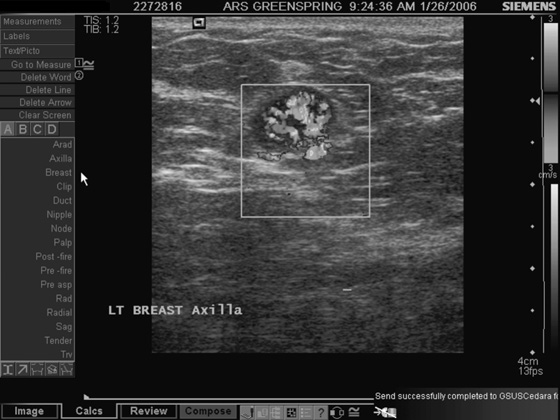
History: A 53-year-old woman presents for her baseline mammogram with a palpable left breast mass. After review of the mammogram, ultrasound was performed.
1. What should be included in the differential diagnosis for the lesion seen on ultrasound? (Choose all that apply.)
2. What is the next step in management of this palpable mass?
A. MRI to differentiate benign from malignant
C. Short-interval follow-up with ultrasound in 6 months
D. PET/CT to evaluate for additional lesions
3. What are the special subtypes of infiltrating ductal carcinoma (IDC)?
A. Medullary, mucinous, tubular, and papillary cancer
B. Invasive ductal carcinoma not otherwise specified (IDC NOS)
D. Lobular carcinoma in situ, atypical lobular hyperplasia, atypical ductal hyperplasia
4. Which of the following statements is true about medullary cancer?
A. It is very rare—about 1% of all breast cancers.
B. It has a worse prognosis than IDC NOS.
C. It may manifest as a benign, soft mass.
D. It is commonly found in men and women.
ANSWERS
CASE 128
Medullary Cancer
1. B, C, and D
2. B
3. A
4. C
References
Meyer JE, Amin E, Lindfors KK, et al. Medullary carcinoma of the breast: mammographic and US appearance. Radiology. 1989;170(1 Pt 1):79–82.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:114.
Comment
Medullary cancer is a subtype of IDC of the breast. It is uncommon, accounting for about 5% to 7% of IDCs. It is seen in slightly younger women compared with generic invasive ductal carcinoma, or NOS. It is well circumscribed, may be soft and mobile on examination, and may have features that suggest a benign etiology on mammography and ultrasound. On mammography, it may be round, oval, or lobulated. Calcifications are rare. On ultrasound, it is round, oval, or lobulated; is hypoechoic; has sharply circumscribed margins; and often has increased through-transmission of sound (see the figures).
On histology, medullary cancer comprises poorly differentiated cells, with a high nuclear grade. A lymphoplasmacytic reaction is seen in the tumor. It has a “pushing” margin, rather than an infiltrative margin, leading to its circumscribed edges, and no spiculation on mammography and ultrasound. It is common to find axillary node enlargement, but the nodes may be reactive rather than metastatic (see the figures). There is an overlap with the appearance of mucinous carcinoma, which is another tumor subgroup of IDC. The differential diagnosis also includes fibroadenoma, phyllodes tumor, and lymphoma.
In this case, the ultrasound features are inconsistent with a fibroadenoma because of the irregular, microlobulated margins. One suspicious ultrasound feature, such as the margins in this mass, means that the mass is a BI-RADS (Breast Imaging Reporting and Data System) 4, and biopsy is indicated. Color Doppler can help evaluate the vascularity of the mass (see the figures) and help differentiate a solid mass from a complex cyst. In this patient, axillary nodes were abnormal: round and hypoechoic, and intensely hypervascular (see the figures). However, the core biopsy of the axillary node revealed reactive cells and no metastases. This is not unusual in medullary carcinoma.
CASE 129
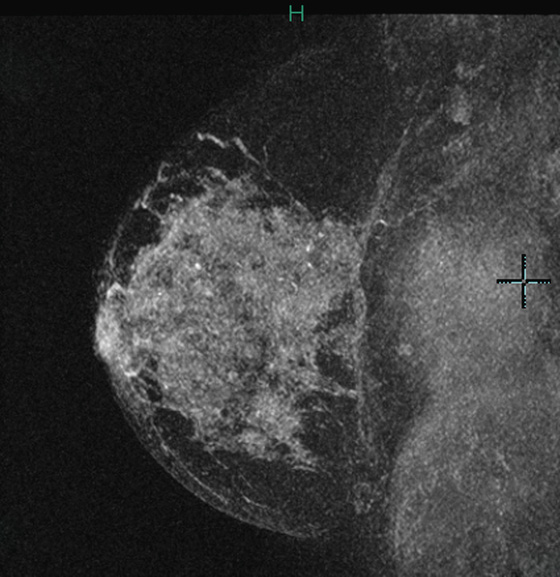
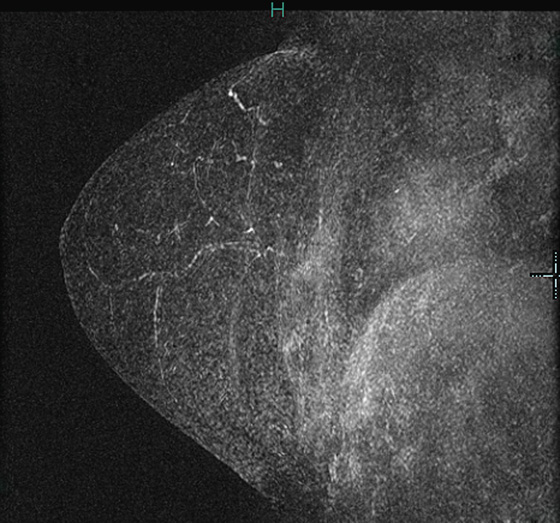
History: A 57-year-old woman underwent image-guided core biopsy, and stage IIIC invasive mammary carcinoma was diagnosed. MRI was performed for extent of disease. She underwent neoadjuvant chemotherapy, and follow-up MRI after chemotherapy.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
A. Complete clinical response to chemotherapy
B. Decrease in size of enhancement after chemotherapy
C. Improved pathologic response to chemotherapy
D. Absence of residual disease
2. Which of the following statements about MRI after chemotherapy is not true?
A. Chemotherapy has an antineovascularity effect.
B. Chemotherapy can break up a larger tumor into smaller clumps of tumor.
C. Tumor is completely eradicated if MRI is negative.
D. Absence of residual tumor on MRI predicts improved survival.
3. Why is neoadjuvant chemotherapy used?
A. To decrease the size of tumors before surgical treatment
B. To negate the need for surgery
C. To reduce the need for radiation therapy
4. Is this patient’s first MRI consistent with inflammatory carcinoma?
A. No, because there is no evidence of skin enhancement
B. No, because the area of enhancement is too large
C. Yes, because of the diffuse enhancement of the entire breast
D. No, because there is no axillary adenopathy
ANSWERS
CASE 129
Response to Neoadjuvant Chemotherapy
1. B and C
2. C
3. A
4. A
References
Chen JH, Feig B, Agrawal G, et al. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112(1):17–26.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:273. 297
Comment
Neoadjuvant chemotherapy is systemic treatment for breast cancer that is given before definitive surgery. The goal is to allow the patient to have breast conservation therapy, rather than mastectomy. Neoadjuvant chemotherapy is typically discussed when a patient presents with locally advanced breast cancer, such as in the present case (see the figures).
Neoadjuvant chemotherapy is an alternative to adjuvant chemotherapy, which is given after the cancer has been resected. Both regimens have been shown to be equivalent in overall survival and in the rate of development of distant metastases. Using chemotherapy before surgery allows the clinician to gauge the response of the patient to the therapy; this serves as an in vivo evaluation of the patient’s response to the chemotherapeutic regimen.
Pathologic response is measured at surgery. Complete pathologic remission is the complete disappearance of cancer in the primary tumor area. MRI predicts complete pathologic response in approximately 70% of cases. The patient in this case had absence of enhancement on follow-up MRI but did not have a complete pathologic response (see the figures).
Because chemotherapy targets tumor angiogenesis, the MRI contrast agent may not reach the tumor, and the enhancement may not accurately reflect the residual tumor present. The radiologist should be cautious about reporting a “complete response” and may want to report only “response to treatment.” MRI is about 90% specific and about 60% sensitive for the presence of residual disease after chemotherapy but performs better than clinical examination, mammography, and ultrasound.
CASE 130
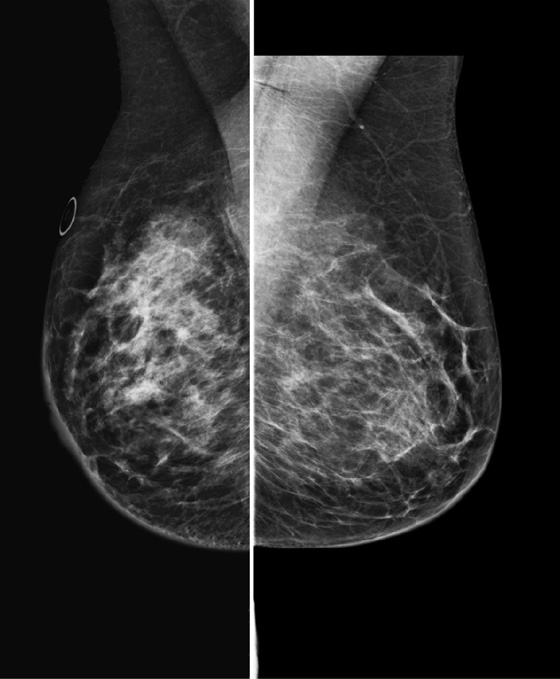
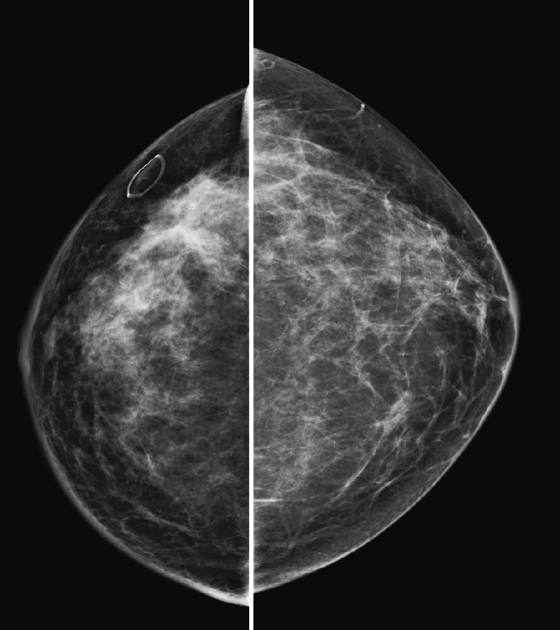
History: A 57-year-old woman presents for her baseline mammogram because of palpable mass in her right breast.
1. What is the differential diagnosis of the four-view mammogram? (Choose all that apply.)
B. Right breast diabetic mastopathy
C. Right breast inflammatory cancer
D. Right breast infiltrating lobular carcinoma
2. What is the next step?
A. Ultrasound of the palpable concern
B. Surgical consultation for biopsy of the palpable mass
3. What histologic type of breast cancer can manifest with increased density and a decrease in the size of the breast on the mammogram?
D. Ductal carcinoma in situ (DCIS)
4. Diagnosis of a large malignancy in the right breast was made. Is breast MRI indicated? Why or why not?
A. No, once the diagnosis is made, the patient should be referred for surgery as soon as possible.
C. Yes, MRI is useful to evaluate for extent of disease in the ipsilateral and contralateral breast.
D. No, MRI is not needed because the patient should be advised to have bilateral mastectomy.
ANSWERS
CASE 130
Invasive Lobular Cancer: The Shrinking Breast
1. B, C, and D
2. A
3. C
4. C
References
Harvey JA. Unusual breast cancers; useful clues to expanding the differential diagnosis. Radiology. 2007;242:683–694.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:101.
Comment
This patient noted a palpable mass, but on review of the mammogram, no discrete mass was seen. The right breast appeared smaller than the left but was not smaller on clinical exam (compare the first and second figures). The right breast was also noted to have ill-defined areas of increased density throughout the central portion (see the figures).
Ultrasound was performed, and there was a large, ill-defined hypoechoic area measuring approximately 10 cm in diameter in the central breast. Ultrasound-guided needle biopsy was performed and showed infiltrating, poorly differentiated mammary carcinoma, with predominantly lobular features. Core biopsy of an axillary node showed metastatic disease.
Invasive lobular carcinoma (ILC) is less common than invasive ductal carcinoma (IDC), comprising less than 10% of all breast cancer. The cells of this cancer spread through the breast in single file or sheets, thus not forming a discrete mass, making it more difficult to detect on the mammogram. Because of this, ILC is more commonly missed on routine screening mammogram and manifests at a later stage than invasive ductal carcinoma. It also manifests more often as muticentric and bilateral disease than does IDC.
When ILC is large, the breast may appear to be shrinking on the mammogram. This is a mammographic finding, not a clinical finding, and may be due to the sheets of tumor cells causing decreased compressibility of the breast. The mammogram can show an irregular mass or asymmetry with ill-defined margins, as in this case.
CASE 131
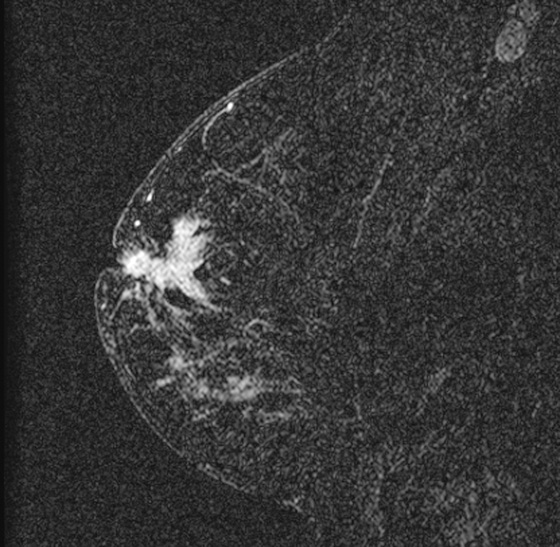
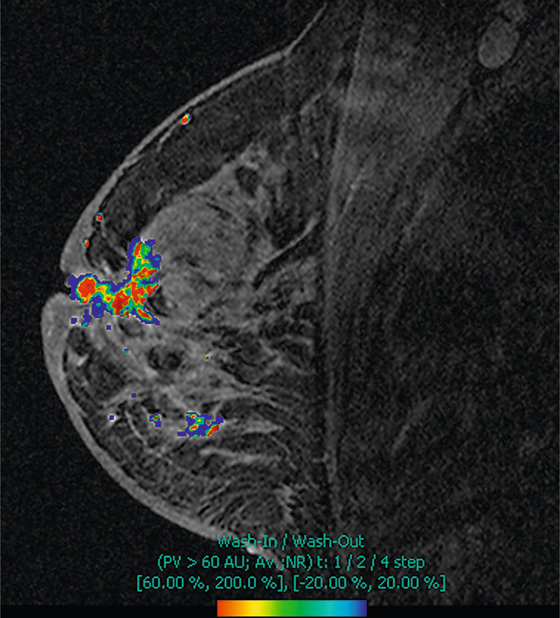
History: A 51-year-old woman presents with nipple retraction in the right breast. Mammogram shows a retracted nipple and skin thickening (not shown). Needle biopsy and bilateral MRI are performed.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
2. What is metaplastic cancer?
A. Synonymous with metastatic cancer, it means cancer that has spread outside the breast
B. Cancer derived from two cell lines, also termed carcinosarcoma
C. Relatively benign-acting tumor that is rare
D. A rare cancer that has only mesenchymal cells and no epithelial cells
3. What is the next management step?
A. Positron emission tomography (PET) scan to check for metastases
B. Positron emission mammography to check for additional sites of cancer in both breasts
C. Referral to surgical and medical oncology
D. Strong recommendation that the patient return in 3 months for follow-up
4. Which of the following statements about breast sarcomas is not true?
A. Sarcomas are unusual in the breast.
B. Sarcomas spread hematogenously.
C. Osteosarcomas can occur in the breast.
D. Sarcomas in the breast generally have a good outcome.
ANSWERS
CASE 131
Metaplastic Cancer
1. B and C
2. B
3. C
4. D
References
Feder JM, de Paredes ES, Hogge JP, et al. Unusual breast lesions: radiologic-pathologic correlation. Radiographics. 1999;19:S11–S26. (Spec No)
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:398.
Comment
Metaplastic carcinoma is a rare form of breast cancer that accounts for less than 0.1% of all breast cancers. Also termed carcinosarcoma, it has carcinomatous (epithelial) and sarcomatous (mesenchymal) features. It is an aggressive form of cancer, behaving similarly to high-grade, receptor-negative adenocarcinomas.
Metaplastic cancer manifests clinically similar to invasive ductal carcinoma as a rapidly growing mass that may be centered on the nipple, as in the patient in this case (see the figures). Rapid wash-in and wash-out kinetics and nipple retraction are noted. The tumor may be locally aggressive, so breast conservation therapy may fail, and mastectomy may be the best surgical option. Chemotherapeutic agents given as neoadjuvant therapy are not as effective in metaplastic carcinoma compared with invasive ductal carcinoma. Recurrence can be rapid, and metastases to the lung are more common than to other organs, making the prognosis poor after metastatic spread occurs.
CASE 132
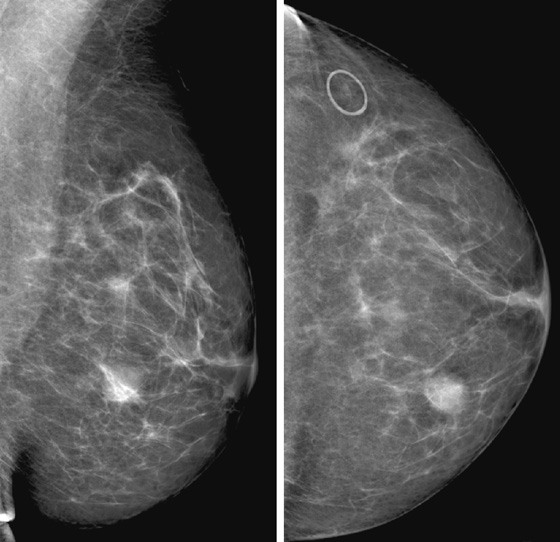
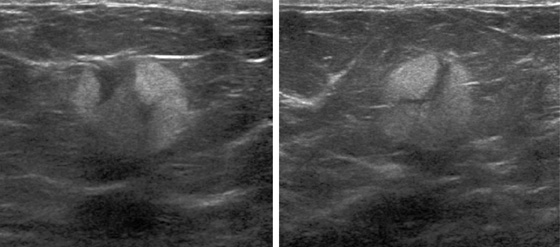
History: A 31-year-old woman presents with a new left palpable lump. Her past medical history is significant for acute myeloid leukemia that was diagnosed 5 years ago, relapsed 1 year ago, and now is in remission.
1. What should be included in the differential diagnosis? (Choose all that apply.)
D. Pseudoangiomatous stromal hyperplasia
2. Which of the following statements regarding metastases to the breast is true?
A. Metastases to the breast are common lesions.
B. Metastases manifest only as bilateral lesions.
C. The most common primaries include melanoma, lymphoma, lung, and ovarian.
D. Breast involvement in leukemia is common.
3. Which of the following is not another name for leukemic infiltrates of the breast?
B. Extramedullary plasmacytoma
D. Extramedullary myeloblastoma
4. Which of the following is an imaging characteristic of leukemic infiltrates in the breast?
A. They always occur as a single mass.
B. They usually appear as a spiculated mass with microcalcifications on mammogram.
C. They usually appear as a predominantly well-defined hypoechoic mass on ultrasound.
D. On MRI, they are easily distinguishable from primary breast cancer.
ANSWERS
CASE 132
Leukemic Infiltrate of the Breast
1. B, C, and D
2. C
3. B
4. C
References
Bayrak IK, Yalin T, Ozmen Z, et al. Acute lymphoblastic leukemia presented as multiple breast masses. Korean J Radiol. 2009;10(5):508–510.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:117.
Comment
Metastases to the breast are uncommon lesions, accounting for 0.5% to 6.6% of all breast malignancies. They can manifest as unilateral or bilateral, single or multiple lesions. Although any malignancy has the potential to metastasize to the breast cancer, melanoma, lymphoma, oat cell carcinoma of the lung, and ovarian cancers are the most common primaries. Other primaries include rhabdomyosarcoma and leukemia. Breast involvement in leukemia is uncommon, with an estimated incidence of less than 1%. It usually occurs by hematologic spread and usually manifests in a setting of diffuse systemic disease. It typically involves younger patients than those with breast involvement in other lymphoproliferative neoplasms and can even affect children.
Leukemic infiltrates, also known as chloromas, extramedullary myeloblastomas, or granulocytic sarcomas, are essentially extramedullary solid tumors composed of granulocytic precursor cells. They are mainly associated with myelogenous leukemia, can occur during either leukemia relapse or remission, and may be the only presentation of the disease. In addition, radiotherapy to the breast can cause acute leukemia in the breast tissue.
Clinically, leukemic infiltrates can mimic primary breast tumors and can be misdiagnosed. Patients may present with single or multiple, unilateral or bilateral breast masses or enlargement, with or without axillary lymphadenopathy. Masses may be hard on palpation. The lesions usually regress after systemic therapy.
Leukemia can manifest on mammogram as a well-defined or ill-defined noncalcified mass or masses (see the figures), ovoid or irregular, or as diffusely increased breast density. The mammogram can also be unremarkable.
The ultrasound appearance is variable. The most frequent presentation is a predominantly well-defined hypoechoic mass, but tumors may also manifest as areas of mixed echogenicity with central anechoic and peripheral hyperechoic areas (see the figures), a large mass with spiculations or angular margins or both, or areas of marked low attenuation with or without acoustic shadowing. Some leukemic breast masses have shown increased vascularity with color Doppler.
MRI findings can be indistinguishable from findings of multicentric carcinoma or malignant lymphoma. The most common presentation is multiple enhancing masses with smooth edges and wash-out kinetics. MRI constitutes an accurate study for monitoring after treatment.
These tumors may manifest as single or multiple, unilateral or bilateral hypermetabolic breast masses on PET/CT (see the figures). They can be widespread and multifocal.
CASE 133
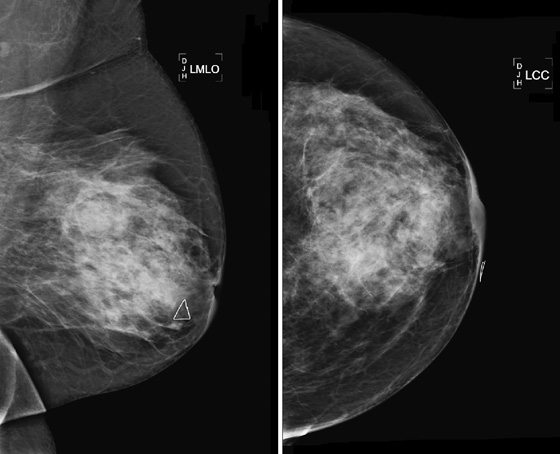
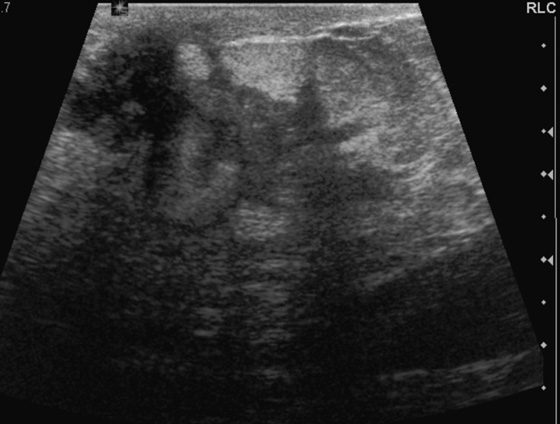
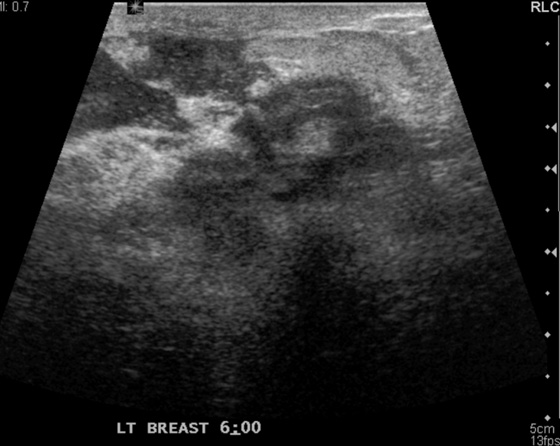
History: A 45-year-old woman presents with a palpable mass and warm, erythematous, tender skin in the central left breast.
1. What should be included in the differential diagnosis of the findings in the left breast on mammography and ultrasound? (Choose all that apply.)
2. What is the next step in management?
A. Biopsy to rule out inflammatory cancer
B. MRI to evaluate for abnormal enhancement pattern of malignancy
D. Short-interval follow-up ultrasound in 1 to 2 weeks
3. What is the etiology of breast abscess?
A. A source of infection is always found.
B. It occurs exclusively in nursing mothers.
C. Infection not related to breastfeeding is more commonly seen in smokers.
D. There is typically a skin wound that undergoes secondary infection.
4. Why does inflammatory breast cancer mimic infection?
A. Inflammatory breast cancer has tumor emboli in the dermal lymphatics.
B. The cancer has become infected.
C. The cancer spreads into the skin.
D. Infection and inflammatory breast cancer manifest very differently.
ANSWERS
CASE 133
Abscess
1. A, C, and D
2. C
3. C
4. A
References
Christensen AF, Al-Suliman N, Nielsen KR, et al. Ultrasound-guided drainage of breast abscesses: results in 151 patients. Br J Radiol. 2005;78(927):186–188.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:140.
Comment
The appearance of breast abscess may be nonspecific. It manifests with findings similar to breast malignancy, and malignancy must always be excluded when a patient presents with a mass. The location of the mass near the nipple is characteristic of breast abscess. The margins of the abscess on mammography may be poorly delineated, as in this patient (see the figures). The mass in this patient merges with the background breast tissue, and the reason is clear on the ultrasound images. On ultrasound, the margins are infiltrative and irregular (see the figures) as the infection tracks along tissue planes. This ultrasound appearance is not unique to infection because malignancy can also manifest as an infiltrative process, rather than a well-defined mass (particularly infiltrating lobular carcinoma). On ultrasound, you may also find low-level, horizontal echoes in the fluid, which may indicate air (see the figures). Color Doppler may show hyperemia in the surrounding tissue.
The abscess may be drained percutaneously using ultrasound guidance. An 18-gauge needle may be needed if the infected fluid is thick, and repeated efforts may be needed. Antibiotics are also necessary, and a trial of antibiotics may serve to differentiate inflammatory breast cancer. If the patient improves after 10 days, biopsy of the mass to exclude cancer may be unnecessary. It is important to observe the patient closely until the mass is completely gone. If there is no improvement on antibiotics, inflammatory cancer must be considered possible, and biopsy must be performed.
In the case of abscess, particularly abscesses greater than 3 cm, percutaneous aspiration may not resolve the infection completely, and surgical incision and drainage may be needed. Rarely, a drain may be placed percutaneously by the radiologist.
CASE 134
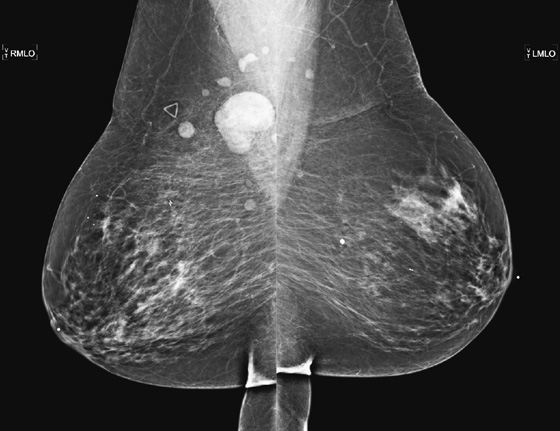
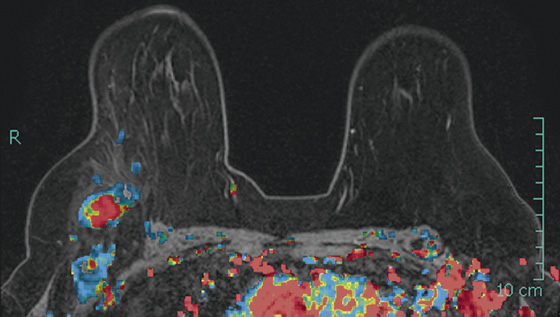
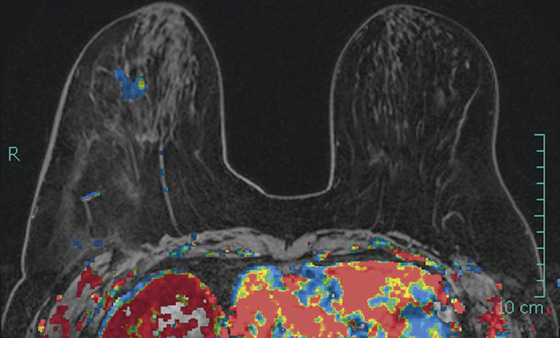
History: A 74-year-old woman with a palpable mass in the right axilla presents for a diagnostic mammogram.
1. What should be included in the differential diagnosis of the mammographic images shown? (Choose all that apply.)
A. Enlarged right axillary nodes owing to metastatic breast cancer
B. Enlarged axillary nodes owing to lymphoma
C. Enlarged reactive axillary lymph nodes
2. What is the next step in management?
B. Spot compression views of right breast
3. How common is axillary metastatic disease as the only manifestation of breast cancer?
A. Very common—more than 50% of breast cancers manifest first with a metastatic node.
B. Common—it is the presentation in about 25% of breast cancer cases.
C. Less common—about 10% of breast cancers manifest first with a metastatic node.
D. Rare—about 1% of all breast cancers have this presentation.
4. If biopsy of the axillary node shows adenocarcinoma, what is the next step in management?
A. PET/CT should be performed to evaluate for other sites of adenocarcinoma.
B. The breast should be evaluated first when adenocarcinoma is found in an axillary node.
D. Chest x-ray and, if needed, chest CT for lung evaluation should be obtained.
ANSWERS
CASE 134
Axillary Node Presentation of Occult Breast Cancer
1. A, B, and C
2. C
3. D
4. B
References
Argus A, Mahoney MC. Indications for breast MRI: case-based review. AJR Am J Roentgenol. 2011;196(3 Suppl):WS1–WS14.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:396.
Comment
It is uncommon for breast cancer to manifest as a palpable axillary node as the only finding. More typically, when breast cancer has invaded the axillary nodes, its presence is not occult. A mass is palpable, or mammographic abnormalities are present by the time the tumor has spread. This presentation occurs in about 1% of all breast cancers (see the figures). In this case, a previous routine mammogram obtained 10 months before the current examination was normal with normal axillary nodes.
Biopsy of the node should be performed first because other etiologies exist for an enlarged palpable axillary node, including granulomatous disease, reactive node, and lymphoma. If adenocarcinoma is found on biopsy, the chances are quite small that the etiology is anything but breast cancer, and work-up of other adenocarcinomas is not needed. The mammogram should be carefully scrutinized for any evidence of abnormality, and additional views should be taken as needed. If no abnormality is seen, MRI is the best next step (see the figures).
According to two published reports, the likelihood of MRI detecting the primary cancer is 75% to 86%. If the cancer is found, patient management is altered. The patient would have likely gone on to mastectomy because the primary was not found. One third of the time, the primary tumor is not found in the mastectomy specimen, likely because it is difficult for the pathologist to examine every square centimeter of the entire breast specimen. Finding the primary tumor allows the patient to have possible breast conservation therapy. The patient in this case had multiple enlarged axillary nodes on MRI and a 1-cm invasive ductal carcinoma grade III on biopsy of the mass, seen in the 6:30 o’clock location on MRI.
CASE 135
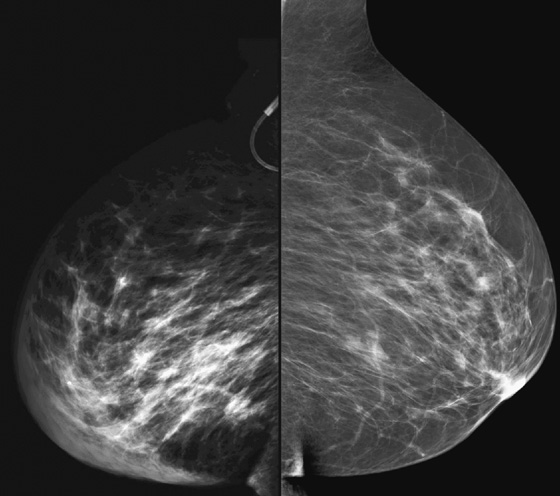
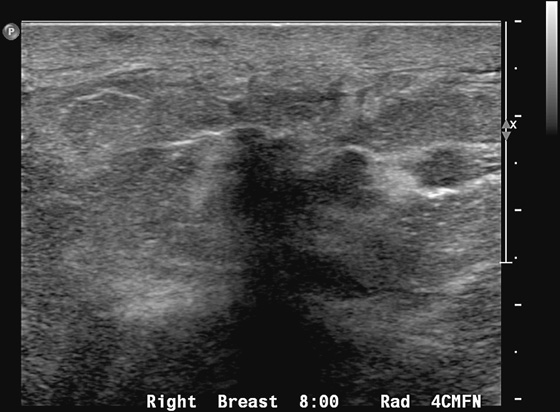
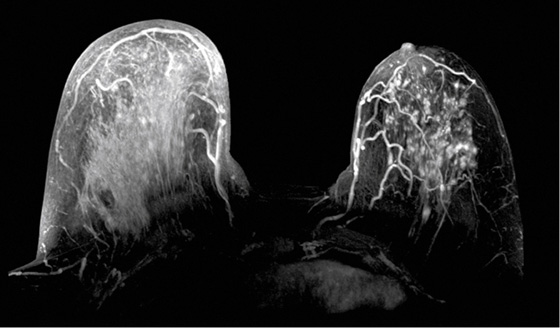
History: A 43-year-old woman with a history of colon cancer and chemotherapy presents with rapid onset of right breast enlargement, associated with peau d’orange and redness, with no discrete palpable mass.
1. What should be included in the differential diagnosis considering the clinical history and mammographic findings? (Choose all that apply.)
2. What is the best next diagnostic step?
A. Follow-up mammogram, ultrasound, and MRI in 6 months
3. Which of the following statements regarding inflammatory breast cancer is true?
A. Invasive ductal carcinoma is the only cause of inflammatory breast cancer.
B. Inflammatory breast cancer is very prevalent.
C. It may be difficult to differentiate from infectious mastitis.
D. The classic clinical presentation includes a discrete palpable mass.
4. What is the best treatment for inflammatory breast cancer?
B. Lumpectomy and radiation therapy
D. Chemotherapy followed by mastectomy and radiation therapy
ANSWERS
CASE 135
Inflammatory Breast Cancer
1. A, C, and D
2. C
3. C
4. D
References
Gunhan-Bilgen I, Ustun EE, Memis A. Inflammatory breast carcinoma: mammographic, ultrasonographic, clinical, and pathologic findings in 142 cases. Radiology. 2002;223(3):829–838.
Yang WT. Advances in imaging of inflammatory breast cancer. Cancer. 2010;116(11 Suppl):2755–2757.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:175. 393
Comment
Inflammatory breast cancer is an aggressive form of invasive carcinoma. It is a rare entity, accounting for 1% to 4% of all breast cancers. Clinically, inflammatory breast cancer is characterized by rapid onset of unilateral breast thickening, edema (which gives a peau d’orange [“orange peel”] appearance to the skin), and erythema but little or no pain and may be associated with a sensation of heat in the affected breast. It frequently manifests in younger patients, with early local and distant metastasis (55% to 58% have axillary adenopathy at presentation), and overall survival is lower compared with other forms of breast cancer. Bilateral involvement has been reported in 1% to 55% of cases.
Any tissue type of cancer may cause inflammatory carcinoma, but invasive ductal carcinoma is the most common. Histology shows tumor infiltration and emboli into the dermal lymphatics, often with nonlymphocytic reaction surrounding the large dilated vessels of the dermis.
The main differential diagnostic consideration is diffuse infectious mastitis, which has a similar clinical presentation except for the presence of fever, localized pain, and leukocytosis, which are not seen in inflammatory cancer. Skin changes in the breast can also be seen after breast surgery and radiation therapy, superior vena cava thrombosis, congestive heart failure, and lymphoma. Clinical history is important to eliminate these causes of breast erythema and edema.
Skin thickening, increased breast density, and trabecular thickening may be seen on mammogram, although the findings may be subtle (see the figures). Masses and calcifications may be present. Ultrasound is a useful imaging tool after mammography to evaluate skin changes and localize a specific parenchymal mass to guide biopsy. The most frequent ultrasound findings include heterogeneous infiltration of the breast tissue or a conglomerate of intraparenchymal masses with overlying skin and subcutaneous edema (see the figures). Lymph nodes can also be easily assessed with ultrasound. MRI can be performed if mammography and ultrasound are inconclusive. Findings on MRI include breast enlargement, diffuse skin thickening, edema, masses, and abnormal parenchymal enhancement (see the figures). MRI is also useful to evaluate tumor response to chemotherapy.
The definitive diagnosis of inflammatory carcinoma requires biopsy. The procedure should include a biopsy specimen of the skin and a specimen of any identifiable discrete mass. Biopsy can be performed with ultrasound guidance. MRI can be also used to guide biopsies of focal areas of suspicious enhancement.
The current standard management of inflammatory breast cancer consists of systemic chemotherapy followed by surgery and radiation therapy. Mastectomy with axillary lymph node dissection is the optimal surgical procedure because the presence of positive margins leads to a poorer prognosis.
CASE 136
History: A 70-year-old woman developed microcalcifications in the left breast. She underwent stereotactic biopsy, with successful retrieval of all the calcifications.
1. What should be included in the differential diagnosis of the mammograms shown? (Choose all that apply.)
2. What is the next step in management of developing calcifications?
A. Short-interval follow-up with magnification views
D. Consultation with a breast surgical specialist regarding advisability of biopsy
3. What would be a case in which a clip would not be needed after biopsy?
A. The lesion is absolutely benign, and no surgery would be needed.
C. The microcalcifications are only moderately suspicious.
D. The calcifications have been completely removed with the biopsy.
4. All of the following are reasons that clips become displaced after biopsy except:
A. Compression of the breast during the biopsy
B. Hematoma formation at the time of biopsy
C. Clip migration secondary to very dense breast tissue
D. Displacement within the biopsy track
ANSWERS
CASE 136
Clip Displacement
1. A, C, and D
2. B
3. B
4. C
References
Esserman LE, Cura MA, DaCosta D. Recognizing pitfalls in early and late migration of clip markers after imaging-guided directional vacuum-assisted biopsy. Radiographics. 2004;24(1):147–156.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:223.
Comment
Needle biopsy is the appropriate management step for indeterminate or suspicious masses or calcifications in the breast (see the figures). When the lesions are small, it is possible that all radiographic evidence of the lesion is removed by the image-guided biopsy, particularly if vacuum-assisted technique is used. For this reason, the site of biopsy is marked after the procedure is completed. The most common way of marking the site is a metal clip or marker. These clips are made of stainless steel or titanium and may have adjacent echogenic absorbable gelatin sponge material (Gelfoam) to allow visualization under ultrasound and to facilitate hemostasis after biopsy. Earlier clips were made to clip the tissue; later devices are termed markers because they are designed to fall into the biopsy cavity.
The clip may be displaced after the biopsy in 20% of patients, according to one series. Clip displacement is most common with stereotactic biopsy, as the breast is compressed during the biopsy procedure and then the compression is released after the sampling. The “accordion effect” allows the clip to travel back along the biopsy track, in the direction of the skin insertion (along the z-axis). Hematoma formation at the time of biopsy can also cause the clip to become displaced.
It is important to obtain standard mammographic views of craniocaudal and lateral projections after the biopsy to assess the location of the clip (see the figures). In the patient in this case, the clip was displaced several centimeters inferiorly, along the z-axis, immediately after the biopsy. Clips can also become displaced several months after the biopsy. Clip displacement is more common in the loose stroma of a fatty breast than in a dense breast. The location of the clip related to the biopsy site should be mentioned in the biopsy report. If subsequent needle localization is needed, the biopsy site, not the clip location, needs to be localized and excised.
CASE 137
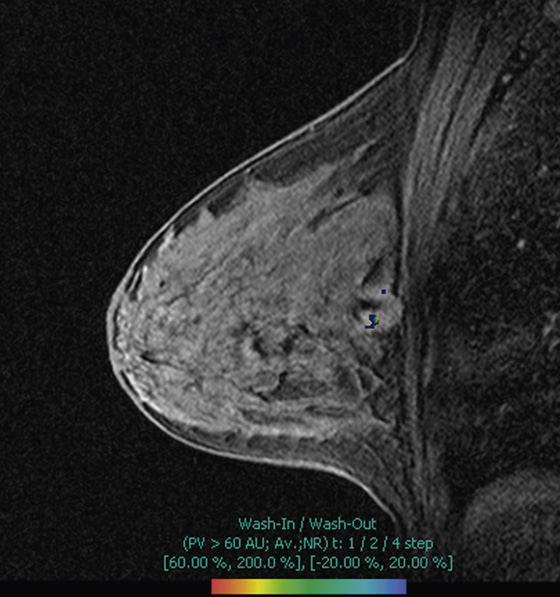
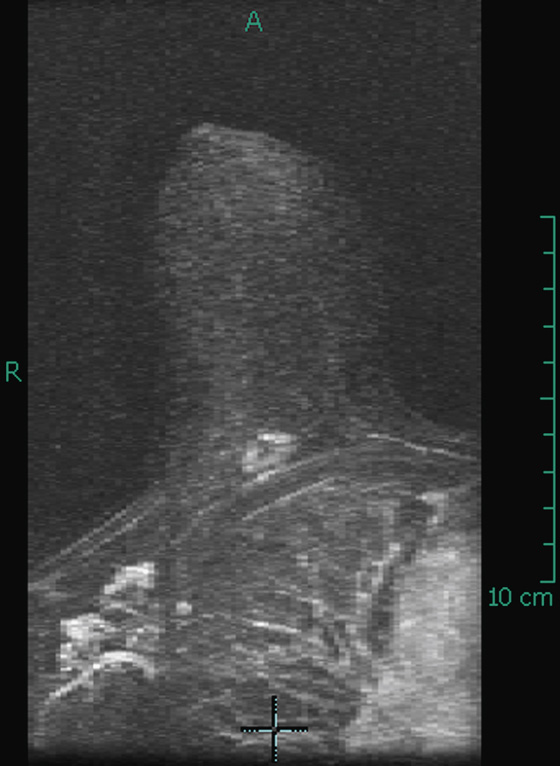
History: In a 58-year-old woman, a posterior right breast mass has been diagnosed and evaluated with needle core biopsy and clip placement.
1. What should be included in the differential diagnosis based on MRI? (Choose all that apply.)
A. Invasive ductal cancer in the posterior breast
C. Fibroadenoma in the posterior breast
2. What is the relationship of the mass to the chest wall?
A. The mass infiltrates the pectoralis muscle.
B. The mass infiltrates the intercostal muscles.
C. The mass abuts the pectoralis but does not invade it.
D. The mass is posterior but is several centimeters away from the pectoralis.
3. What is the best imaging tool for evaluating the relationship of the posterior mass and the chest wall?
D. Positron emission mammography (PEM)
4. How does chest wall involvement affect staging of breast cancer?
A. There is no effect on staging.
B. Enhancement of the pectoralis muscle indicates stage IV disease.
C. Enhancement of the intercostal muscles indicates at least stage IIIB disease.
D. Tumor enhancement of both the pectoralis and the intercostal muscles increases stage.
ANSWERS
CASE 137
Posterior Mass on MRI
1. A, B, and C
2. C
3. B
4. C
References
Mahoney MC, Argus A. Indications for breast MRI: case-based review. AJR Am J Roentgenol. 2011;196(3 Suppl):WS1–WS14.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:271.
Comment
MRI is the best imaging tool for evaluating the extent of disease beyond the breast by direct extension into the pectoralis muscle and into serratus muscle and intercostal muscles. The distinction between extension into the pectoralis major muscle and into the underlying chest wall is important: Pectoralis extension alone does not affect staging, but chest wall involvement is designated as at least stage IIIB.
To diagnose muscle involvement, one should look for enhancement of the muscle—more than the physiologic enhancement normally seen in nearby areas of muscle. The enhancement may be a diffuse involvement or may show enlargement of the muscle. Obliteration of the fat plane between the malignant mass and the pectoralis major muscle is not a definite indication of involvement with tumor, and it may be seen in tumors that abut the pectoralis but do not involve it (see the figures).
CASE 138
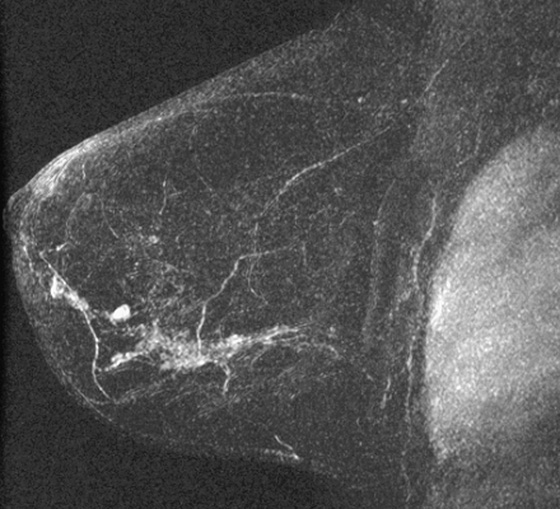
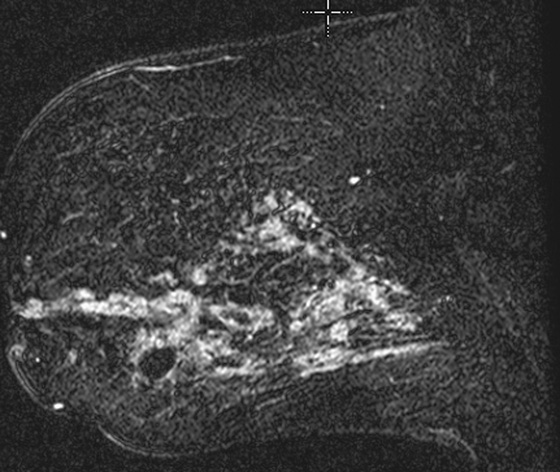
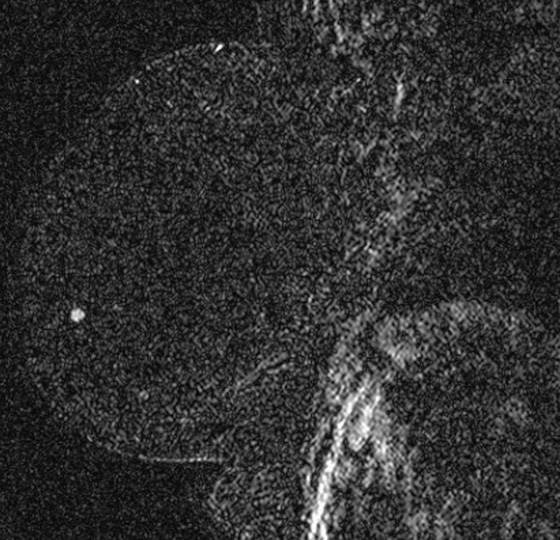
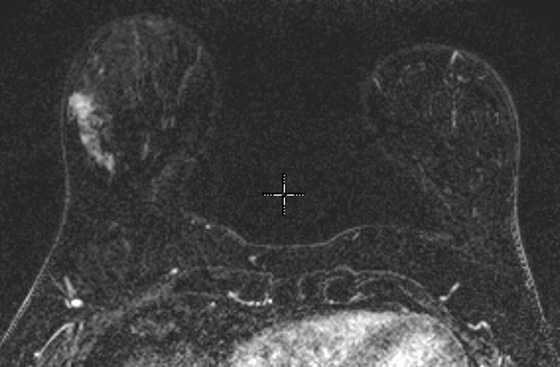
History: Four patients underwent MRI after receiving a diagnosis of invasive or intraductal malignancy on image-guided core biopsy.
1. What should be included in the differential diagnosis for the images shown? (Choose all that apply.)
A. Atypical ductal hyperplasia
B. Ductal carcinoma in situ (DCIS)
2. What is the best imaging tool for assessing extent of disease after DCIS is diagnosed on mammography?
A. Mammography for additional sites of calcifications
C. Positron emission mammography
3. Which is more important in MRI assessment of DCIS—kinetics or morphology?
A. Kinetics is more important because DCIS rarely forms a mass.
D. Morphology is more important because DCIS may not enhance.
4. What is the most common presentation of DCIS on MRI?
A. Small foci scattered throughout the breast
B. Rim-enhancing masses in a ductal distribution
C. Non-masslike enhancement in a segmental distribution
ANSWERS
CASE 138
MRI of Ductal Carcinoma In Situ
1. A, B, and D
2. D
3. C
4. C
References
Liberman L, Morris EA, Lee MJ, et al. Breast lesions detected on MR imaging: features and positive predictive value. AJR Am J Roentgenol. 2002;179(1):171–178.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:265.
Comment
The sensitivity of MRI in detecting DCIS has been reported to range from 40% to 100%. However, in more recent studies, the sensitivity was consistently approximately 90%. MRI is consistently better at detecting DCIS compared with the gold standard of mammography. The sensitivity of mammography for DCIS is approximately 27%. Mammography depends on microcalcifications to detect DCIS, and not all DCIS calcifies. Other presentations of DCIS include asymmetric density and focal mass. In a dense or heterogeneously dense breast, these manifestations are difficult to detect on mammography but are seen better on MRI, which is not affected by breast density.
The manifestations of DCIS on MRI are varied. Non-masslike enhancement is seen in 60% to 90% of cases and is more common than a mass (see the figures). Within this category, clumped enhancement in a linear or segmental distribution is most highly correlated with DCIS (see the figures). However, DCIS was seen as a mass in 30% of cases in one study (see the figures). MRI enhancement is related to neovascularity. In intraductal disease, the increased abnormal vascularity is present in the periductal region and in the stroma surrounding the abnormal ducts, not within the duct itself. It has been shown that high-grade DCIS, also known as DCIS with comedonecrosis, is more likely to enhance compared with low-grade or intermediate-grade DCIS. A study showed that not only the detection of DCIS but also the assessment of tumor size was affected by the DCIS grade. All of the patients illustrated here had grade II DCIS.
Morphology is more reliable than kinetics for detection in DCIS. Kinetics varies from brisk to slow initial uptake, with approximately 50% to 70% of cases having rapid initial uptake. Delayed kinetics can be wash-out, plateau, or persistent. In one study, the delayed kinetics was equally distributed among the three patterns; in another study, wash-out kinetics was seen in only 9% of DCIS. Using computer-assisted detection (CAD) systems, with color-coded information to draw the reader’s attention to only lesions with wash-out, may cause DCIS, which is more likely to have persistent kinetics, to be missed.
It is important to assess extent of disease when DCIS is diagnosed. If DCIS is found on mammography as developing calcifications, MRI is better than mammography for assessing tumor size and multifocal and multicentric disease, which is important in surgical planning (see the figures).
CASE 139
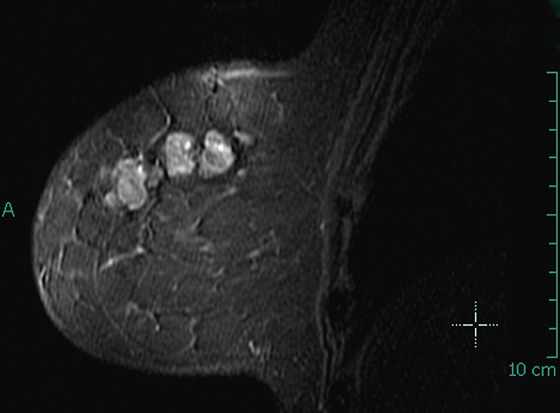
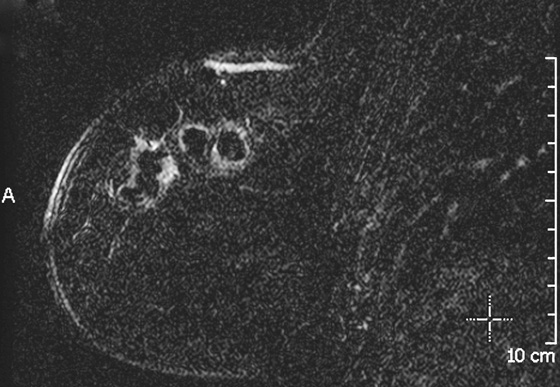
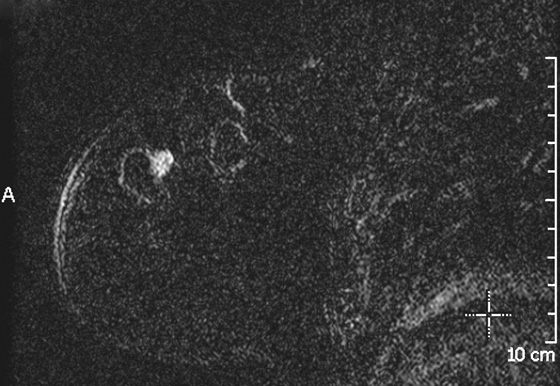
History: A 53-year-old woman found to have suspicious calcifications on screening mammogram underwent stereotactic biopsy of three sites in the right breast, with diagnosis of DCIS in all three sites. MRI is requested to evaluate for extent of disease.
1. What should be included in the differential diagnosis, based on MRI? (Choose all that apply.)
A. Normal, expected enhancement at the seroma
B. Residual ductal carcinoma in situ (DCIS) after biopsy
C. Invasive malignancy adjacent to seroma
D. Enhancing hematoma after biopsy
2. What is the reason for performing MRI after a malignant stereotactic biopsy?
A. Not indicated because the area of cancer is documented, and the patient should have surgery first
B. Not indicated because the patient may be unable to tolerate the examination, and it is expensive
C. To document extent of disease and help guide surgical management
3. How common is detection of additional ipsilateral disease after malignant image-guided biopsy?
A. It is relatively uncommon, occurring in less than 10%.
B. It is more common to find additional disease in the contralateral breast.
C. It is very common, seen in more than 50% of patients.
D. It is detected about 15% to 27% of the time.
4. Which pathology result on core biopsy is more likely to have additional disease detected on MRI (unsuspected on mammogram)?
C. Infiltrating lobular carcinoma
ANSWERS
CASE 139
MRI for Disease Extent after Biopsy
1. B and C
2. C
3. D
4. C
References
American College of Radiology: ACR practice guideline for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. Revised 2008
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:305.
Comment
Breast MRI is the most accurate tool for showing extent of disease in women who have undergone image-guided biopsy with a malignant result. The incidence of ipsilateral and contralateral cancer found on MRI that was unsuspected on conventional imaging ranges from 15% to 30% for the ipsilateral breast and 3% to 6% for the contralateral breast. This information is important to obtain before surgery so that all of the disease can be excised in a single operation. The finding of significant multicentric disease (cancer in multiple quadrants of the breast) may alter the surgical plan from breast conservation therapy to mastectomy. DCIS was upgraded to invasive carcinoma in 18% of patients in one series, as it was in the patient in the present case.
In this patient with dense breasts on mammography, there were suspicious calcifications in three sites in the right breast, and she underwent stereotactic biopsy of three sites, with histology on all core samples showing DCIS grade II. No masses were seen on mammography. MRI was performed (see the figures). The first figure shows the seromas from a recent biopsy on a T2-weighted image. The second and third figures show an enhancing mass adjacent to the seromas, suspicious for invasive carcinoma or additional DCIS on subtracted, T1-weighted, fat-suppressed images.
MRI has limited use in patients with calcifications. Biopsy should be performed of suspicious calcifications regardless of enhancement pattern (MRI is approximately 85% sensitive for detection of DCIS that is calcified on mammogram). However, MRI has a high negative predictive value for invasive cancer, according to the ACRIN trial 6667, so if MRI is negative in a breast with DCIS on stereotactic biopsy, it is highly likely that no invasive carcinoma would be found at excisional biopsy. However, if there is enhancement, as in this patient, invasion is more likely, and the surgeon can be directed to excise the area of enhancement. Excision appears to reduce the rate of local recurrence after breast conservation therapy.
CASE 140
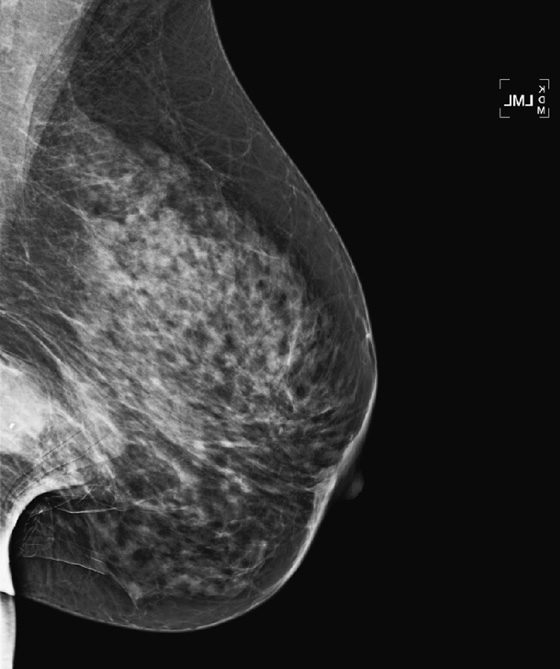
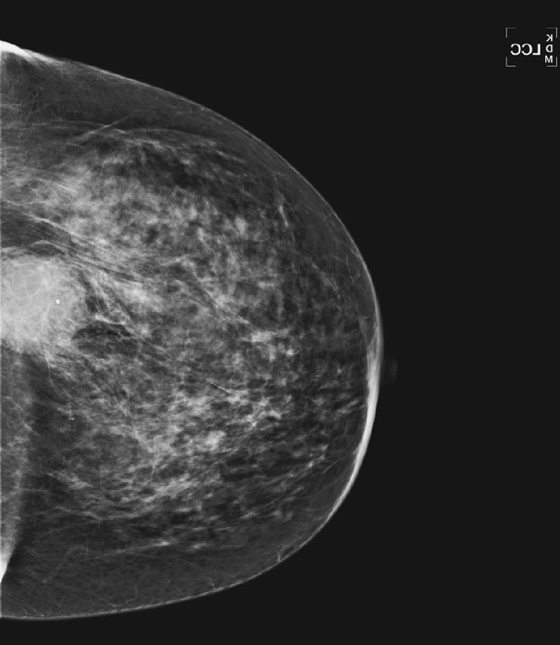
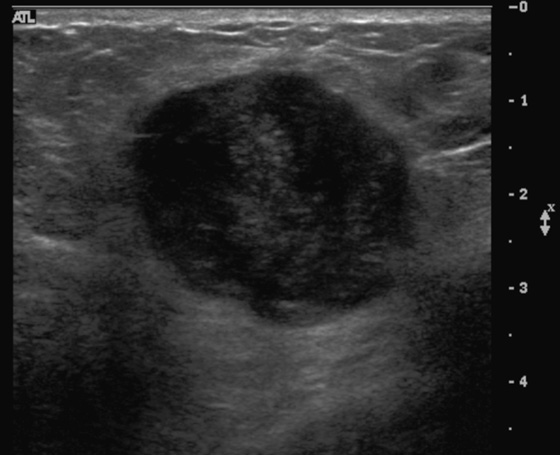
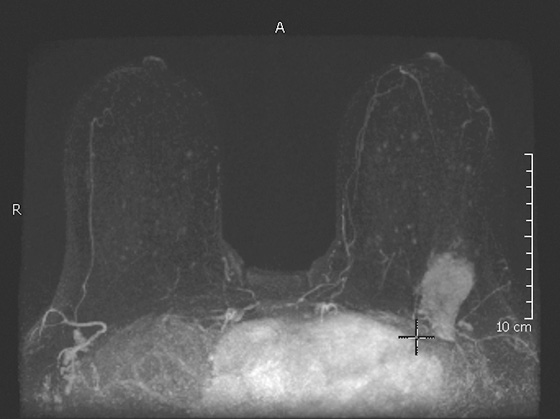
History: A 52-year-old woman presents for a baseline mammogram because of a palpable mass in the left breast.
1. What should be included in the differential diagnosis for the mammogram views shown? (Choose all that apply.)
2. What is the next step in evaluation?
A. Ultrasound and ultrasound-guided biopsy
3. What is a desmoid tumor?
A. A malignant mass of the chest wall with malignant potential
B. Related to diabetic mastopathy and likely related to inflammation
C. Aggressive fibromatosis that may arise from the pectoralis fascia
D. A mass related to fat necrosis
4. What is the management?
A. Surgical excision with axillary node sampling should be performed.
B. Close clinical follow-up is needed after diagnosis is made with core biopsy.
C. Wide local excision should be performed.
ANSWERS
CASE 140
Desmoid Tumor
1. B, C, and D
2. A
3. C
4. C
References
Erguvan-Dogan B, Dempsey PJ, Ayyar G, et al. Primary desmoid tumor (extraabdominal fibromatosis) of the breast. AJR Am J Roentgenol. 2005;185(2):488–489.
Glazebrook KN, Reynolds CA. Mammary fibromatosis. AJR Am J Roentgenol. 2009;193(3):856–860.
Cross-Reference
Ikeda D. Breast Imaging. In: THE REQUISITES. 2nd ed Philadelphia: Saunders; 2010:401.
Comment
Desmoid tumor is a rare, benign tumor of fibroblasts that can occur in the breast and in other organs. It is also termed aggressive fibromatosis or extraabdominal desmoid. Desmoid tumors account for less than 0.2% of all breast tumors. This tumor does not spread beyond the breast but is locally aggressive and can recur after local excision in 29% of cases. It has been reported to be associated with implants, perhaps arising in the fibrous capsule of the implant, or with prior surgery or trauma, although the etiology is uncertain. Desmoid tumor was initially reported in a patient with familial polyposis, or Gardner’s syndrome, although few additional cases in that subset have been reported.
The presentation of desmoid tumor is similar to that of breast cancer, and because of its rarity, it should not be the first choice when a mass is seen in the breast. The tumor has irregular margins, is dense, and typically does not calcify. It is often posterior because it may arise from the pectoralis fascia (see the figures). On ultrasound, it is hypoechoic, with irregular margins, usually without posterior shadowing (see the figures). MRI is a valuable imaging tool after the diagnosis has been made by needle biopsy. Although signal characteristics may vary on T1-weighted and T2-weighted images, and variable enhancement characteristics have been reported, MRI remains the best way to evaluate the tumor relative to the chest wall and thus aid the surgeon in planning excision (see the figures).
In this patient, the mass was initially thought to represent a malignant mass until needle core histology revealed fibromatosis. The patient underwent wide excision, and the histology was concordant. No further treatment was needed, and the patient returned to routine mammography.