CASE 106
1. Which lesions might have been treated with the device shown? (Choose all that apply.)
C. Partial anomalous pulmonary venous connection
2. Which complication of this procedure is most readily apparent on radiographs?
B. Migration
3. Which of the following statements regarding limitations of the performance of cardiac MRI in this patient is true?
A. MRI is safe to perform, and there are no limitations related to the device.
B. MRI is safe to perform, but the device might cause an artifact.
C. MRI is not safe to perform because of potential heating of the device.
D. MRI is not safe to perform because of potential migration of the device.
4. Which MRI artifact would most likely be caused by the device?
A. Aliasing
C. Ringing
ANSWERS
References
Burney K, Thayur N, Husain SA, et al. Imaging of implants on chest radiographs: a radiological perspective. Clin Radiol. 2007;62(3):204–212.
Marie Valente A, Rhodes JF. Current indications and contraindications for transcatheter atrial septal defect and patent foramen ovale device closure. Am Heart J. 2007;153(4 suppl):81–84.
Shellock FG. Patent ductus arteriosus (PDA), atrial septal defect (ASD), ventricular septal defect (VSD) occluders, and patent foramen ovale closure devices. MRIsafety.com (accessed February 8, 2013).
Comment
Indications and Complications
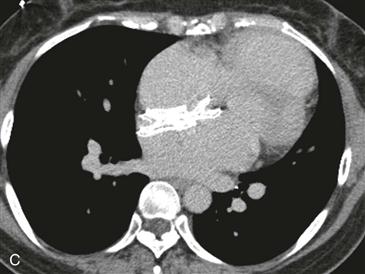
An atrial septal occluder device can be used for transcatheter closure of a secundum atrial septal defect (ASD). Contraindications include a mild shunt (pulmonary-to-systemic flow ratio <1.5 : 1), an ASD that is larger than the device, a defect other than a secundum ASD, and severe pulmonary hypertension. Complications include unsatisfactory placement, migration, pericardial effusion, and thrombus formation. The device can be used to close a patent foramen ovale, but there is some controversy related to this issue.
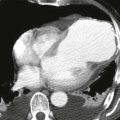
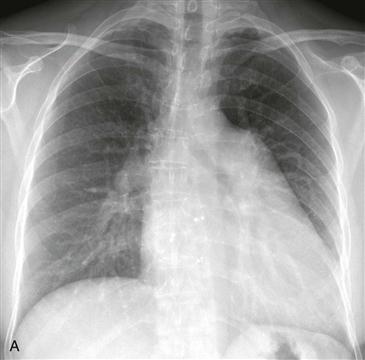
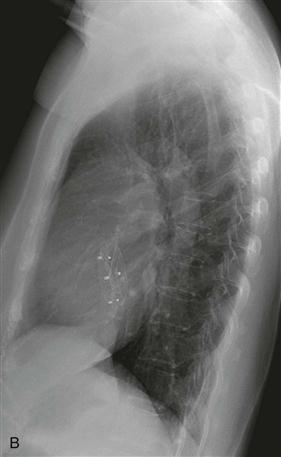
Imaging
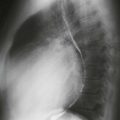
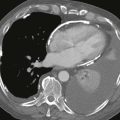
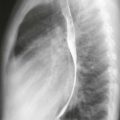
Chest radiographs are capable of identifying the device (Figs. A and B) and assessing the possibility of migration. Other complications are more difficult to ascertain with radiographs. CT (Fig. C) can be performed to identify complications, but it has not been extensively studied for this indication. As of 2012, MRI can be performed safely, but metallic artifact can limit evaluation of the area of the device.