CHAPTER 104
Myofascial Pain Syndrome
Martin K. Childers, DO, PhD; Jeffery B. Feldman, PhD; H. Michael Guo, MD, PhD
Definition
Myofascial pain syndrome (MPS) is a painful disorder characterized by the presence of myofascial trigger points (MTrPs), distinct sensitive spots in a palpable taut band of skeletal muscle fibers [1] that produce local and referred pain. Thus, MPS is characterized by both a motor abnormality (a taut or hard band within the muscle) and a sensory abnormality (tenderness and referred pain) [2] (Fig. 104.1). In addition to pain, the disorder is accompanied by referred autonomic phenomena as well as by anxiety and depression. The pathophysiologic mechanism of MPS is not clearly understood in part because of the scarcity of reliable valid studies. Moreover, concomitant disorders and frequent behavioral and psychosocial contributing factors in patients with MPS contribute to the complexity of human studies. Symptoms of MPS are generally associated with physical activities that are thought to contribute to “muscle overload,” either acutely by sudden overload or gradually with prolonged repetitive activity [3]. MPS is reported to be prevalent in regional musculoskeletal pain syndromes; however, the syndrome can be classified as regional or generalized. Some authors broaden the definition of myofascial pain to include a regional pain syndrome of any soft tissue origin. Thus, MPS may be considered either a primary disorder causing local or regional pain syndromes or a secondary disorder that occurs as a consequence of some other condition, such as a radiculopathy.
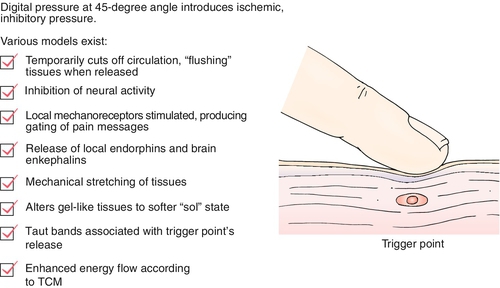
The MTrP is generally considered the hallmark of MPS; therefore, much attention has been given to characteristic features of MTrPs in skeletal muscle [4,5]. One such feature of the MTrP is the so-called twitch response. This local response is considered a characteristic finding of the MTrP. Mechanical stimulation (“snapping” palpation, pressure, or needle insertion) can elicit a local twitch response that frequently is accompanied by referred pain [6]. The twitch response is accompanied by a burst of electrical activity (“end-plate noise”) within the muscle band that contains the activated trigger point, whereas no activity is seen at other muscle bands. End-plate noise is significantly more prevalent in MTrPs than in sites that lie outside of the MTrP but still within the end-plate zone [7]. This observation has been attributed to a spinal reflex [4,6], as the response is abolished by motor nerve ablation or infusion of local anesthetic. Moreover, spinal cord transection above the neurologic level of the MTrP fails to permanently alter the characteristic response.
A number of hypotheses [8,9] have been put forward to explain the findings observed in MTrPs. One theory proposes that MTrPs are found only at the muscle spindle in an attempt to explain beneficial effects of α-adrenergic antagonists. However, this idea does not fully explain the electromyographic findings recorded at the MTrP. Further, there appears to be little evidence that painful muscle areas, such as MTrPs, are associated with any structural changes, such as an alteration in the appearance of the muscle spindle. Another theory is related to excessive release of acetylcholine in abnormal end plates [4], as the electromyographic activity recorded at trigger points resembles findings described at the end-plate region [7]. This idea has led some clinicians to study effects of botulinum toxin injection into MTrPs in an attempt to reduce release of excessive acetylcholine. To date, results of small cohort studies [10] examining effects of botulinum toxin on MTrPs have yielded inconsistent findings.
Central neurologic processes are increasingly viewed as essential factors in chronic pain syndromes. The medial thalamus is the principal relay of nociceptive input to the anterior cingulate cortex, and persistent stimulation of this pathway by pain in peripheral tissues has been demonstrated to change neurons in the cingulate cortex [11]. Thus, persistent pain is associated with long-term changes in the morphology, neurochemistry, and gene expression of the anterior cingulate cortex, which has the most direct connection with autonomic arousal, thereby contributing to the maintenance and exacerbation of pain [12].
Such central sensitization is characterized by an enhanced pain response to normally painful stimuli (hyperalgesia), a decrease in pain threshold to normally nonpainful stimuli (allodynia), and an increase in spontaneous activity (spontaneous pain). This process is clinically seen in MPS as the pain–muscle tension (in response to autonomic arousal and affective distress)–increased pain–increased tension and distress cycle.
Fibromyalgia (see Chapter 101), a chronic musculoskeletal pain condition that predominantly affects women, is characterized by diffuse muscle pain, fatigue, sleep disturbance, depression, and skin sensitivity [13]. Fibromyalgia may fit the classification of MPS as the diagnosis includes the presence of 11 of 18 tender points [14]. Furthermore, treatment of MPS and fibromyalgia is similar as evidence supports the role of exercise, cognitive-behavioral therapy, education, and social support in the management of both fibromyalgia and chronic MPS. However, there is controversy as to whether fibromyalgia and MPS represent specific pathologic processes or are descriptive terms of clinical conditions. Objective evidence of muscle abnormalities in fibromyalgia has been demonstrated by histologic studies showing disorganization of Z bands and abnormalities in the number and shape of muscle mitochondria. Biochemical studies and magnetic resonance spectroscopy have also shown inconstant abnormalities of adenosine triphosphate and phosphocreatine levels. It is unclear whether these abnormalities are a result of physical deconditioning or if abnormalities are due to problems in energy metabolism. There are no clear biochemical markers that distinguish patients with fibromyalgia. Thus, whereas the pathogenesis is still unknown, there has been evidence of increased corticotropin-releasing hormone and substance P in the cerebrospinal fluid of fibromyalgia patients as well as increased substance P and interleukins 6 and 8 in their serum [14]. One hypothesis supports the idea that fibromyalgia is an immunoendocrine disorder in which increased release of corticotropin-releasing hormone and substance P from neurons triggers local mast cells to release proinflammatory and neurosensitizing molecules. This hypothesis fits well with recent discoveries of neuropeptides found in the muscles of patients with active MTrPs [15].
Symptoms
The patient with MPS generally complains about dull or achy pain, sometimes poorly localized, particularly occurring during repetitive activities or during activities requiring sustained postures. Symptoms are exacerbated with digital pressure over tender areas of muscle with reproduction of the patient’s usual pain. Symptoms are relieved with rest or cessation of repetitive activities. In contrast, the patient with fibromyalgia typically presents with sleep disturbances, depressed mood, and fatigue.
Physical Examination
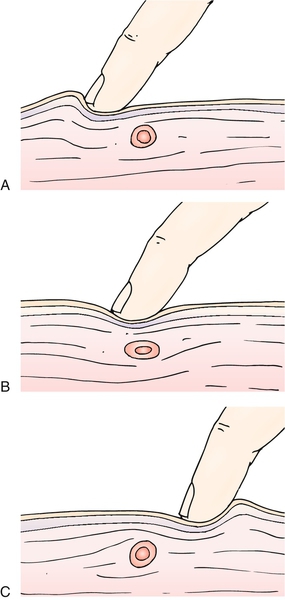
The most important part of the physical examination is generally considered to be finding and localizing MTrPs to provide an accurate diagnosis of MPS. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual [16] is considered the criterion standard reference on locating and treating MTrPs. Active MTrPs, attributed to cause pain, exhibit marked localized tenderness and may refer pain to distant sites, disturb motor function, or produce autonomic changes. Specific clinical training is required to become adept at identifying MTrPs as evidence suggests that “non-trained” clinicians do not reliably detect the taut band and local twitch response [17]. To clinically identify MTrPs, the clinician palpates a localized tender spot in a nodular portion of a taut, rope-like band of muscle fibers. Manual pressure over a trigger point should elicit pain at that area and may also elicit pain at a distant site (referred pain) from the point under the fingertip (Fig. 104.2). MTrPs, when palpated, should also elicit pain that mirrors the patient’s experience. Applied pressure often reproduces the pain. Insertion of a needle, abrupt palpation, or even a brisk tap with the fingertip directly over the trigger point may induce a brief muscle contraction detectable by the examiner. This rapid contraction of muscle fibers of the ropy taut band is termed a local twitch response [3]. In muscles that move a relatively small mass or are large and superficial (such as the finger extensors or the gluteus maximus), the response is easily seen and may cause the limb to visibly move when the examiner introduces a needle into the trigger point. Localized abnormal response from the autonomic nervous system may cause piloerection, localized sweating, or even regional temperature changes in the skin attributed to altered blood flow [8,18,19].
Regional Examination of the Lower Extremity for Piriformis Syndrome
To evaluate individuals for piriformis syndrome [20], their usual buttock, hip, and lower limb pain may be reproduced during the following maneuvers: palpation over a point midway between the sacrum and greater trochanter of the femur, active hip abduction in the lateral recumbent position, and rectal palpation of the ipsilateral side of the involved limb [21,22]. Beatty described a maneuver [23,24] performed with the patient’s lying with the painful side up, the painful leg flexed, and the knee resting on the table. Buttock pain is produced when the patient lifts and holds the knee several inches off the table. A positive finding in at least two of the preceding maneuvers is sufficient to confirm a diagnosis of piriformis syndrome, provided other potential causes have been eliminated from the differential diagnosis.
Diagnostic Studies
No definitive laboratory test or imaging method is diagnostic of MPS. Thus, diagnosis is made primarily by history and physical examination. Whereas no specific laboratory tests confirm (or refute) a diagnosis of MPS [25,26], some tests can be helpful in looking for predisposing conditions, such as hypothyroidism, hypoglycemia, and vitamin deficiencies. Specific tests that may be helpful include complete blood count, chemistry profile, erythrocyte sedimentation rate, and levels of vitamins C, B1, B6, B12, and folic acid. If clinical features of thyroid disease are present, an assay for thyrotropin may be indicated [27].
Treatment
Initial
Therapeutic modalities such as biofeedback, ultrasound, lasers, and massage may be useful adjuncts in relieving initial pain to allow participation in an active exercise program [28]. Data suggest that addition of therapeutic physical modalities [1,3,25,29], such as heat, and various forms of muscle and nerve stimulation are beneficial in the initial treatment of MPS.
Nonsteroidal anti-inflammatory drugs (NSAIDs) may be a useful adjunct to active exercise-based treatment of MPS, but NSAIDs are generally considered beneficial when they are used in conjunction with an active treatment program. However, no randomized placebo-controlled clinical trials exist to support efficacy of NSAIDs in this condition. Interestingly, the NSAID diclofenac, when it is injected into the MTrP, was shown to be superior to lidocaine in one small clinical trial [30]. Low-dose amitriptyline is widely used in patients with fibromyalgia and is thought to help improve the patient’s sleep cycle [31]. Muscle relaxants may also provide benefit to patients with MPS. For example, cyclobenzaprine hydrochloride, a commonly prescribed muscle relaxant, is indicated as an adjunct to rest and physical therapy for the relief of muscle spasm associated with acute, painful musculoskeletal conditions. In contrast to low-dose (5 mg three times daily) cyclobenzaprine, a higher dose (10 mg three times daily) is associated with more somnolence and dry mouth. Importantly, there does not appear to be a relationship between somnolence and pain relief. A large, multicenter, community-based trial of patients with acute pain and muscle spasm evaluated low-dose cyclobenzaprine (5 mg three times daily) alone compared with combination therapy with two doses of ibuprofen. It is possible that low-dose cyclobenzaprine or high-dose ibuprofen alone may be sufficient to relieve acute musculoskeletal pain and that no additional benefit is incurred by adding another medication. Future trials comparing various doses of muscle relaxants and NSAIDs alone or in combination will be required to address these questions in the treatment of patients with MPS.
Rehabilitation
Physical therapy techniques that focus on correction of muscle shortening by targeted stretching, strengthening of affected muscles, and correction of aggravating postural and biomechanical factors are generally considered to be the most effective treatment of MPS [32–34]. This idea is supported by a line of evidence examining the relationship between muscle overload and MTrPs [33,35], suggesting a direct relationship between exercise and MPS.
The goal for the treatment of MPS is to engage patients in active therapy to prevent the development of chronic pain syndrome or to rehabilitate patients from its disabling interacting symptoms if it has developed. Chronic MPS is not a diagnosis but a descriptive term for individuals who not only report persistent pain but also evidence poor coping, self-limitations in functional activities, significant life disruption, and dysfunctional pain behavior [36]. Other common symptoms of chronic pain syndrome related to an accompanying disuse syndrome include the multiple physical systems effects of deconditioning as well as insomnia, fatigue, anxiety, and depression [37]. A central feature of chronic MPS is a disability conviction and resulting avoidance of activity based on the fear that engaging in functional activity will increase pain (fear-avoidance) [37]. The critical importance of addressing such a belief is underlined by prior studies indicating that patients’ beliefs about their pain are the best predictors of task performance [38], medical utilization [39], and long-term rehabilitation [40].
Preventing the development of such a disability conviction begins by assisting patients to shift from a biomedical perspective, in which there is an ongoing search for the cause of an illness to be “cured” or “fixed,” to a biopsychosocial rehabilitation perspective [37,40]. This perspective views MPS as a multifactorial condition that need not be disabling if it is actively managed by the patient. Cognitive-behavioral therapy is the psychological approach that focuses on changing dysfunctional beliefs or “schemas” by which individuals process, store, and act on information [41]. For individuals with chronic pain to successfully participate in a functionally oriented rehabilitation approach, they need to understand or to believe the following:
2. The rehabilitation approach involving physical activity and conditioning will increase functional capabilities and eventually reduce suffering.
3. The hurt engendered through physical conditioning will not cause harm.
4. Reinjury or worsening of the painful condition is unlikely, and it is in the individual’s best interest to become more functional.
The first point most often can be addressed by the physician in the office, but the critical shift in belief that hurt will not cause harm generally requires the patient to have repeated experiences that contradict the prior life experience that if something hurts, you should stop doing it. For a patient with MPS to exercise consistently and sufficiently to contradict the common sense to avoid pain, an interdisciplinary team approach is often required. In such an approach, the physical therapist educates and guides the patient through a progressive physical reconditioning regimen. The physician periodically reevaluates the patient, reassuring and encouraging the patient that there is no problematic change in condition while adjusting medications to facilitate involvement in the program. Concurrently the psychologist provides training in stress management, pacing, and pain coping strategies. This is often best done in a group setting that normalizes the reactions and experience of the patient and where the social support and encouragement of the patient’s peers is of significant benefit. Ultimately, though, it is the patient’s repeated mild increases in pain without harm, as functioning improves, that change beliefs about pain and fear-avoidance of activity. It is largely for this reason that multidisciplinary pain programs that include a cognitive-behavioral approach have been found to be most effective for individuals with chronic pain on a range of key outcomes [42]. A cognitive-behavioral, functional restoration approach is particularly effective for chronic MPS because unlike in many other chronic pain conditions, one can be fairly certain that the hurt experienced through increased activity will not only not cause harm but will lead to long-term benefit.
Hypnosis
In addition to the cognitive changes noted, patients should be educated on the interacting effects of pain leading to increased sympathetic arousal (“stress response”) that leads to increased muscle tension and increased pain. They therefore can reduce pain by reducing their reactivity to pain as well as other stressors in their life. Techniques for doing so include relaxation training, progressive muscle relaxation, mindfulness meditation, and hypnosis. Hypnosis is increasingly being integrated into multidisciplinary treatment approaches because it enables one to train patients to develop a relaxation response while also interspersing suggestions to encourage the changes in thinking noted before [43–51]. In other words, hypnosis is a tool that can be used not only to assist patients in reducing their attention to the sensation of pain but, more important, to reduce their affective distress and autonomic reactivity.
Mindfulness Meditation
Another approach to reducing affective distress and autonomic reactivity in response to pain that has received increasing attention is mindfulness meditation. Mindfulness has been defined as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally.” [52] Zeidan and colleagues [53] expanded on this description to operationally define mindfulness as involving “(a) regulated, sustained attention to the moment-to-moment quality and character of sensory, emotional and cognitive events, (b) the recognition of such events as momentary, fleeting and changeable (past and future representations of those events being considered cognitive abstractions), and (c) a consequent lack of emotional or cognitive appraisal and/or reactions to these events.” Pragmatically, this tends to involve training in focused attention (Samatha meditation) and open awareness (Vipassana meditation). One is taught that such thoughts are momentary and fleeting and that one need not react to them. Whereas mindfulness-based stress reduction has been viewed as the “gold standard,” there have been variations including mindfulness-based cognitive therapy [54]. For example, brief mindfulness training can have a significant impact on experimentally induced pain and cognition [53]. Although the specific mechanisms remain unclear, accruing evidence indicates that through processes of neuroplasticity, significant changes can occur in brain structures associated with the processing of pain, especially the prefrontal and anterior cingulate cortices [55]. In this way, one can therapeutically use the neuroplasticity of the brain to enhance pain control, in a manner that is the reverse of central sensitization described before.
Procedures
In combination with other therapies, interventional techniques can be an effective adjunct in the multidisciplinary management of patients with MPS [56]. In the treatment of MPS, other than trigger point injections, interventional procedures (e.g., epidural steroid injections, sacroiliac joint injections, and medial branch blocks) are usually not employed. However, at times, myofascial pain is associated with or caused by other underlying conditions. For instance, lumbar myofascial pain may also have some component of lumbar facet arthropathy. Lumbar medial branch blocks and radiofrequency denervation, alone or in combination with the other therapies (e.g., muscle relaxants), may work together to relieve myofascial pain. Similarly, epidural steroid injection may provide lumbar pain relief in a patient with spondylosis. It has been suggested that epidural steroid injections may be used to treat cervical MPS if conservative treatments fail [57]. Therefore, underlying disease may respond to more aggressive interventional methods and in turn synergistically provide pain relief to the patient with MPS.
MTrP injections should be individualized for both the patient and the clinician. Alcohol, if it is used to clean the skin, should be allowed to dry completely to prevent additional pain. Use of operating rooms or special procedure (sterile) rooms equipped with monitoring devices for the purpose of intramuscular injections with small-caliber needles is not necessary. Most patients can be treated safely in an office setting by experienced clinicians. The diagnostic skill required to find active MTrPs depends on considerable innate palpation ability, authoritative training, and extensive clinical experience [3]. Application of trigger point injection begins by determining the equipment needs according to the needs of the patient, the clinician’s training, and the anatomic target for injection. Typically, a 1.0-mL tuberculin-type syringe with < ?xml:namespace prefix = "mml" />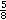 -inch 25-gauge needle is adequate for superficial muscles. For small muscles (e.g., facial muscles), a 1-inch 30-gauge needle is sufficient. For larger muscles, a 1-inch or 11⁄2-inch 25-gauge needle is adequate. After the patient is placed in a position in which the desired muscle can be relaxed, the MTrP is located. In the prone position, the MTrP is ascertained by gentle pressure from the end of a fingertip or a ballpoint pen applied at regular 1-cm intervals. The patient is observed closely during the palpation because pressure on the markedly tender MTrP usually causes the patient to jump, to wince, or to cry out. Each muscle has a characteristic elicited referred pain pattern that, for active MTrPs, is familiar to the patient. Thus, the patient will respond that this pressure reproduces the usual pain and, when questioned, will describe painful sensations at a site slightly distant to the point under the examiner’s finger. Once the MTrP has been located, the skin is marked, and the site is injected with saline, anesthetic agent, or corticosteroid solution. Once the MTrP has been located, the skin is marked and prepared. The site is injected with no more than 1 mL injectate per site after negative aspiration for blood. When the needle is advanced to the MTrP, it may elicit a local twitch response, although a local twitch response is not observed all the time despite significant improvement of myofascial pain with trigger point injection. Other findings that may help determine the needle entrance to the MTrP are the patient’s confirmation of reproduction of the usual pain pattern and the clinician’s sensation of increased resistance as the needle is advanced from normal muscle tissue to the taut band.
-inch 25-gauge needle is adequate for superficial muscles. For small muscles (e.g., facial muscles), a 1-inch 30-gauge needle is sufficient. For larger muscles, a 1-inch or 11⁄2-inch 25-gauge needle is adequate. After the patient is placed in a position in which the desired muscle can be relaxed, the MTrP is located. In the prone position, the MTrP is ascertained by gentle pressure from the end of a fingertip or a ballpoint pen applied at regular 1-cm intervals. The patient is observed closely during the palpation because pressure on the markedly tender MTrP usually causes the patient to jump, to wince, or to cry out. Each muscle has a characteristic elicited referred pain pattern that, for active MTrPs, is familiar to the patient. Thus, the patient will respond that this pressure reproduces the usual pain and, when questioned, will describe painful sensations at a site slightly distant to the point under the examiner’s finger. Once the MTrP has been located, the skin is marked, and the site is injected with saline, anesthetic agent, or corticosteroid solution. Once the MTrP has been located, the skin is marked and prepared. The site is injected with no more than 1 mL injectate per site after negative aspiration for blood. When the needle is advanced to the MTrP, it may elicit a local twitch response, although a local twitch response is not observed all the time despite significant improvement of myofascial pain with trigger point injection. Other findings that may help determine the needle entrance to the MTrP are the patient’s confirmation of reproduction of the usual pain pattern and the clinician’s sensation of increased resistance as the needle is advanced from normal muscle tissue to the taut band.
Surgery
Surgery is not indicated in the treatment of patients with MPS.
Potential Disease Complications
Patients with MPS may go on to develop chronic pain syndrome. Treatment of individuals with chronic MPS is described earlier. Perhaps the biggest complication of untreated and progressive MPS is development of a syndrome of physical inactivity that may lead to cardiovascular disease. Evidence of a dose-response relation between physical activity and cardiovascular disease endpoints has been proposed [58], although the majority of the literature in this area has relied on prospective observational studies, and few randomized trials of physical activity and cardiovascular disease as a clinical outcome have been reported. This notwithstanding, evidence indicates that cardiovascular disease incidence and mortality are causally related to physical activity in an inverse, dose-response fashion. Thus, left untreated, patients with MPS who go on to develop chronic pain and lack physical activity are at high risk for cardiovascular disease and early death.
Potential Treatment Complications
The greatest risk of treatment of the patent with MPS is related to MTrP injections in the thoracic area. Because of the anatomic location of the apex of the lung to the proximity of the upper trapezius muscle or the scalene muscles, the clinician must be aware of the potential for pneumothorax as a result of MTrP injection in this area. The use of long (> 1 inch) small-gauge needles should be avoided because long, thin needles can easily bend once they are inserted into the muscle, and the tip can inadvertently puncture the pleura. Rather, short (< 1 inch) needles should be used for MTrP injections anywhere near the apex of the lung. In addition, the needle should be directed away from structures at risk of inadvertent puncture. To provide additional proprioceptive feedback during injection, grasping the muscle between the thumb and forefinger will allow the clinician to palpate the thickness of the tissue to be injected. Thin patients or those with reduced lung capacity from underlying diseases are particularly at risk, and thus the clinician should use extra precautions when performing MTrP injections in these patients.