CHAPTER 103
Intercostal Neuralgia
Susan J. Dreyer, MD; William J. Beckworth, MD
Definition
Intercostal neuralgia is pain in the thoracic region emanating from an intercostal nerve. The pain is typically a sharp, shooting, or burning pain radiating around the chest wall. It can be accompanied by altered sensitivity to touch, such as allodynia or an area of hyperalgesia. Intercostal neuralgia occurs commonly after thoracotomy [1–4]. It can also be seen in elderly debilitated patients without a known precipitating event [5]. Other causes include rib trauma, very rarely benign periosteal lipoma [6], and pregnancy [7].
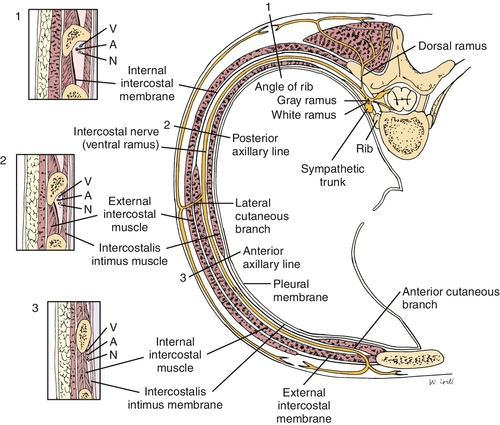
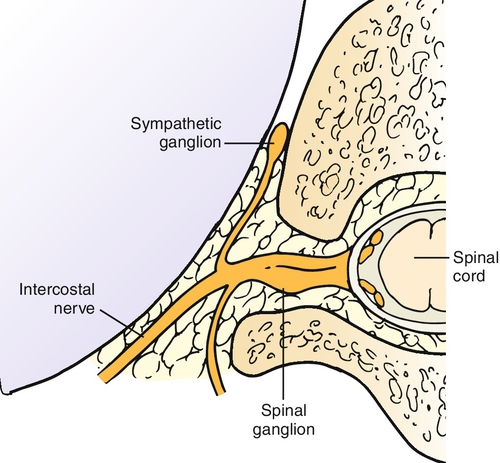
Intercostal nerves are peripheral nerves that run along with the vascular bundle on the inferior surface of each rib (Fig. 103.1). Intercostal nerves are derived from the ventral rami of the first through twelfth thoracic nerves (Fig. 103.2), with the first, second, third, and twelfth being atypical on the basis of anatomic differences. Only 17% of intercostal nerves were found in the classic subcostal position in one study [8]. In Hardy’s study, a midcostal location was the most prevalent at 73%; an additional 10% were supracostal. The intercostal nerve gives off four main branches as it travels anteriorly: gray rami communicantes, posterior cutaneous branch, lateral cutaneous division, and anterior cutaneous division.
Symptoms
Chest pain is the cardinal symptom. Because intercostal neuralgia involves a peripheral nerve, the pain is neuropathic rather than nociceptive. Neuropathic pain is often unrelenting, shooting, burning, and deep. The International Association for the Study of Pain defines neuropathic pain as “pain initiated or caused by a primary lesion or dysfunction in the nervous system.” [9] Neuropathic pain is characterized by three symptoms: dysesthesia, paroxysmal pain, and allodynia [10]. Dysesthetic pain is an abnormal sensation described as unpleasant. Patients commonly use terms such as aching, cramping, pressure, and heat to describe a dysesthetic pain [11]. Paroxysmal pain is pain that comes in waves and is often described as lancinating or electric. Allodynia is the abnormal perception of pain after a normally nonpainful mechanical or thermal stimulus [11]. Patients with allodynia may respond to light touch with an exaggerated pain response or report a sensation of heat when a cold stimulus is applied. Intercostal neuralgia pain is unilateral. It is common (up to 81% of patients) after thoracotomy for coronary artery bypass grafting to the internal thoracic artery and after thoracotomy for tumor excision [3,4]. During thoracotomy (either open or video-assisted thoracoscopic surgery), the intercostal nerve may be directly injured during rib resection, compressed by a retractor, or later entrapped by a healing rib fracture. Intercostal neuralgia may follow other forms of chest trauma. It may mimic the pain of shingles (herpes zoster) but without the rash and can occur without significant trauma in the elderly.
The mechanism of neuropathic pain may be due to ectopic signals from neural “sprouts” after axonal injury. This new nerve growth may become a pain generator, especially if it becomes entrapped in scar tissue, forming a neuroma. Another mechanism may be compression or disruption of the nervi nervorum afferents in the connective tissue covering, producing a peripheral neuropathic pain.
Physical Examination
Much of the physical examination in intercostal neuralgia is done to exclude other sources of pain. First, it is important to exclude cardiac and other visceral sources of chest pain (Table 103.1). Although point tenderness is uncommon during myocardial infarction, the presence of point tenderness does not exclude significant cardiac disease. In intercostal neuralgia, there are no constitutional signs, such as fever, dyspnea, diaphoresis, or shortness of breath. Cardiopulmonary examination findings should be normal or stable if prior cardiovascular or pulmonary disease exists.
Intercostal neuralgia is common after thoracotomy [1–3]. However, chest pain that recurs after a pain-free period following a thoracotomy for tumor resection is likely (90%) to be due to tumor recurrence. On the other hand, pain that persists for months or years after thoracotomy is most likely (70%) intercostal neuralgia [12].
Once the chest pain has been determined to be neuromusculoskeletal and nonvisceral, the task becomes one of differentiation of intercostal neuralgia from thoracic radiculopathy, herpes zoster, rib fracture, costochondritis, and local contusion. History of trauma, ecchymosis, crepitus, and point tenderness over a rib suggests rib fracture. If the trauma was minor, a contusion or intercostal neuralgia may be the source of discomfort. Contusions typically improve quickly during a period of weeks and are responsive to simple analgesics, such as acetaminophen and nonsteroidal anti-inflammatory medications. In contrast, pain from intercostal neuralgia persists and can be refractory to acetaminophen, nonsteroidal anti-inflammatory drugs, and even low-dose narcotics.
Careful palpation along the thoracotomy scar or rib may reveal a neuroma with the presence of a Tinel sign. Larger neuromas can often be visualized on magnetic resonance imaging. Sensory examination often reveals a small (1 to 2 cm) band of dermatomal sensory loss.
Examination of the thoracic spine in patients with intercostal neuralgia reveals full active range of motion without tenderness. In contrast, thoracic radiculopathy may be accompanied by pain with range of motion and at times thoracic spinal tenderness. Still, pain from thoracic radiculopathy is similar in quality and distribution to intercostal neuralgia.
Intercostal neuralgia is distinct from postherpetic neuralgia (shingles), and no herpes zoster virus can be identified in cases of intercostal neuralgia. Furthermore, in most cases of shingles, the chest pain is followed within a matter of days to weeks by a vesicular, linear eruption. The more debilitating pain of postherpetic neuralgia follows the skin lesions of shingles.
Functional Limitations
The pain of intercostal neuralgia is commonly mild to moderate but can be debilitating because it may interfere with one’s ability to comfortably wear clothes. In one study, nearly 10% of post-thoracotomy patients observed for a mean of 19.5 months had moderate to severe pain that required daily analgesics, nerve blocks, relaxation therapy, acupuncture, or referral to a pain clinic [4]. The pain may also interfere with sleep. Trunk motion may stimulate the intercostal nerve, especially if a neuroma has formed. As a result, patients may begin restricting their activities.
Diagnostic Studies
Final diagnosis of intercostal neuralgia is often one of exclusion. Depending on which intercostal nerve is involved, chest pain from intercostal neuralgia may require an initial workup such as electrocardiography, cardiac enzyme analysis, cardiac computed tomography or magnetic resonance imaging, and other testing to exclude a cardiac source. Electromyographic recording of the paraspinal muscles is normal in intercostal neuralgia, whereas thoracic radiculopathy from thoracic disc herniation or foraminal stenosis may reveal active denervation potentials in the paraspinal muscles. Advanced imaging (thoracic magnetic resonance imaging, computed tomography, or computed tomographic myelography) also aids in demonstrating thoracic disc herniations or spinal stenosis as the source of the neural compression and confirms a diagnosis of thoracic radiculopathy. Larger intercostal neuromas may be visualized on magnetic resonance imaging of the ribs.
In the case of pain with a history of malignant disease, chest radiography, computed tomography, and bronchoscopy may be necessary to exclude recurrence of tumor. In patients whose history includes chest trauma, rib radiographs and at times bone scans help identify rib fractures.
Thoracic magnetic resonance imaging can exclude a disc herniation in an anatomically related area. Recall that it is not uncommon to identify asymptomatic disc herniations. In cases in which the disc protrusion does not cause clear compression but is abutting a thoracic nerve in the painful distribution, electrodiagnostic testing can exclude thoracic radiculopathy. Lesions of the intercostal nerve do not cause denervation in the thoracic paraspinal muscles.
When a postoperative neuroma is suspected, careful palpation often reveals a Tinel sign. Magnetic resonance imaging of the suspected area may reveal the neuroma. Small neuromas may be missed on magnetic resonance examination only to be identified later at the time of surgical resection.
Historical red flags including history of malignant disease, unexplained weight loss, malaise, and severe night pain raise the index of suspicion for rib metastases rather than intercostal neuralgia as the cause of the patient’s chest pain. Appropriate oncologic workup, such as bone scans, laboratory investigation, and at times positron emission tomographic scans, should be performed.
Treatment
Initial
Thoracic radiculopathy, postherpetic neuralgia, and intercostal neuralgia are all forms of neuropathic pain, and they share many initial conservative treatment options. Principles of treatment of neuropathic pain in general, rather than of a specific disease state, are employed [13,14]. Typically, drugs have not been specifically tested on populations of patients with intercostal neuralgia. Instead, physicians are left to extrapolate the data from treatment of other sources of neuropathic pain and to apply those principles in choosing medication to be used for this population. Often, neuropathic pain responds poorly to acetaminophen, nonsteroidal anti-inflammatory drugs, and low-dose narcotics, in contrast to nociceptive pain [15,16]. However, if the pain is mild to moderate, these agents are often tried first. Topical agents may be effective when there is significant allodynia or dysesthesia. The patient may need a family member to help apply the topical agent because it may be difficult to reach the involved area. Capsaicin creams are available over-the-counter, and a newer capsaicin patch (Qutenza) is available by prescription (the actual indication is for postherpetic neuralgia). The cream requires frequent application three or four times a day and may initially cause an exacerbation of pain as substance P is depleted. Topical lidocaine applied as a gel, eutectic mixture of local anesthetics (EMLA), or patch (Lidoderm) is another alternative [17].
Tricyclic antidepressants (e.g., amitriptyline, nortriptyline, desipramine) have been used for neuropathic pain for decades [18]. The dosage for analgesia is typically lower than that required to treat depression. Onset of action may be in days to weeks. The mechanism of action in neuropathic pain is believed to be modulation of descending inhibitory pathways by selective inhibition of norepinephrine or serotonin reuptake. Tricyclic antidepressants have anticholinergic effects and may cause confusion, increase the risk of falls and injury, and result in urinary retention. For these reasons, it is recommended that amitriptyline be avoided in persons older than 65 years. Despite this, amitriptyline (Elavil) is still a widely prescribed tricyclic antidepressant for neuropathic pain. It is usually started at 10 mg at bedtime and titrated upward as tolerated at 2- to 3-day intervals. Serotonin and norepinephrine reuptake inhibitors (SNRIs) such as duloxetine (Cymbalta), milnacipran (Savella), and venlafaxine (Effexor) are commonly used for neuropathic pain conditions, although only duloxetine is approved for a neuropathic diagnosis (painful diabetic neuropathy). Nausea can be seen with SNRIs. Milnacipran has a preference for norepinephrine reuptake inhibition and may lead to an increase in blood pressure. The SNRIs may be a better choice than the tricyclic antidepressants for elderly or debilitated patients with neuropathic pain.
Anticonvulsants are another mainstay for treatment of neuropathic pain. Anticonvulsants may work in several ways, including suppression of paroxysmal discharges and overall neuronal hyperexcitability [19]. Older agents such as carbamazepine and phenytoin have a narrow therapeutic window and risks of serious adverse drug reactions. These agents require careful monitoring if they are used. Bone marrow suppression occurs in up to 1% of patients, and severe dermatologic reactions such as Stevens-Johnson syndrome and epidermal necrolysis have also been reported with carbamazepine. Newer anticonvulsants, such as gabapentin (Neurontin) and pregabalin (Lyrica), are often chosen for their more favorable side effect profiles, efficacy, and limited interaction with other drugs. Gabapentin and pregabalin commonly cause sedation and may cause edema; both are indicated for neuropathic pain of diabetic peripheral neuropathy. These agents do not require blood samples for drug levels to be monitored. Dosing typically begins low and at night. For example, many practitioners start with gabapentin 300 mg at bedtime and titrate to 600 to 800 mg two or three times daily. At times, even higher doses are prescribed. Dosages are reduced in patients with renal insufficiency. Pregabalin can also be started at a low 50-mg bedtime dose and titrated to 300 mg twice daily, although it is uncommon to exceed 150 mg twice daily. Gabapentin and other anticonvulsants should be tapered before discontinuance. Like gabapentin and pregabalin, topiramate has also been used off-label for intercostal neuralgia [20].
Narcotics are often required for intercostal neuralgia. Adequate analgesia is needed to promote continued activity levels and to prevent deconditioning from disuse. Initial treatment should be with short-acting agents such as hydrocodone 5 mg with acetaminophen 325 mg every 4 hours as needed. Treatment of intercostal neuralgia can be protracted, and patients are often switched to long-acting agents for maintenance. If the pain is progressive, one should reevaluate the diagnosis and reconsider the possibility of occult tumor.
In refractory cases of neuropathic pain, clonidine, an adrenergic agonist, and ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, may be prescribed by pain specialists [10].
Rehabilitation
Physical and occupational therapy can be instrumental in combating disuse deconditioning. Also, desensitization techniques may be employed. Psychological consultation in patients who exhibit signs of anxiety, depression, or panic disorder may be beneficial to address the negative impact of these psychological stressors on the pain or the patient’s behavior in response to the pain. Relaxation therapy and acupuncture have also been employed with anecdotal success [4].
If pain is severe, intercostal neuralgia may lead to avoidance of activity and deconditioning. It is important to keep patients physically active early in the rehabilitation process. The muscles of persons on strict bed rest lose 1.0% to 1.5% of their strength per day during a 2-week period. The loss is greatest during the first week of inactivity. Thus, if the patient is physically limited by pain, early prescription of a structured therapy program focusing on pain modulation helps prevent deconditioning. Desensitization techniques, heat, cold, and transcutaneous electrical nerve stimulation are all ways by which the therapist may assist in pain control while encouraging activity.
Psychological consultation in patients who exhibit signs of fear-avoidance, anxiety, and depression related to the pain may be beneficial later in the rehabilitation process. Biofeedback and relaxation therapy techniques are sometimes used. Biofeedback uses instrumentation to provide feedback on a variety of physiologic responses, such as muscle tension. It is typically used to facilitate relaxation and to enhance self-regulation. Two common relaxation therapies are autogenic training and progressive muscle relaxation. Relaxation training and biofeedback are thought to be equally effective in pain modulation.
Procedures
Local infiltration of a neuroma with corticosteroids and local anesthetics, intercostal nerve blocks, indwelling epidural catheters, and spinal nerve injections have all been used to control the pain associated with intercostal neuralgia when adequate relief is not achieved with oral and topical medications [2].
If a focal neuroma is identified, infiltration with several milliliters of local anesthetic with 40 mg of a long-acting corticosteroid, such as methylprednisolone (Depo-Medrol) or triamcinolone (Kenalog), often results in significant analgesia. In addition to its potent anti-inflammatory effect, the corticosteroid may also reduce neural discharges. Care must be taken during the injection to avoid pneumothorax.
Intercostal nerve blocks should be performed with appropriate monitoring. There are several techniques well described in interventional pain management books [21]. One common technique is to position the patient prone and to identify the angle of the rib just lateral to the sacrospinalis muscles (Fig. 103.3). The skin is moved up over the rib, and the needle is introduced down onto the posterior periosteum of the rib and then walked inferior. The advantage of moving the skin cephalad over the rib before needle insertion is that it allows the needle to be easily moved inferior after the needle contacts bone. At the angle of the rib, the rib is typically 8 mm thick. Great care must be taken not to overpenetrate and cause a pneumothorax; 3 to 5 mL of local anesthetic is instilled along with a long-acting corticosteroid, such as triamcinolone, methylprednisolone, betamethasone, or dexamethasone. The needle is then withdrawn slightly and moved back over the rib before safe removal. Another approach is midaxillary and may be preferred in postoperative and acutely ill patients who cannot be easily positioned prone.
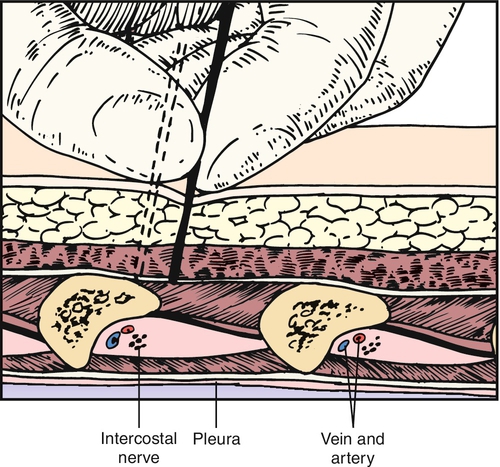
If symptoms are well controlled with intercostal nerve blocks but recur, neurolytic injection of phenol or alcohol has been used to denervate the peripheral nerve [21]. Indwelling epidural catheters have been used to provide regional anesthesia with minimal drug use. A report of pregnant patients who developed intercostal neuralgias found this technique safe and effective [7].
Cryotherapy involves another way of interrupting the peripheral nerve’s ability to transmit pain by freezing it. Cryotherapy has been used on neuromas and the involved intercostal nerve [22].
Spinal cord stimulators have been implanted for intercostal neuralgia but with less success than in neuropathic pain due to diabetic peripheral neuropathy and causalgia [23]. Chronic post-thoracotomy pain and intercostal neuralgia can be difficult to control. In one study, up to 40% of post-thoracotomy patients required pain procedures including trigger point injections, intercostal nerve blocks, epidural steroid injections, and stellate ganglion blocks [2].
Surgery
Surgical resection of a neuroma can be effective but should be reserved for cases that have failed more conservative care and have demonstrated a temporary response to intercostal blocks. Dorsal root entry zone ablation involves surgical destruction of nociceptive secondary neurons in the spinal cord when pain is not adequately controlled with medical therapy [24,25]. The technique involves laminectomy with intradural exposure of the spinal cord. With an operating microscope, a radiofrequency probe is used to heat the dorsal horn of the affected side with a series of lesions.
Potential Disease Complications
Untreated upper intercostal neuralgia can potentially lead to a frozen shoulder as patients limit their use of the arm in response to the pain. Intercostal neuralgia can also cause a chronic pain syndrome with its comorbidities. Psychosocial dysfunction associated with chronic neuropathic pain includes impaired sleep, decreased appetite, and diminished libido.
Potential Treatment Complications
All medications carry risks. Many of the medications used for neuropathic pain (anticonvulsants, tricyclic antidepressants, narcotics) cause sedation. Injections in the area carry the risk of pneumothorax, bleeding, and infection. Surgical techniques may result in further nerve damage and pain, infection, bleeding, and pneumothorax.







