Anatomy
Thorax
Before describing the anatomy of the heart, it is helpful to review other anatomic features of the thoracic cavity and organs.
The thorax proper constitutes the upper part of the body or trunk, with a shape between a barrel and a truncated cone that is functionally favorable. Although the intrathoracic pressure is often subatmospheric, the chest wall is still able to retain its integrity by means of rather thin, lightweight skeletal elements. The thoracic cavity occupies only the upper part of the thoracic cage. The abdominal (peritoneal) cavity reaches upward as high as the lower tip of the sternum, affording protection to large, easily injured abdominal organs such as the liver, spleen, stomach, and kidneys.
The thoracic and abdominal cavities are separated by the dome-shaped diaphragm, a sheet of tissue consisting of a peripheral muscular part and a central tendinous part that closes the thoracic cavity interiorly. Superiorly, the narrow upper thoracic aperture—bounded by the upper part of the sternum, the short stout first ribs, and the body of the first thoracic vertebra (T1)—gives access to the root of the neck and is not closed by a specific structure. The thorax is bounded posteriorly by the bodies of the 12 thoracic vertebrae and the posterior portions of the ribs, anteriorly by the sternum, costal cartilages, and anterior portions of the ribs, and laterally by the remaining parts of the ribs. The spaces between successive ribs are bridged by the intercostal muscles.
The sternum (breastbone) lies anterior in the midline and superficially. The clavicles and the first seven pairs of ribs articulate with it. The sternum consists of three parts: the bony manubrium and corpus sterni and the small, cartilaginous xiphoid process. The clavicles articulate with the manubrium on its upper border, and the notch between these joints is the interclavicular (or suprasternal) notch. Just below the sternoclavicular joints, the cartilages of the first ribs are attached to the sternum. No joint spaces are present here. The manubrium and the body of the sternum are united by fibrocartilage. The junction between the manubrium and the body of the sternum usually forms a prominent ridge, accentuated by the two parts of the sternum forming a slight angle with each other, the sternal angle of Louis. This is an important landmark because the cartilages of the second ribs articulate with the sternum at this point. The third, smallest part of the sternum is the xiphoid cartilage, a thin, spoon-shaped process attached to the lower end of the sternal body.
Most of the bony thorax is formed by the ribs, usually 12 on each side of the trunk. The ribs consist of a series of thin, curved, rather elastic bones that articulate posteriorly with the thoracic vertebrae and terminate anteriorly in the costal cartilages. The first seven pairs of ribs attach to the sternum by means of their cartilages, whereas the eighth, ninth, and tenth pairs articulate with each other and do not reach the sternum. The 11th and 12th pairs are small and poorly developed, ending in free cartilaginous tips. The ribs are thickest posteriorly; they flatten out and widen as they curve forward. Along the inferior and inner surface of the posterior part of each rib, a groove—the sulcus costae—affords protection to the intercostal vessels and nerve.
The first two and last two ribs differ somewhat from the previous description. The first rib (see Plate 1-2) is very short and relatively heavier than the other ribs. On the superior surface of the first rib, two grooves are divided by a tubercle—the tuberculum scaleni—that forms the point of insertion of the anterior scalene muscle. The groove in front of the muscle is occupied by the subclavian vein, whereas the subclavian artery follows the groove behind the tubercle. The second rib is longer than the first and resembles the other ribs except the small 11th and 12th ribs.
The spaces between successive ribs are occupied by intercostal muscles (see Plate 1-1). Each external intercostal muscle arises from the lower border of the rib above, runs obliquely downward and medially, and inserts into the upper border of the rib below. Each internal intercostal muscle arises from the lower border of the rib above and runs downward and outward to insert on the upper border of the rib below. Between these two muscle layers lie the intercostal vessels, whereas the intercostal nerves lie between the internal and the innermost intercostal muscles.
Many muscles of the upper extremities originate from the chest wall, including the pectoralis major (see Plate 1-1) and pectoralis minor muscles and the serratus anterior muscle, which originate from the anterior and lateral portions of the chest wall.
Several neck muscles originate from the upper rim of the thoracic cage. The sternohyoid and sternothyroid (see Plate 1-1) are thin, straplike muscles that arise from the superior border and posterior surface of the sternum and insert into the hyoid bone and the thyroid cartilage, respectively. The sternocleidomastoid muscle (SCM) arises (see Plate 1-1) as a stout sternal head from the upper border of the sternum, adjacent to the sternoclavicular joint, and as a second clavicular head from the medial third of the clavicle. The interval between the two heads is usually visible as a slight depression, behind which the apex of the lung rises from the thorax into the root of the neck. Above this interval the two heads of the SCM unite to form a single muscular belly that passes obliquely upward, backward, and laterally to insert into the lateral surface of the mastoid process and occipital bone.
Superficial to the SCM, the external jugular vein passes perpendicularly downward from its origin at the lower border of the parotid gland, crosses the SCM, and penetrates the deep fascia of the neck to empty into the subclavian vein.
Of the deeper neck muscles, the three scalene muscles originate from the transverse processes of the cervical vertebrae. The anterior scalene muscle inserts into the scalene tubercle of the first rib; the medial scalene muscle also attaches to the upper surface of the first rib, but more posteriorly. The posterior scalene muscle inserts on the second rib. The components of the cervical nerve plexus emerge from the groove between the anterior and middle scalene muscles. The anterior scalene muscle is crossed laterally and anteriorly by the phrenic nerve, which originates from the cervical plexus and runs downward and behind the subclavian vein to enter the thoracic cavity. The groove between the anterior and middle scalene muscles widens inferiorly to form a triangular opening through which emerge the components of the brachial plexus and the subclavian artery. After ascending from the thoracic cavity, the subclavian artery crosses the upper surface of the first rib, lying in the groove posterior to the scalene muscle, and enters the axilla. The subclavian vein runs parallel to the subclavian artery but in front of the anterior scalene muscle.
Deep in the lower portion of the neck under the SCM, a narrow space is bordered anteriorly by the omohyoid and strap muscles, posteriorly by the anterior scalene muscle and prevertebral fascia, and medially by the pharynx, esophagus, trachea, and thyroid gland (see Plate 1-1). In this space the common carotid artery, internal jugular vein, and vagus nerve are enclosed in a common connective-tissue sheath; the jugular vein runs most superficially and the vagus nerve lies beneath, between the common carotid artery and internal jugular veins. On the left side the thoracic duct (see Plate 1-1) crosses over the subclavian artery and runs anteriorly to empty into the proximal subclavian vein.
Blood for the chest wall is supplied by the intercostal arteries and the internal thoracic (internal mammary) arteries. After originating from the aorta, the posterior intercostal arteries cross the vertebral bodies and enter their corresponding intercostal spaces, passing along the inferior border of the ribs between the internal and external intercostal muscles. The vessels are well protected posteriorly by the subcostal groove. The internal thoracic arteries originate from the inferior surface of the subclavian arteries and run downward, lateral to, and (for a short distance) with the phrenic nerve, reaching the posterior surface of the anterior chest wall. The arteries continue their downward course for approximately 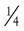 inch laterally to the edges of the sternum, dividing just above the diaphragm into their two terminal branches: the musculophrenic and superior epigastric arteries. Along their course the internal thoracic arteries give rise to branches to the thymus, mediastinum, and pericardium posteriorly; to the perforating branches to the skin and subcutaneous tissues anteriorly; and finally to the lateral branches that pass along the rib cartilages and anastomose with the posterior intercostal arteries.
inch laterally to the edges of the sternum, dividing just above the diaphragm into their two terminal branches: the musculophrenic and superior epigastric arteries. Along their course the internal thoracic arteries give rise to branches to the thymus, mediastinum, and pericardium posteriorly; to the perforating branches to the skin and subcutaneous tissues anteriorly; and finally to the lateral branches that pass along the rib cartilages and anastomose with the posterior intercostal arteries.
The veins of the thoracic wall correspond in their course with the arteries. The 10 lower intercostal veins on the right enter the azygos vein, and the upper two intercostal veins enter either the azygos or the brachiocephalic (innominate) vein. The lower intercostal veins on the left side enter the hemiazygos or accessory hemiazygos vein. The three left superior intercostal veins enter the left brachiocephalic vein by a common stem, the left superior intercostal vein.
The chest wall receives its nerve supply from the intercostal nerves, which accompany the intercostal vessels.
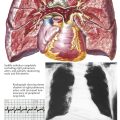
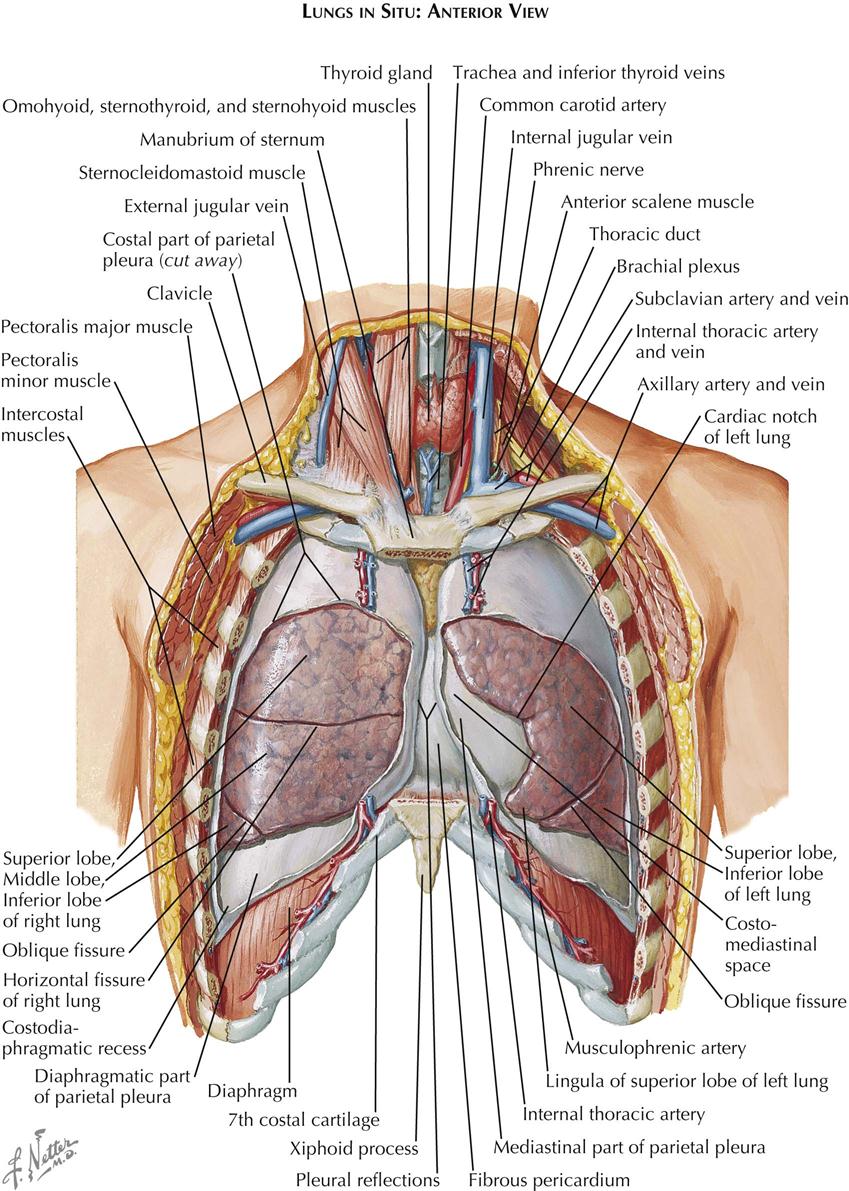
Most of the thoracic cavity is occupied by the two lungs, each of which is enclosed by its pleura. Each pleura forms a closed sac invaginated by the lung so that part of it covers (and is adherent to) the inner surface of the chest wall, the diaphragm, and the mediastinum, known as the costal, the diaphragmatic, and the mediastinal pleura, respectively, and collectively as the parietal pleura (see Plate 1-2). That part of the mediastinal pleura that covers the pericardium is called the pericardial pleura; the remainder (visceral pleura) covers the lung. The virtual space between the visceral and parietal pleurae contains a tiny amount of clear fluid. The pleural reflections (see Plate 1-1), between the costal and diaphragmatic portions of the parietal pleura, lie lower than the corresponding lower edge of the lung. The resulting space normally is not completely filled by the lung, even on deep inspiration, and is called the recessus costodiaphragmaticus.
The right lung consists of three lobes—the superior, middle, and inferior lobes—and is somewhat larger than the left lung, which has two—the superior and inferior lobes (see Plate 1-1). The smaller size of the left lung results from the eccentric position of the heart, which encroaches on the left pleural cavity. The two pleural cavities almost meet behind the upper sternum, but the left costomediastinal reflection deviates laterally below the fourth rib cartilage, exposing a small triangular portion of the pericardium that is not covered by pleura. At the same level, the anteroinferior portion of the left superior lobe recedes even more, leaving a portion of the pericardial pleura that is not covered by lung tissue.
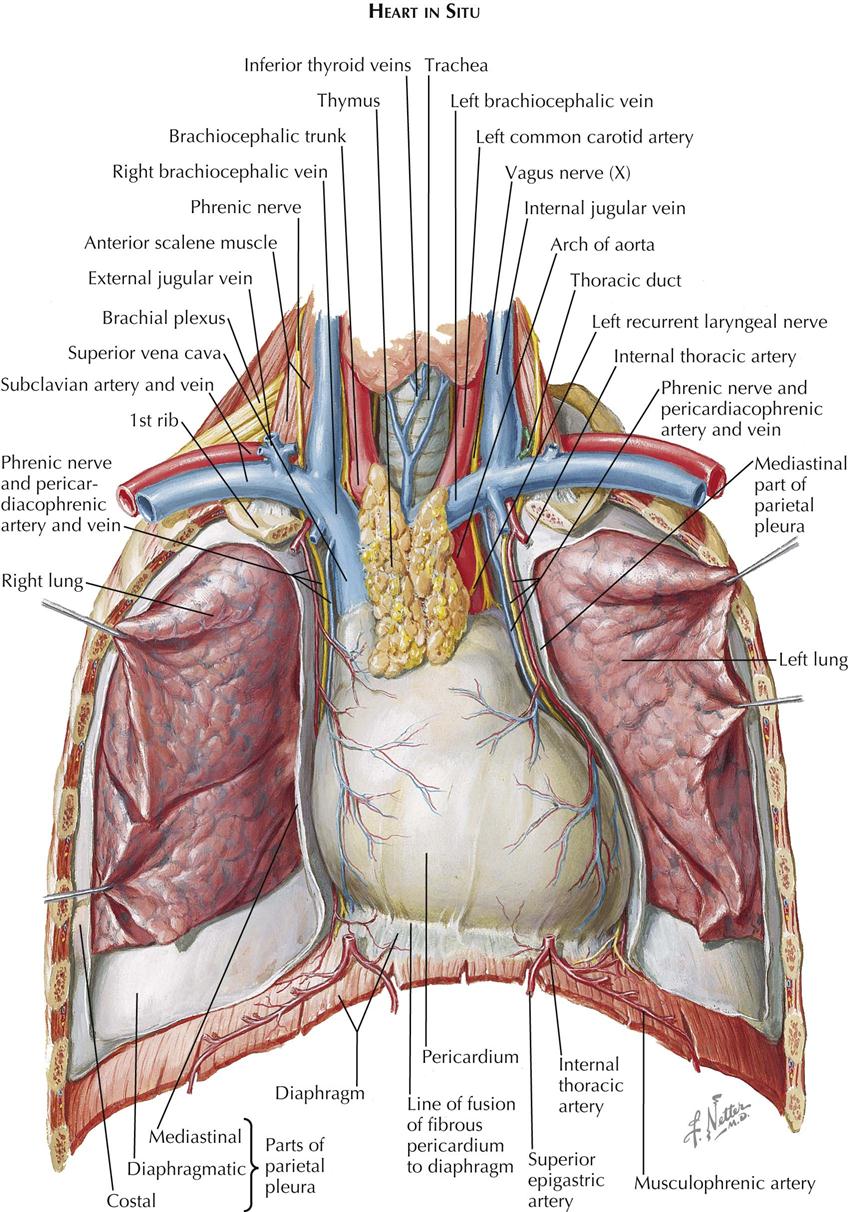
The central space between the two pleural cavities is the mediastinum. The mediastinum is divided arbitrarily into superior, anterior, middle, and posterior mediastina. The shallow anterior mediastinum contains a portion of the left internal thoracic vessels and the vestigial transverse thoracic muscle. The superior mediastinum contains the thymus gland (see Plate 1-2), which largely disappears by about age 12 years leaving a small pad of fat and areolar tissue, and the brachiocephalic veins, which join each other on the right to form the superior vena cava (see Plate 1-5). Posterior to the brachiocephalic veins, the phrenic and vagus nerves descend from the neck. The phrenic nerves, accompanied by the pericardiacophrenic vessels, run laterally, anterior to the lung roots and along the pericardium, until they reach the diaphragm.
The aortic arch ascends from the heart into the superior mediastinum, almost reaches the upper border of the manubrium sterni, courses obliquely backward and to the left over the left main bronchus, and continues as the descending aorta downward, anteriorly, and slightly to the left of the vertebral column. Originating from the convexity of the arch, from the proximal to the distal position, are the brachiocephalic, left common carotid, and subclavian arteries.
The right vagus nerve (see Plate 1-5) passes between the subclavian artery and vein and gives off the right recurrent nerve, which loops around the subclavian artery to ascend along the trachea. The left vagus nerve runs between the subclavian vein and the aortic arch, giving rise to the left recurrent nerve (see Plate 1-5), which similarly loops around the arch to ascend along the trachea.
The trachea descends from the neck behind the aortic arch and bifurcates into right and left main bronchi at the level of the sternal angle. Behind the trachea runs the normally collapsed esophagus (see Plate 1-4), joined by the vagus nerves just beyond the branching off of the recurrent nerves from the vagi. Behind the esophagus, between the azygos vein and the descending aorta, the thoracic duct (see Plate 1-2) ascends, coursing behind the aortic arch to enter the neck, where it empties into the left subclavian vein.
Against the necks of the ribs, the sympathetic trunks descend from the neck, first giving off the greater thoracic splanchnic nerve (major splanchnic nerve) (see Plate 1-3) at about the level of the sixth rib and then the minor or lesser and lowest thoracic splanchnic nerves.
The posterior mediastinum is a shallow space containing the lower portions of the esophagus, vagus nerves, descending aorta, azygos and hemiazygos veins, thoracic duct, and sympathetic nerve chains. The remaining and largest part of the mediastinum, the middle mediastinum, contains the pericardium, heart, lung roots, and phrenic nerves.
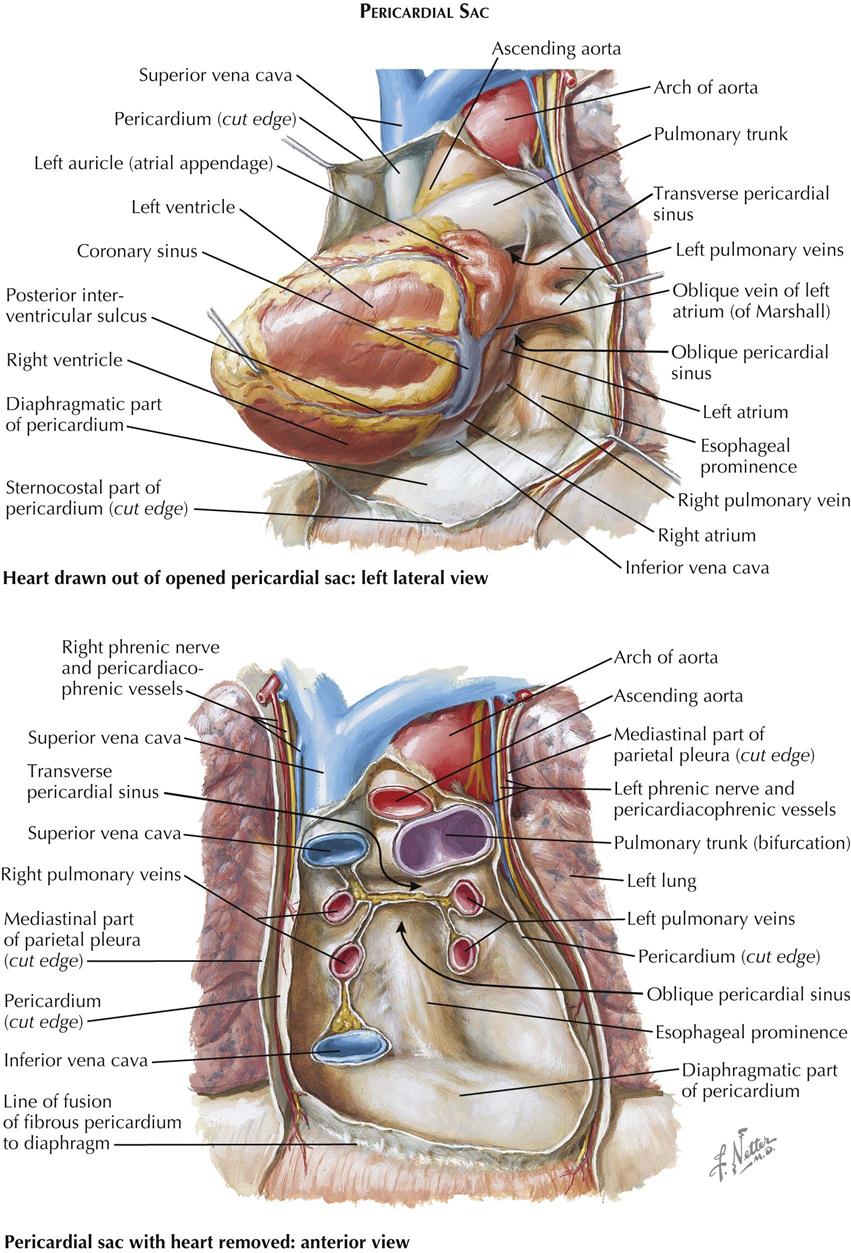
The pericardial cavity is the third serous cavity contained in the chest, with the two pleural cavities. The pericardial cavity is conical in shape, with the base of the cone lying posteriorly to the right and the apex anteriorly to the left. It completely invests the heart and the proximal portions of the great vessels. As with the pleura, a visceral portion of the pericardium is distinguished overlying the heart and proximal great vessels, usually called the epicardium, as is a parietal portion. The inferior part of the parietal pericardium is densely adherent to the middle tendinous part of the diaphragm. Most of the lateral and anterior portions are contiguous but not normally adherent to the pleura. A small triangular part of the anterior portion of the parietal pericardium lies directly behind the sternum, separated only by areolar and fatty tissue (endothoracic fascia) and the transverse thoracic muscle.
The great vessels enter and leave the pericardial cavity at its base. A curved, transversely running passageway between the arterial and venous poles of the heart is called the transverse pericardial sinus. Posteriorly, a blind recess of the pericardial cavity is bordered by the pericardial reflection between the pulmonary veins and inferior vena cava, called the oblique pericardial sinus. Small recesses exist between the superior and inferior pulmonary veins on each side and behind the fold of the left vena cava (ligament of Marshall), a small crease of pericardium running from the left aspect of the pulmonary trunk to the left atrium, between the neck of the left auricle and the left pulmonary veins. The left vena cava fold contains the vestigial remains of the left common cardinal vein.
Exposure of the Heart
Sternocostal Aspect
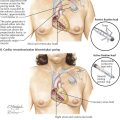
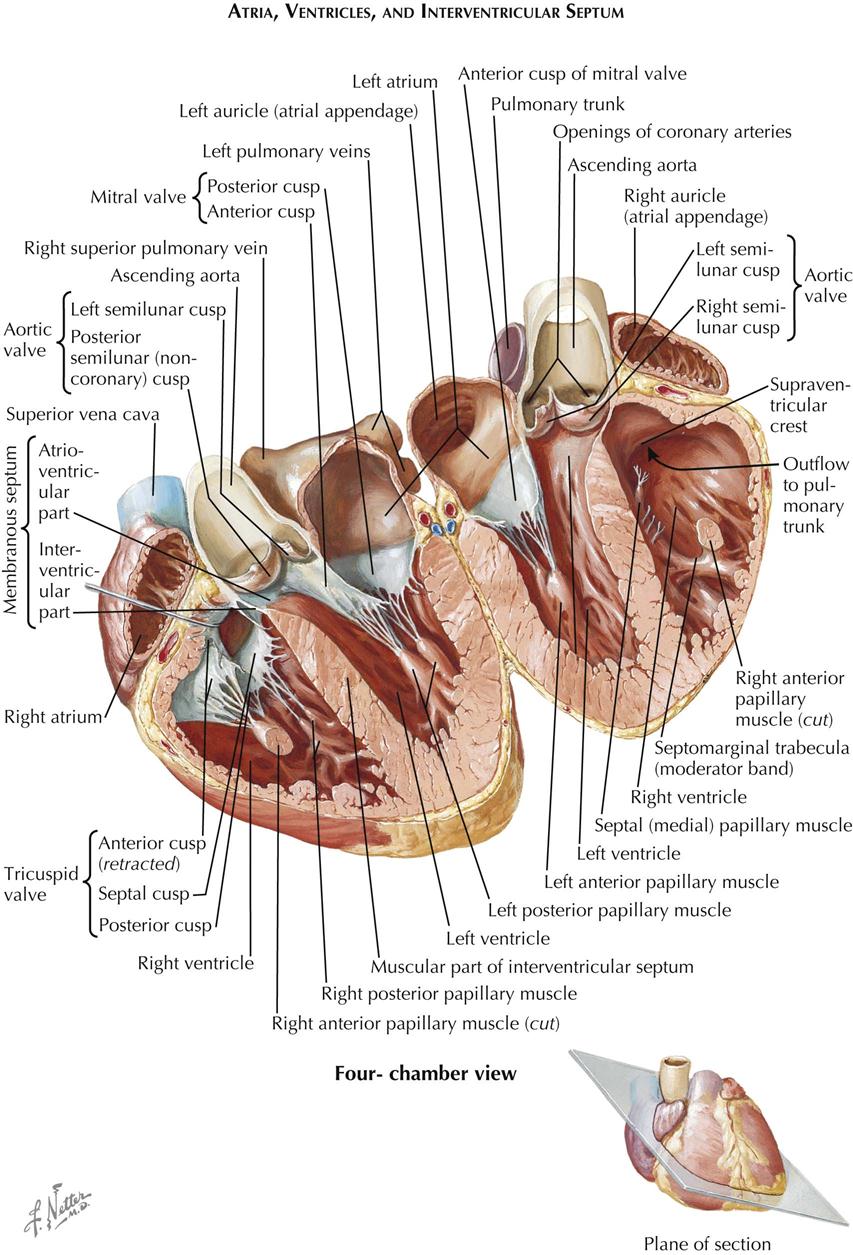
Within the pericardium lies the heart, a hollow, muscular, four-chambered organ suspended at its base by the great vessels. In situ the heart occupies an asymmetric position, with its apex pointing anteriorly, inferiorly, and about 60 degrees toward the left. Its four chambers are arranged in two functionally similar pairs, separated from each other by the cardiac septum (see Plate 1-5). Each pair consists of a thin-walled atrium and a thicker-walled ventricle.
The anatomic nomenclature of the heart removes it from the body and places it on its apex, and thus the cardiac septum is in a sagittal plane. This practice has led to misconceptions and difficulties in orientation among cardiologists and surgeons. On a chest radiograph, for example, the left cardiac border is formed by the left ventricle, but the right border is formed by the right atrium, not the right ventricle, which lies anterior. The major and important part of the left atrium lies directly posterior and in the midline in front of the spine and esophagus, allowing the pulmonary veins to be as short as possible.
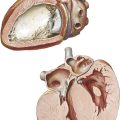
On removing the anterior chest wall and opening the pericardium, most of the presenting part of the heart is formed by the right ventricle, with its exposed surface triangular in shape. The right atrium lies to the right of the right ventricle.
The term “auricle” is often improperly used instead of atrium. The true auricle is then regrettably called “auricular appendage” instead of atrial appendage, which is morphologically correct. The term “auricular fibrillation” is clinically incorrect and should be atrial fibrillation.
The right atrium and right ventricle are separated by the right atrioventricular (coronary) sulcus, through which runs the right coronary artery, embedded in a variable amount of fat. To the left of the right ventricle, a small segment of the left ventricle is visible, separated from it by the anterior interventricular sulcus (groove). The anterior interventricular (descending) branch of the left coronary artery (see Plate 1-5) lies in this groove, again embedded in fat.
Superiorly, the pulmonary trunk is seen originating from the right ventricle and leaving the pericardium just before it bifurcates into its two main branches: the right and left pulmonary arteries. To the right of the pulmonary trunk lies the intrapericardial portion of the ascending aorta, the base of which is largely covered by the right auricle (right atrial appendage). The base of the aorta, including the first part of the right coronary artery, is surrounded by lobules of fatty tissue called Rindfleisch folds, the largest and uppermost of which is rather constant.
Posterior and Diaphragmatic Aspects
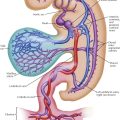
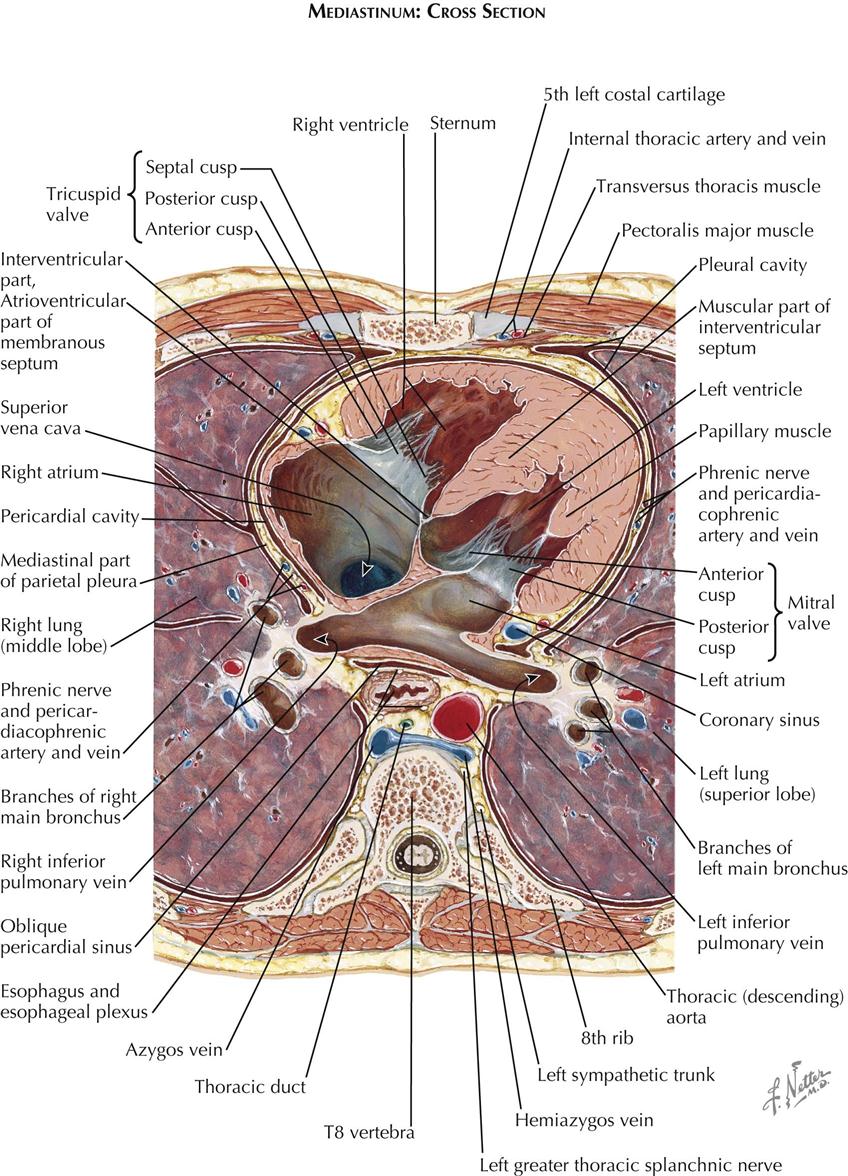
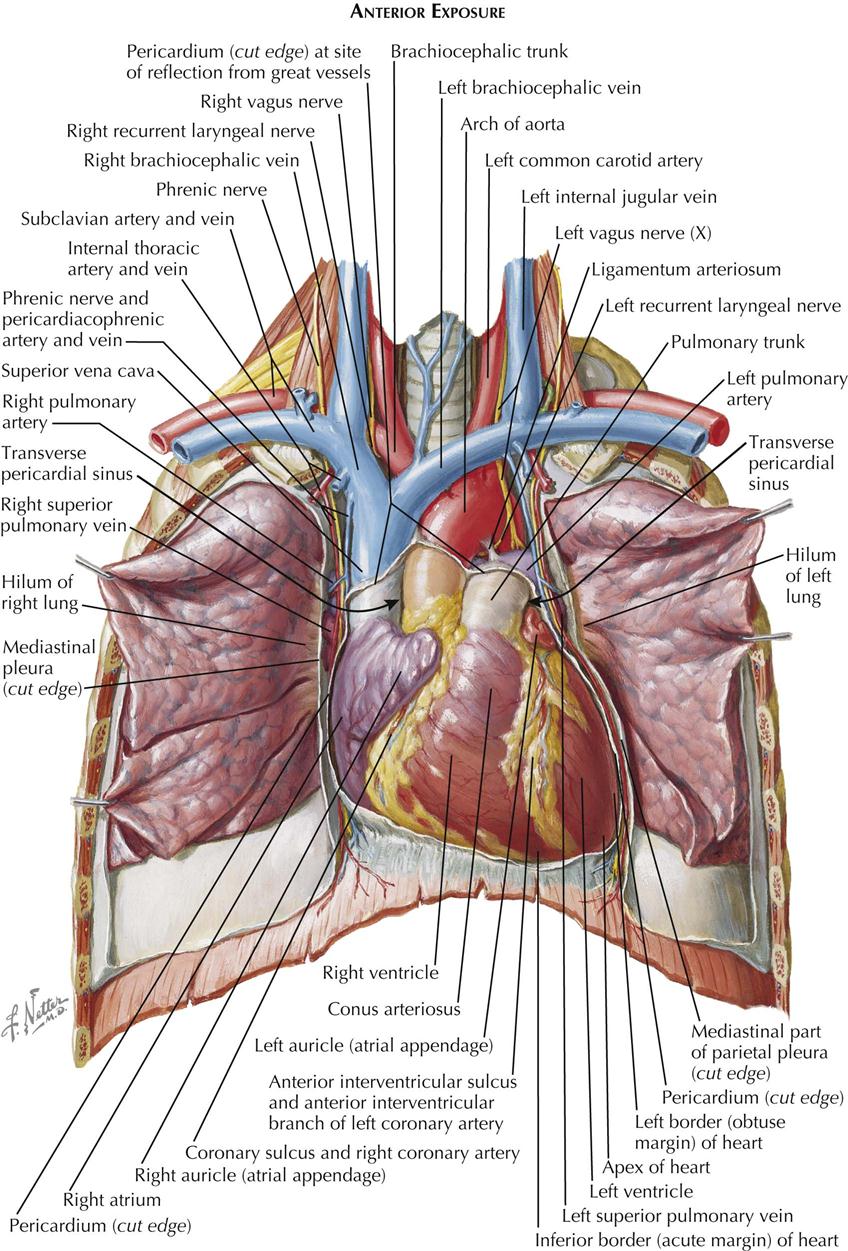
After removal of the heart from the pericardium, its posterior (basilar) and diaphragmatic aspects can be inspected. The superior vena cava (SVC) and inferior vena cava (IVC) enter the right atrium, with the long axis of both cavae inclined slightly forward and the IVC in a more medial position. A pronounced groove, the sulcus terminalis, separates the right aspect of the SVC from the base of the right auricle. As this groove descends along the posterior aspect of the right atrium, it becomes less distinct.
The right pulmonary veins (usually two but occasionally three) arise from the right lung and cross the right atrium posteriorly to enter the right side of the left atrium. The two left pulmonary veins enter the left side of the left atrium, sometimes by a large common stem. The posterior wall of the left atrium forms the anterior wall of the oblique pericardial sinus. Normally, the left atrium is not in contact with the diaphragm.
The bifurcation of the pulmonary trunk lies on the roof of the left atrium. The left pulmonary artery courses immediately toward the left lung, and the right pulmonary artery runs behind the proximal SVC and above the right pulmonary veins to the right lung.
The aortic arch crosses the pulmonary artery bifurcation after giving off its three main branches: the brachiocephalic (innominate), left common carotid, and left subclavian arteries. Variations in this pattern occur and usually are not significant.
The coronary sinus lies between the left atrium and the left ventricle in the posterior (diaphragmatic) portion of the left atrioventricular groove (coronary sulcus). The cardiac veins enter the coronary sinus, which has the appearance of a short, wide vein. However, its wall consists of cardiac muscle, and because of its embryonic origin, the coronary sinus should be considered a true cardiac structure. Its right extremity turns forward and upward to enter the right atrium.
The diaphragmatic surfaces of the right ventricle and the left ventricle are separated by the posterior interventricular sulcus (groove). This sulcus is continuous with the anterior interventricular groove just to the right of the cardiac apex, which in a normal heart is formed by the left ventricle. The posterior interventricular (descending) artery and middle cardiac vein lie in the posterior interventricular sulcus, embedded in fat.
Atria and Ventricles
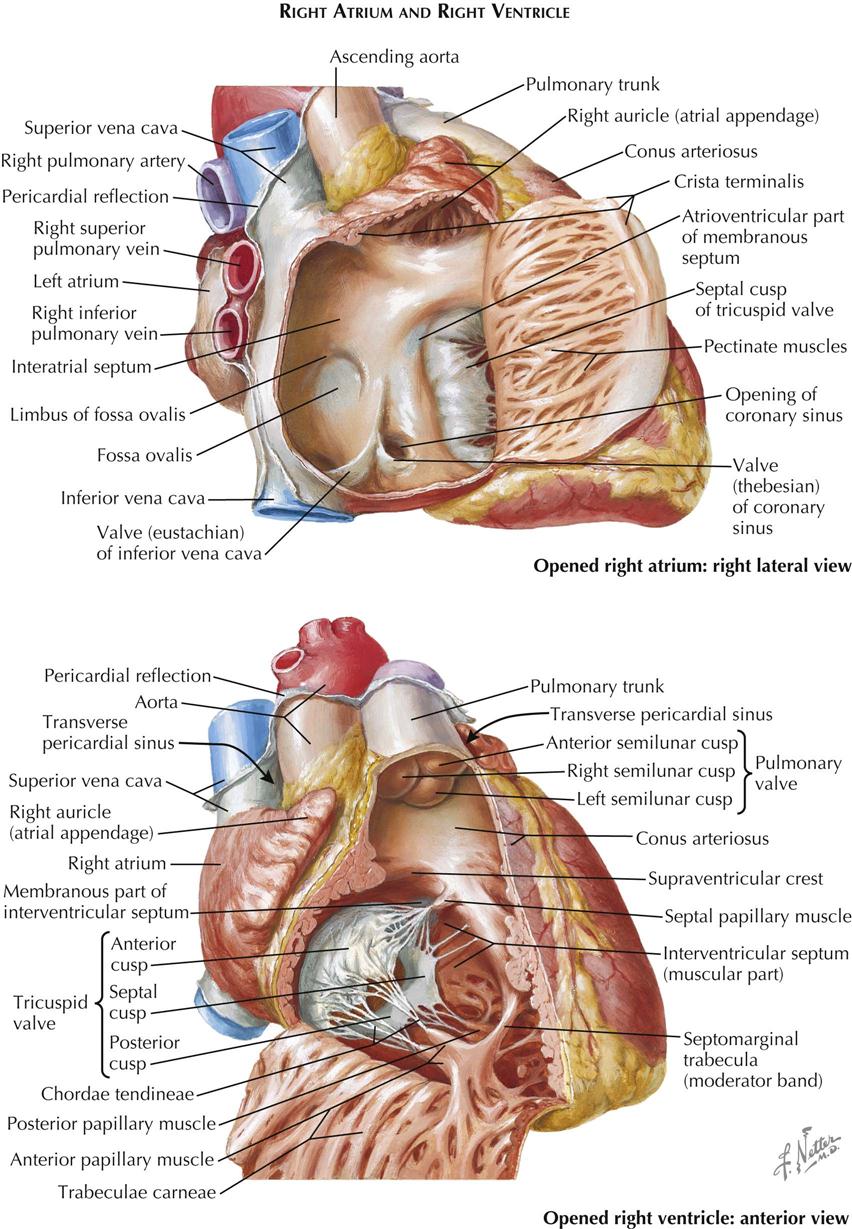
Right Atrium
The right atrium consists of two parts: (1) a posterior smooth-walled part derived from the embryonic sinus venosus, into which enter the superior and inferior venae cavae, and (2) a thin-walled trabeculated part that constitutes the original embryonic right atrium. The two parts of the atrium are separated by a ridge of muscle. This ridge, the crista terminalis (see Plate 1-7), is most prominent superiorly, next to the SVC orifice, then fades out to the right of the IVC ostium. Its position corresponds to that of the sulcus terminalis externally (see Plate 1-6). Often described as a remnant of the embryonic right venous valve. the crista terminalis actually lies just to the right of the valve.
From the lateral aspect of the crista terminalis, a large number of pectinate muscles run laterally and generally parallel to each other along the free wall of the atrium. The atrial wall is paper-thin and translucent between the pectinate muscles. The triangular-shaped superior portion of the right atrium—the right auricle—is also filled with pectinate muscles. One pectinate muscle originating from the crista terminalis is usually larger than the others and is called the taenia sagittalis.
The right auricle usually is not well demarcated externally from the rest of the atrium. The right auricle is a convenient, ready-made point of entry for the cardiac surgeon and is used extensively.
The anterior border of the IVC ostium is guarded by a fold of tissue, the inferior vena cava (eustachian) valve, which varies greatly in size and may even be absent. When large, the IVC valve is usually perforated by numerous openings, forming a delicate lacelike structure known as the network of Chiari. The coronary sinus enters the right atrium just anterior to the medial extremity of the IVC valve. The eustachian valve’s orifice may also be guarded by a valvelike fold, the coronary sinus (thebesian) valve. Both IVC valves and coronary sinus valves are derived from the large, embryonic right venous valve.
The posteromedial wall of the right atrium is formed by the interatrial septum, which has a thin, fibrous, central ovoid portion. The interatrial septum forms a shallow depression in the septum called the fossa ovalis. The remainder of the septum is muscular and usually forms a ridge around the fossa ovalis, the limbus fossae ovalis. A probe can be passed under the anterosuperior part of the limbus into the left atrium in some cases, and the foramen (fossa) ovalis is then “probe patent.” Anteromedially, the tricuspid valve gives access to the right ventricle.
Right Ventricle
The right ventricular cavity (see Plate 1-7) can be divided arbitrarily into a posteroinferior inflow portion, containing the tricuspid valve, and an anterosuperior outflow portion, from which the pulmonary trunk originates. These two parts are separated by prominent muscular bands, including the parietal band, the supraventricular crest (crista supraventricularis), the septal band, and the moderator band. These bands form a wide, almost circular orifice with no impediment to flow in the normal heart.
The wall of the inflow portion is heavily trabeculated, particularly in its most apical portion. These trabeculae carneae enclose a more or less elongated, ovoid opening. The outflow portion of the right ventricle, often called the infundibulum, contains only a few trabeculae. The subpulmonic area is smooth walled.
A number of papillary muscles anchor the tricuspid valve cusps to the right ventricular wall through many slender, fibrous strands called the chordae tendineae. Two papillary muscles, the medial and anterior, are reasonably constant in position but vary in size and shape. The other papillary muscles are extremely variable in all respects. Approximately where the crista supraventricularis joins the septal band, the small medial papillary muscle receives chordae tendineae from the anterior and septal cusps of the tricuspid valve. Often well developed in infants, the medial papillary muscle is almost absent in adults or is reduced to a tendinous patch. An important surgical landmark, the medial papillary muscle is also of diagnostic value to the cardiac pathologist with its interesting embryonic origin. The anterior papillary muscle originates from the moderator band and receives chordae from the anterior and posterior cusps of the tricuspid valve. In variable numbers, the usually small posterior papillary muscle and septal papillary muscle receive chordae from the posterior and medial (septal) cusps. The muscles originating from the posteroinferior border of the septal band are important in the analysis of some congenital cardiac anomalies.
The pulmonary trunk arises superiorly from the right ventricle and passes backward and slightly upward. It bifurcates into right and left pulmonary arteries (see Plate 1-7) just after leaving the pericardial cavity. A short ligament—the ligamentum arteriosum (see Plate 1-8)—connects the upper aspect of the bifurcation to the inferior surface of the aortic arch (arch of aorta; see Plate 1-6). It is a remnant of the fetal ductus arteriosus (duct of Botallo).
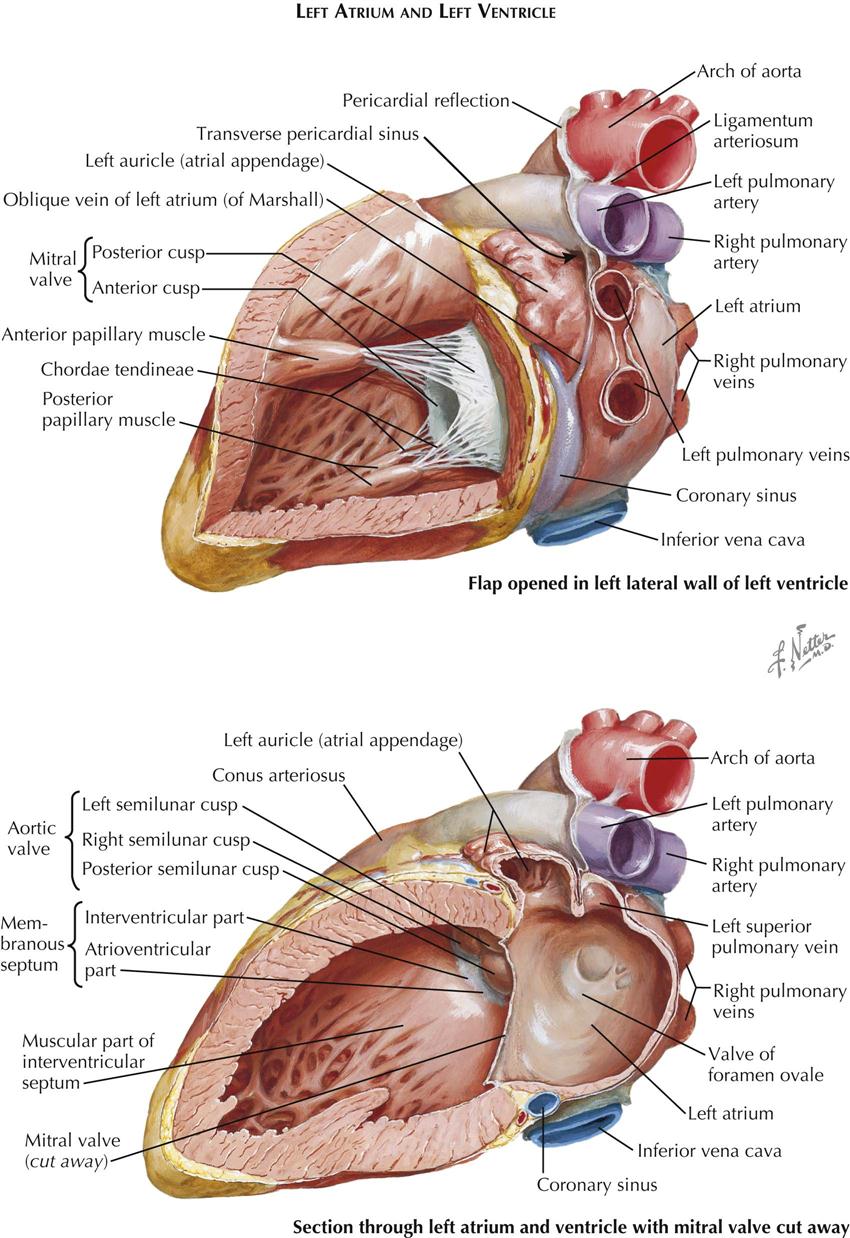
Left Atrium
The left atrium consists mainly of a smooth-walled sac with the transverse axis larger than the vertical and sagittal axes. On the right, two or occasionally three pulmonary veins enter the left atrium; on the left there are also two (sometimes one) pulmonary veins. The wall of the left atrium is distinctly thicker than that of the right atrium. The septal surface is usually fairly smooth, with only an irregular area indicating the position of the fetal valve of the foramen ovale. A narrow slit may allow a probe to be passed from the right atrium to the left atrium.
The left auricle is a continuation of the left upper anterior part of the left atrium. The auricle’s variable shape may be long and kinked in one or more places. Its lumen contains small pectinate muscles, and there usually is a distinct waistlike narrowing proximally.
Left Ventricle
The left ventricle (see Plate 1-8) is egg shaped with the blunt end cut off, where the mitral valve and aortic valve are located adjacent to each other. The valves are separated only by a fibrous band giving off most of the anterior (aortic) cusp of the mitral valve and the adjacent portions of the left and posterior aortic valve cusps. The average thickness of the left ventricular (LV) wall is about three times that of the right ventricular (RV) wall. The LV trabeculae carneae are somewhat less coarse, with some just tendinous cords. As in the right ventricle, the trabeculae are much more numerous and dense in the apex of the left ventricle. The basilar third of the septum is smooth.
Usually there are two stout papillary muscles. The dual embryonic origin of each is often revealed by their bifid apices; each receives chordae tendineae from both major mitral valve cusps. Occasionally a third, small papillary muscle is present laterally.
Most of the ventricular septum is muscular. Normally it bulges into the right ventricle, showing that a transverse section of the left ventricle is almost circular. The muscular portion has approximately the same thickness as the parietal LV wall. The ventricular septum consists of two layers, a thin layer on the RV side and a thicker layer on the LV side. The major septal arteries tend to run between these two layers. In the human heart a variable but generally small area of the septum immediately below the right and posterior aortic valve cusps is thin and membranous.
The demarcation between the muscular and the membranous part of the ventricular septum is distinct and is called the limbus marginalis. As seen from the opened right ventricle (see Plate 1-7, bottom), the membranous septum lies deep to the supraventricular crest and is divided into two parts by the origin of the medial (septal) cusp of the tricuspid valve. As a result, one portion of the membranous septum lies between the left ventricle and the right ventricle—the interventricular part—and the other between the left ventricle and the right atrium—the atrioventricular part.
On sectioning of the septum in an approximately transverse plane, the basilar portion of the ventricular septum, including the membranous septum, is seen to deviate to the right, so that a plane through the major portion of the septum bisects the aortic valve. It must be emphasized that the total cardiac septum shows a complex, longitudinal twist and does not lie in any single plane.
Valves
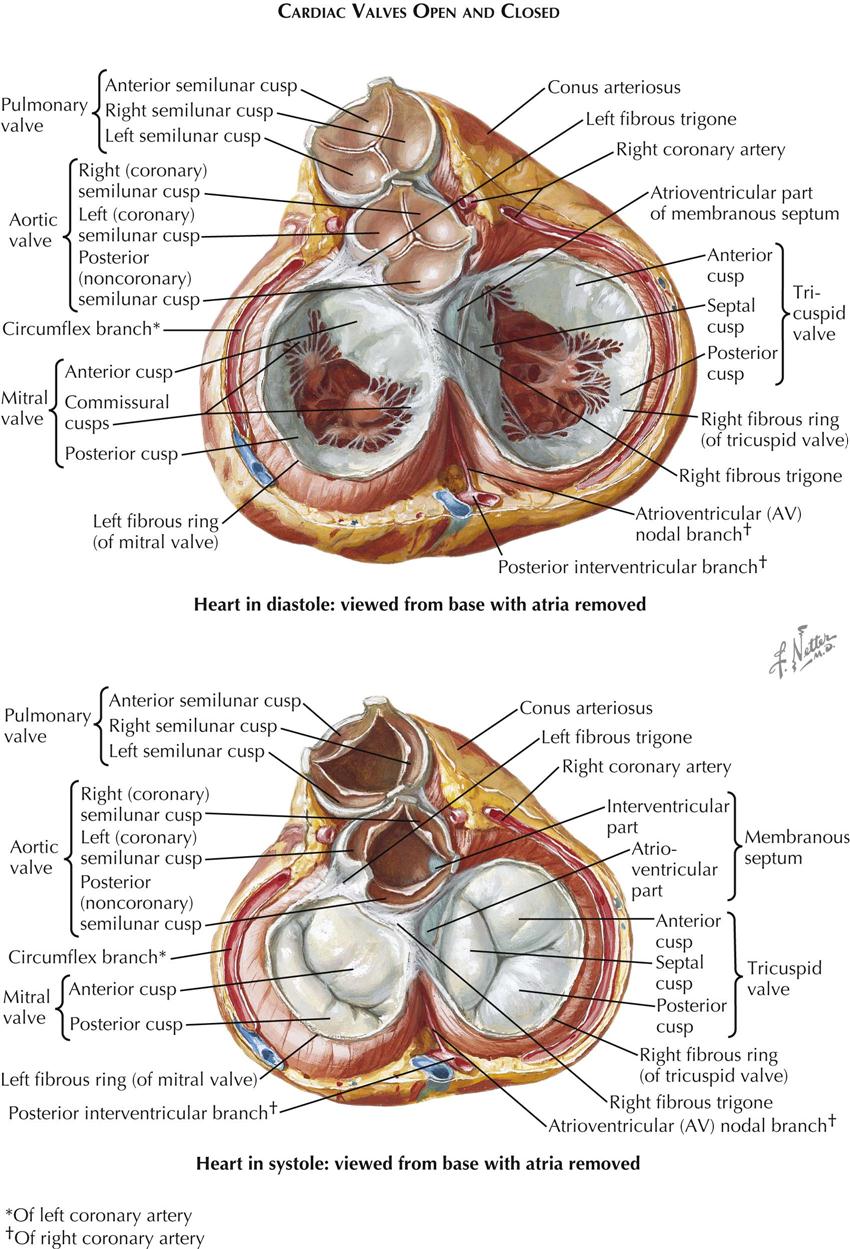
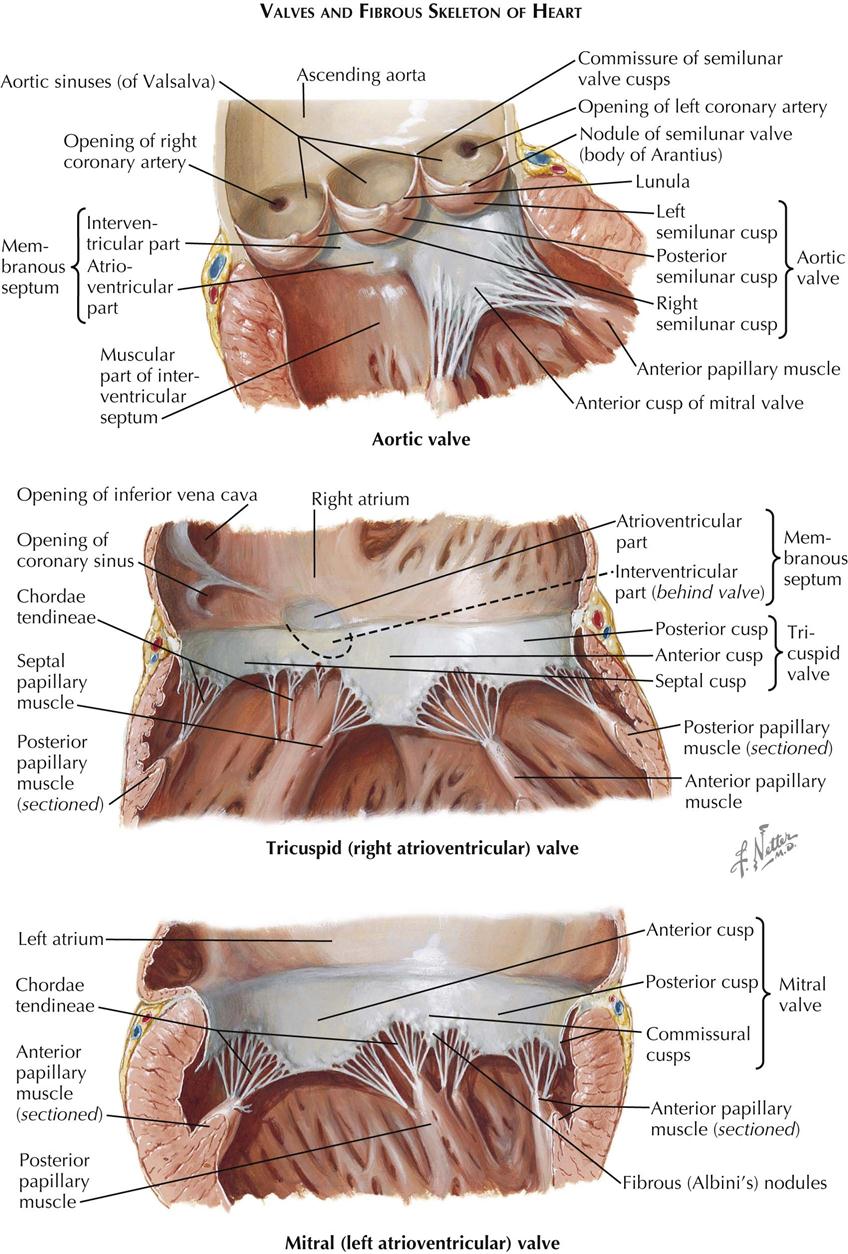
Each atrioventricular (AV) valve apparatus consists of a number of cusps, chordae tendineae, and papillary muscles. The cusps are thin, yellowish white, glistening trapezoid-shaped membranes with fine, irregular edges. They originate from the annulus fibrosus, a poorly defined and unimpressive fibrous ring around each AV orifice. The amount of fibrous tissue increases only at the right and left fibrous trigones.
The atrial surface of the AV valve is rather smooth (except near the free edge) and not well demarcated from the atrial wall. The ventricular surface is irregular because of the insertion of the chordae tendineae and is separated from the ventricular wall by a narrow space.
The extreme edges of the cusps are thin and delicate with a sawtooth appearance from the insertion of equally fine chordae. Away from the edge, the atrial surface of the cusps is finely nodular, particularly in small children. These nodules are called the noduli Albini. On closure of an AV valve, the narrow border between the row of Albini nodules and the free edge of each cusp presses against that of the next, resulting in a secure, watertight closure. The chordae tendineae may be divided into the following three groups:
The first two groups originate from or near the apices of the papillary muscles. They form a few strong, tendinous cords that subdivide into several thinner strands as they approach the valve edges. Occasionally, particularly on the left side, the chordae of the first two orders may be wholly muscular, even in normal hearts, so that the papillary muscle seems to insert directly into the cusp. This is not surprising because the papillary muscles, the chordae tendineae, and most of the cusps are derived from the embryonic ventricular trabeculae and therefore were all muscular at one time.
The tricuspid valve consists of an anterior, a medial (septal), and one or two posterior cusps. The depth of the commissures between the cusps is variable, but the commissures never reach the annulus, so the cusps are only incompletely separated from each other.
The mitral (bicuspid) valve actually is made up of four cusps: two large ones—the anterior (aortic) and posterior (mural) cusps—and two small commissural cusps. Here, as in the tricuspid valve, the commissures are never complete, and they should not be so constructed in the surgical treatment of mitral stenosis.
The arterial or semilunar valves differ greatly in structure from the AV valves. Each consists of three pocketlike cusps of approximately equal size. Although, functionally the transition between the ventricle and the artery is abrupt and easily determined, this cannot be done anatomically in any simple manner. There is no distinct, circular ring of fibrous tissue at the base of the arteries from which these and the valve cusps arise; rather, the arterial wall expands into three dilated pouches, the sinuses of Valsalva, whose walls are much thinner than those of the aorta or pulmonary artery. The origin of the valve cusps is therefore not straight but scalloped.
The cusps of the arterial semilunar valve are largely smooth and thin. At the center of the free margin of each cusp is a small fibrous nodule called the nodulus Arantii. On each side of the nodules of Arantius, along the entire free edge of the cusp, there is a thin, half-moon–shaped area called the lunula that has fine striations parallel to the edge. The lunulae are usually perforated near the insertion of the cusps on the aortic wall. In valve closure, because the areas of adjacent lunulae appose each other, such perforations do not cause insufficiency of the valve and are functionally of no significance.
Specialized Conduction System of Heart
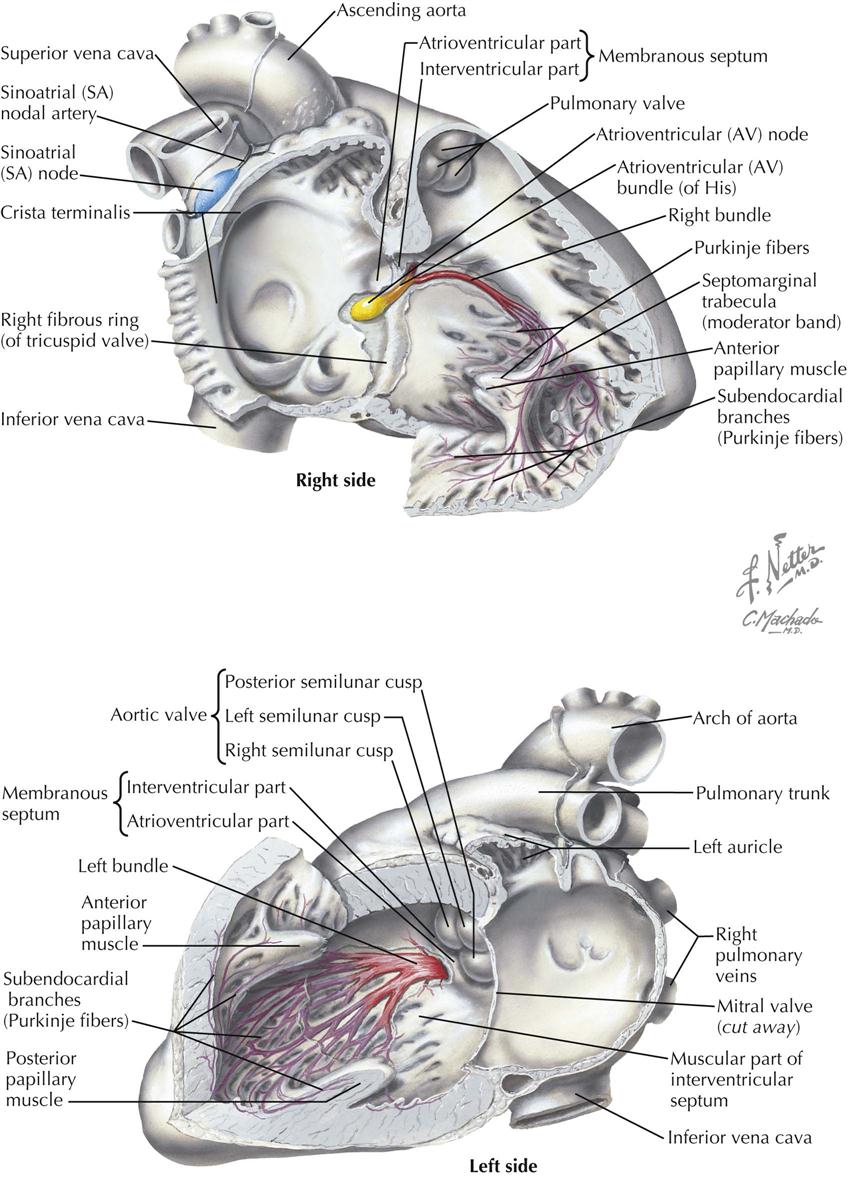
The specialized heart tissues include the sinoatrial (SA) node, atrioventricular (AV) node, common atrioventricular bundle or bundle of His, right and left bundle branches, and peripheral ramifications of these bundle branches, which make up the subendocardial and intramyocardial Purkinje network. In addition, other fiber groups in the atria meet some of the histologic and electrophysiologic criteria for specialization. These tissues constitute Bachmann’s bundle and the internodal conducting paths of the right atrium.
The body of the SA node is in the wall of the right atrium, at the junction between the atrium proper and the superior vena cava. At the lower end, the nodal fibers change and form the common bundle. The common bundle divides into right and left bundle branches, which extend subendocardially along both septal surfaces. The left bundle branch rapidly subdivides, forming a broad sheet of fascicles sweeping over the left interventricular septal surface. The right bundle branch extends for a distance without subdivision; one branch usually passes through the moderator band, and other parts extend over the endocardial surface of the ventricle. Peripherally, both bundle branches subdivide and form the subendocardial network of Purkinje fibers, which extend a variable distance into the ventricular walls and are in direct continuity with fibers of the ventricular muscle.
In definitive histologic studies of the human atrium, James demonstrated the existence of three discrete internodal paths and the relationship of one of these to Bachmann’s bundle. The anterior internodal tract leaves the head of the sinus node and spreads to the left, dividing to form two branches: One extends along the dorsal aspect of the interatrial band to ramify over the left atrium. This subdivision constitutes the specialized fibers of Bachmann’s bundle. The other branch curves across the interatrial septum to the region of the AV node, where it merges with fibers from other nodal tracts. The middle internodal tract leaves the posterodorsal margin of the sinus node and crosses the interatrial septum to merge at the AV node with other specialized atrial fibers. This tract corresponds to the bundle described by Wenckebach. The posterior internodal tract extends from the tail of the sinus node along the crista terminalis, through the eustachian ridge, the right superior margin of the AV node. A description of the interconnections of internodal tracts with the atrium and AV node follows.
Physiologic evidence suggests that the spread of the sinus impulse to the left atrium and from the sinus node to the AV node normally depends primarily on activation of the anterior internodal tract and Bachmann’s bundle. The physiologic significance of these tracts is also described here.
The only normal anatomic communication between the atria and ventricles of the mammalian heart is the atrioventricular node with the common bundle of His. On the atrial side, the AV node communicates with the atrium through the branched and interweaving fibers of the internodal tracts and perhaps through connections with ordinary atrial musculature. In addition, in studies of the canine AV node, fiber tracts appear to bypass the nodal body and connect with distal portions close to the junction of nodal fibers and the common AV bundle. Similar “bypass” fibers can be demonstrated in studies of the human AV node.
Coronary Arteries and Cardiac Veins
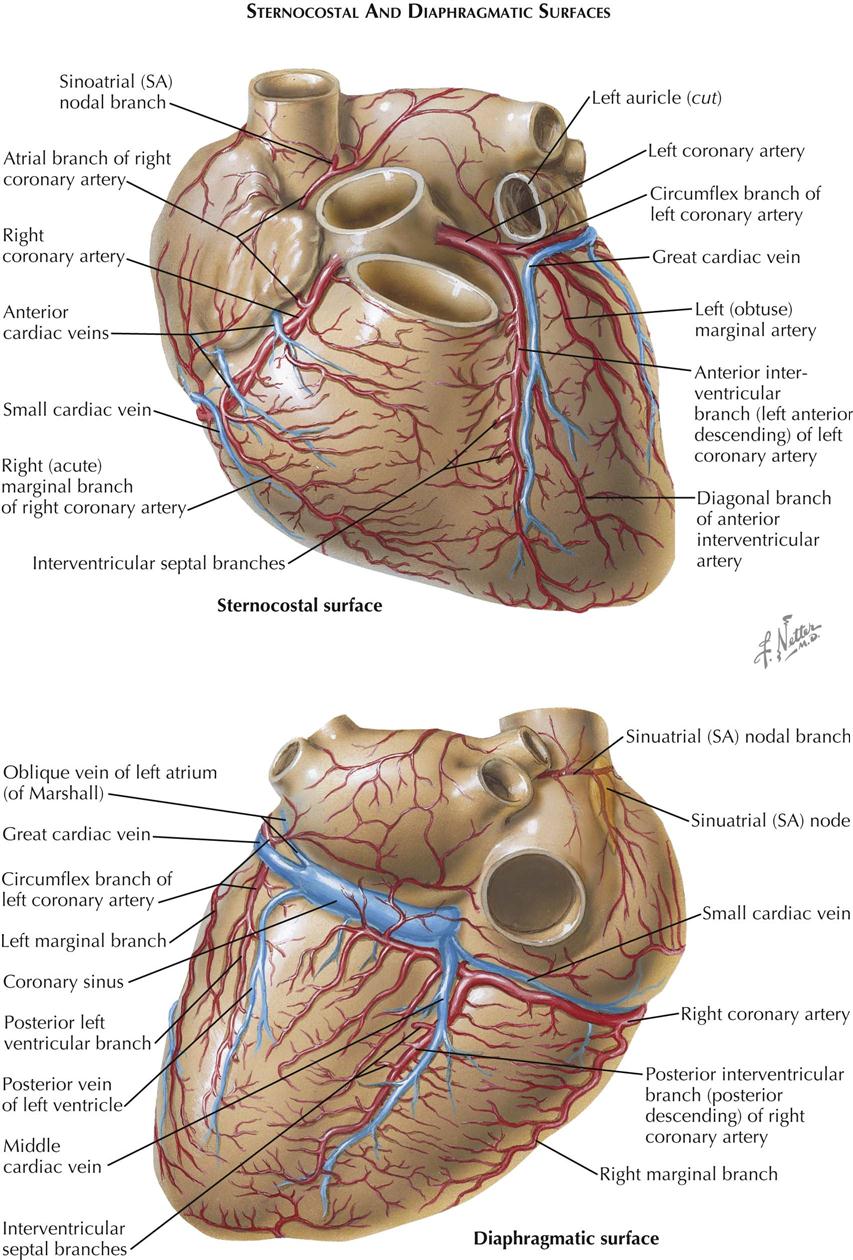
Blood Supply of the Heart
The normal heart and the proximal portions of the great vessels receive their blood supply from two coronary arteries. The left coronary artery (LCA) originates from the left sinus of Valsalva near its upper border, at about the level of the free edge of the valve cusp. The LCA usually has a short (0.5-2 cm) common stem that bifurcates or trifurcates. One branch, the anterior interventricular (descending) branch, courses downward in the anterior interventricular groove (largely embedded in fat), rounds the acute margin of the heart just to the right of the apex, and ascends a short distance up the posterior interventricular groove.
The left anterior descending branch of the LCA gives off branches to the adjacent anterior RV wall (which usually anastomose with branches from the right coronary artery) and septal branches (which supply anterior two thirds and apical portions of septum), as well as a number of branches to the anteroapical portions of the left ventricle, including the anterior papillary muscle.
One septal branch originating from the upper third of the anterior interventricular branch is usually larger than the others and supplies the midseptum, including the bundle of His and bundle branches of the conduction system. This branch also may supply the anterior papillary muscle of the right ventricle through the moderator band. The second, usually smaller circumflex branch of the left coronary artery runs in the left AV sulcus and gives off branches to the upper lateral left ventricular wall and the left atrium. The circumflex branch usually terminates at the obtuse margin of the heart, but it can reach the crux (junction of posterior interventricular sulcus and posterior AV groove). In this case the circumflex branch supplies the entire left ventricle and ventricular septum with blood, with or without the right coronary artery.
In cases where the LCA trifurcates, the third branch, coming off between the anterior interventricular and the circumflex branches, is merely an LV branch that originates from the main artery.
The right coronary artery (RCA) arises from the right anterior sinus of Valsalva of the aorta and runs along the right AV sulcus, embedded in fat. The RCA rounds the acute margin to reach the crux in the majority of cases, and it gives off a variable number of branches to the anterior RV wall. A usually well-developed and large branch runs along the acute margin of the heart. The posterior interventricular (descending) branch descends along the posterior interventricular groove, not quite reaching the apex, and supplies the posterior third or more of the interventricular septum. The diaphragmatic part of the right ventricle is largely supplied by small, parallel branches from the marginal and posterior descending arteries, not from the parent vessel itself. The latter generally crosses the crux, giving off the posterior interventricular branch and a small branch to the atrioventricular node. It terminates in a number of branches to the LV wall.
The posterior papillary muscle of the left ventricle usually has a dual blood supply from both the left and the right coronary artery.
Of the right atrial branches of the right coronary artery, one is of great importance. This branch originates from the RCA shortly after its takeoff and ascends along the anteromedial wall of the right atrium. It enters the upper part of the atrial septum, reappears as the superior vena cava branch (nodal artery) posterior and to the left of the SVC ostium, rounds the ostium, and runs close to (or through) the sinoatrial node (see Plate 1-13), giving off branches to the crista terminalis and pectinate muscles.
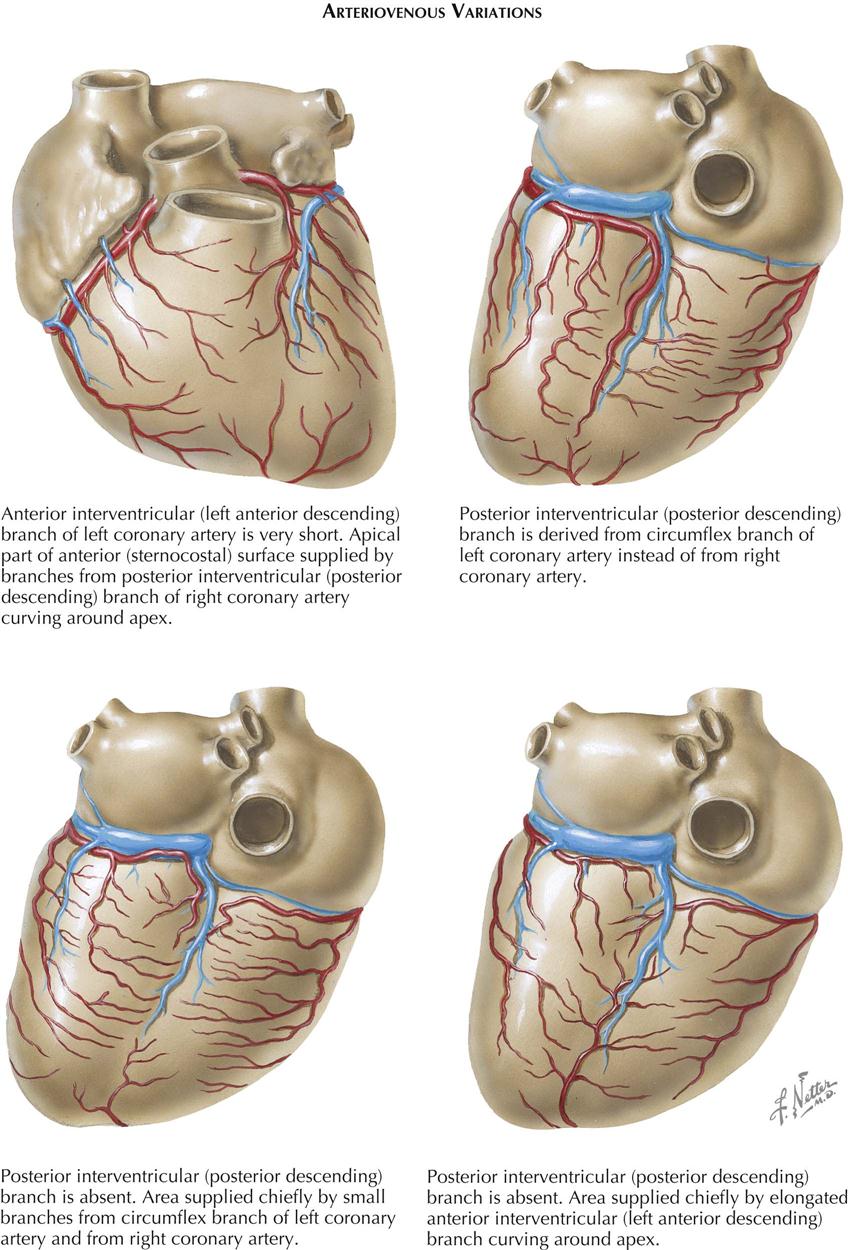
Variations in the branching pattern are extremely common in the human heart. In about 67% of cases the RCA crosses the crux and supplies part of the LV wall and the ventricular septum. In 15% of cases (as in dogs and many other mammals) the LCA circumflex branch crosses the crux, giving off the posterior interventricular branch and supplying the entire left ventricle, the ventricular septum, and part of the RV wall. In about 18% of cases, both coronary arteries reach the crux. No real posterior interventricular branch may exist, but the posterior septum is penetrated at the posterior interventricular groove by many branches from the LCA, RCA, or both. In about 40% of cases the SVC branch is a continuation of a large anterior atrial branch of the LCA rather than of the anterior atrial branch of the RCA.
Also, the first branch of the RCA may originate independent of the right sinus of Valsalva rather than from the parent artery. Rarely, the second or even the third RCA branch arises independently.
Most of the cardiac or coronary veins enter the coronary sinus. The three largest veins are the great cardiac vein, middle cardiac vein, and posterior left ventricular vein. The ostia of these veins may be guarded by fairly well-developed unicuspid or bicuspid valves. The oblique vein of the left atrium (of Marshall) enters the sinus near the orifice of the great cardiac vein, and its ostium never has a valve. The small cardiac vein may enter the right atrium independently, and the anterior cardiac veins always do.
Small venous systems in the atrial septum (and probably in ventricular walls and septum) enter the cardiac chambers directly, called the thebesian veins. The existence of so-called arterioluminal and arteriosinusoidal vessels is debatable and the evidence inconclusive.
Innervation of Heart
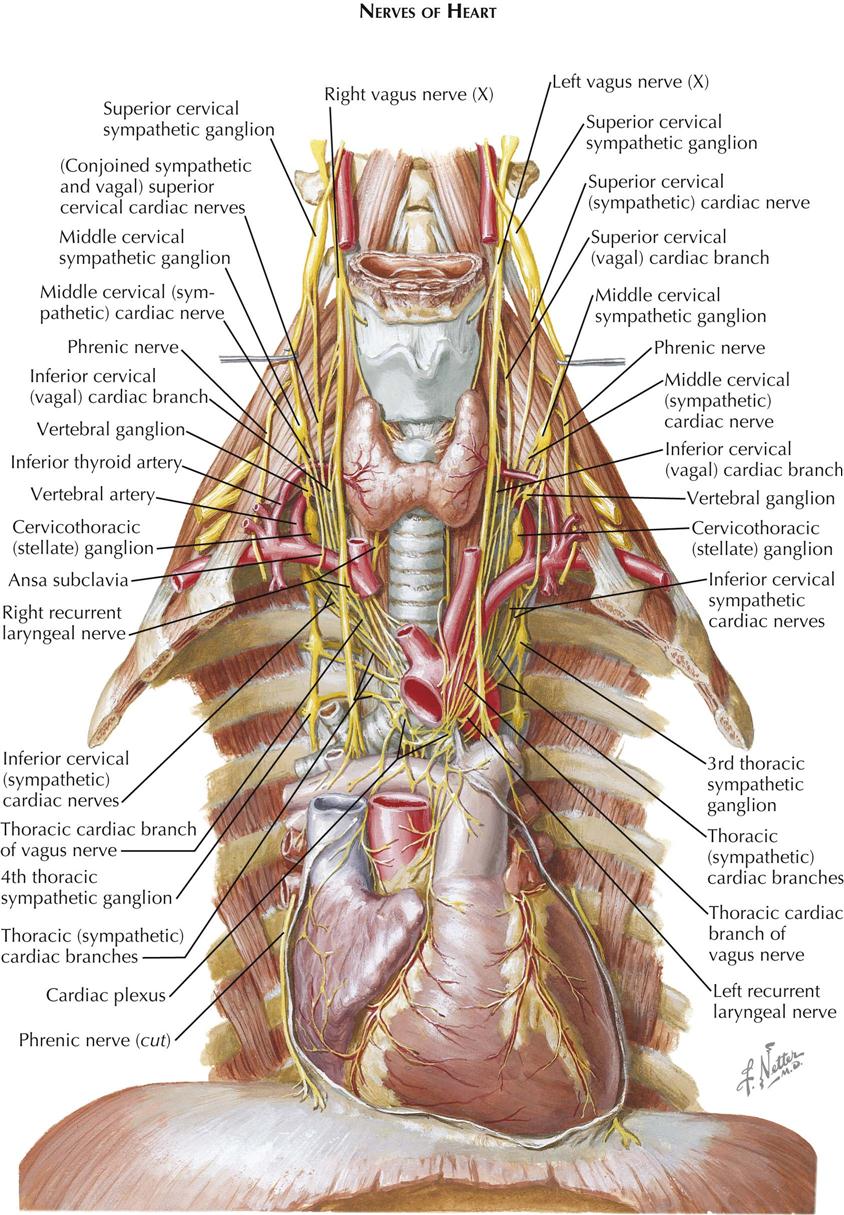
The heart is supplied by sympathetic and parasympathetic nerves that arise primarily in the cervical region because initially the heart develops in the neck. Later the heart migrates downward into the thorax, along with its nerves.
The cervical and upper thoracic sympathetic trunk ganglia contribute cardiac branches, all of which pass through the cardiac plexus, usually without forming synapses. These ganglia are ultimately distributed to the various layers of the heart wall through the coronary plexuses. Three pairs of sympathetic cardiac nerves are derived from the cervical ganglia of the sympathetic trunks, and others arise from the upper thoracic ganglia.
The superior cervical sympathetic cardiac nerve originates by several rootlets from the corresponding ganglion. It often unites with the superior vagal cardiac nerve(s), and this conjoined nerve then descends behind the carotid sheath, communicating en route through slender rami with the pharyngeal, laryngeal, carotid, and thyroid nerves. On the right side, the conjoined nerve passes posterolateral to the subclavian and brachiocephalic arteries and aortic arch; on the left it curves downward across the left side of the aortic arch.
The middle cervical sympathetic cardiac nerve is often the largest of the cervical cardiac nerves. It is formed by filaments from the middle and vertebral ganglia of the sympathetic trunk. This cardiac nerve usually runs independent of the cardiac plexus but may unite with other cardiac nerves, and it is interconnected with tracheal, esophageal, and thyroid branches of the sympathetic trunks.
The inferior cervical sympathetic cardiac nerves consist of filaments arising from the stellate (cervicothoracic) ganglion and ansa subclavia. These cardiac nerves often combine with each other or with other cardiac nerves before reaching the cardiac plexus, and inconstant communications exist between these nerves and the phrenic nerves.
The thoracic sympathetic cardiac nerves are four or five slender branches on each side that arise from the corresponding upper thoracic sympathetic trunk ganglia. These cardiac nerves run forward and medially to the cardiac plexus. Some enter the plexus directly, whereas others are united for variable distances with filaments destined for the lungs, aorta, trachea, and esophagus.
The vagal (parasympathetic) cardiac branches vary in size, number, and arrangement but can be grouped as superior and inferior cervical and thoracic vagal cardiac nerves. The superior cervical vagal cardiac nerve forms from two or three filaments that leave the vagus in the upper part of the neck and usually unites with the corresponding sympathetic cardiac nerve. This conjoined nerve then descends to the cardiac plexus (see earlier). The inferior cervical vagal cardiac nerve(s), one to three in number, arise in the lower third of the neck and often join or communicate with the cardiac branches from the middle cervical sympathetic ganglia and the vertebral and/or stellate sympathetic ganglia. If they remain separate, these cardiac nerves lie posterolateral to the brachiocephalic artery and aortic arch on the right side and lateral to the left common carotid artery and aortic arch on the left side.
The thoracic vagal cardiac nerves are a series of filaments arising from the vagus nerve of each side, at or below the level of the thoracic inlet, and also from both recurrent laryngeal nerves, with the left contributing more filaments than the right. These often unite with other cardiac nerves in their passage to the cardiac plexus.
Cardiac Plexus
All the vagal and the sympathetic cardiac nerves converge on the cardiac plexus, and filaments from the right and left sides of the plexus surround and accompany the coronary arteries and their branches. The cardiac plexus lies between the concavity of the aortic arch and the tracheal bifurcation and is sometimes described as consisting of superficial and deep parts, although their depths vary minimally, and they are intimately interconnected. However, a superficial tenuous preaortic plexus exists over the ascending aorta.
A proportion of the vagal fibers relay in several ganglia present in the cardiac plexus. The largest, the ganglion of Wrisberg, lies below the aortic arch between the division of the pulmonary trunk and the tracheal bifurcation. Other, smaller collections of parasympathetic cells—the intrinsic cardiac ganglia—are located mainly in the atrial subendocardial tissue, along the AV sulcus and near the roots of the great vessels. Relatively few cardiac ganglia are found over the ventricles, but enough exist to question the view that the ventricular innervation is entirely or predominantly sympathetic.
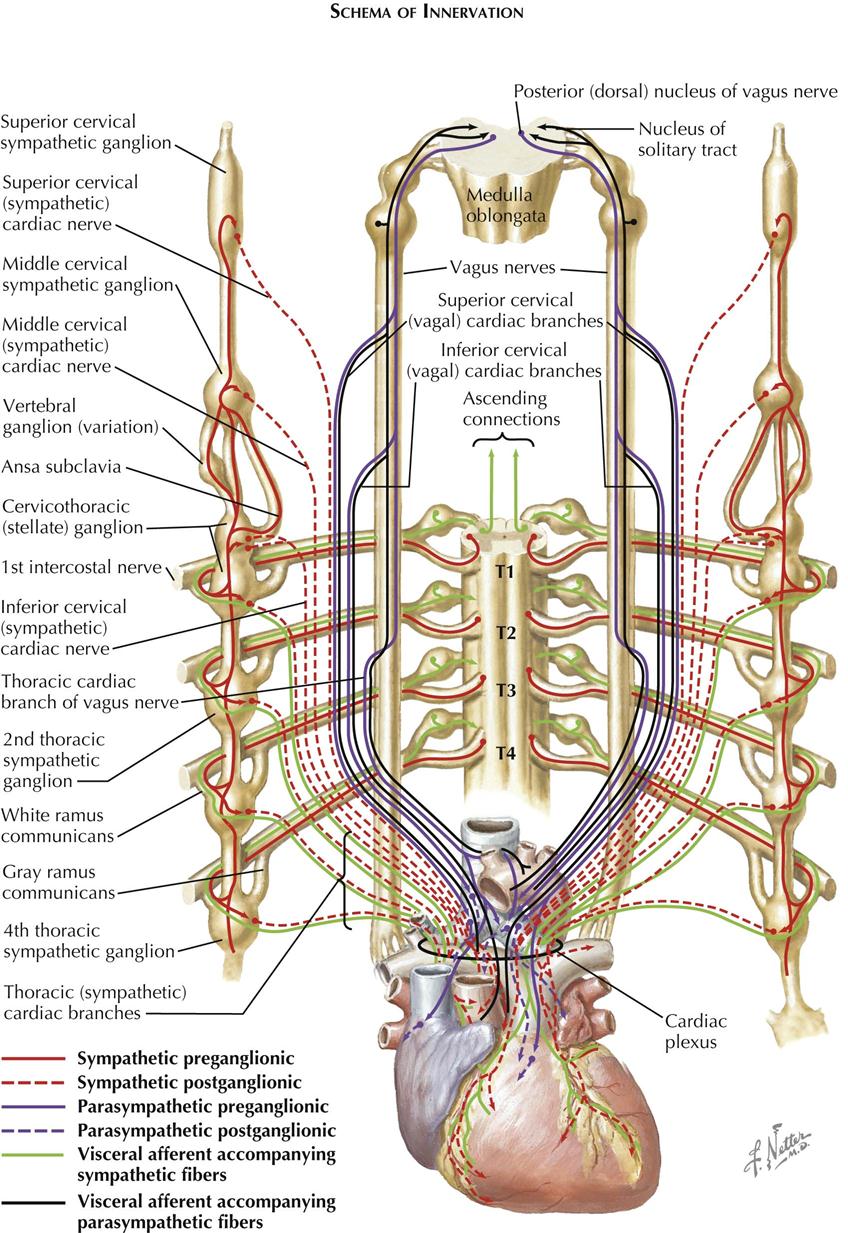
The cardiac sympathetic and parasympathetic nerves carry both afferent and efferent fibers. The afferents transmit impulses to the central nervous system from discrete cardiac receptor endings and terminal networks plentiful in these reflexogenous zones, such as the endocardium around openings of the caval and pulmonary veins, over the interatrial septum, and in the AV valves. The efferents carry impulses that are modified reflexively by afferent impulses from the heart and great vessels. Efferent fibers are under the overall control of the higher centers in the brain, the hypothalamus, and the brainstem.
The more important pathways are illustrated in Plates 1-15 and 1-16. Afferents from the heart and the great vessels are shown traveling to the cord via the sympathetic cardiac nerves, whereas others are carried upward to nuclei in the medulla oblongata by the vagus nerves. The efferents pursue similar routes but travel in a centrifugal direction. The cell bodies of the afferent neurons are situated in the dorsal root ganglia of the upper four or five thoracic nerves and in the inferior vagal ganglia.
The preganglionic parasympathetic fibers are the axons of cells in the dorsal vagal nuclei, and these fibers relay in cardiac plexus or intrinsic cardiac ganglia. The preganglionic sympathetic fibers are the axons of cells located in the lateral gray columns of the upper four or five thoracic segments. These fibers enter the corresponding spinal nerves and leave them in white rami communicantes. which pass to adjacent ganglia in the sympathetic trunks. Some fibers relay in these ganglia, however, and the postganglionic fibers (the axons of ganglionic cells) are conveyed to the heart in the thoracic sympathetic cardiac nerves. Others ascend in the sympathetic trunks to form synapses with cells in the superior, middle, and vertebral ganglia, and the postganglionic fibers reach the heart via cardiac branches of these ganglia. Therefore the parasympathetic relays occur in ganglia near or in the heart, whereas the sympathetic relays are located in ganglia at some distance from the heart. Consequently, the parasympathetic postganglionic fibers are relatively short and circumscribed in their distribution.
Afferent and efferent fibers probably run in all the sympathetic and the parasympathetic cardiac nerves, although afferents may not be present in the superior cervical sympathetic cardiac nerves. Many afferent vagal fibers from the heart and great vessels are involved in reflexes depressing cardiac activity, and in some animals these fibers are aggregated in a separate “depressor nerve” and in humans may run in cardiac branches of the laryngeal nerves.
Despite their insignificant size, the thoracic sympathetic cardiac nerves carry many efferent accelerator and afferent fibers to and from the heart and great vessels. Other cardiac pain afferents run in the middle and inferior cervical sympathetic cardiac nerves, but after entering the corresponding cervical ganglia, they descend within the sympathetic trunks to the thoracic region before passing through rami communicantes into the upper four or five thoracic nerves, then to the spinal cord. Because many cardiac pain fibers run through the preaortic plexus, some advocate excision of this plexus as a simpler, safer alternative to upper thoracic sympathetic ganglionectomy for relief of angina pectoris.
Afferent fibers from the pericardium are carried mainly in the phrenic nerves, although afferents from the visceral serous pericardium are conveyed in the coronary plexuses.