Case 1 Acne vulgaris/rosacea
Description of acne vulgaris/rosacea
Definition
Acne is an integumentary disorder that affects the pilosebaceous units (acne vulgaris) and/or the underlying blood vessels (rosacea) of the skin. Some of the variants of the disease include acne vulgaris, acne conglobata, acne fulminans, acne excorié, mature onset, pyoderma faciale, rosacea, neonatal and infantile acne.
Epidemiology
Males and females of all ages can be affected by acne;1 however, the type of acne can vary between sexes. The vulgaris, conglobata and fulminant variants of acne, for example, are most prevalent among adolescent males, while acne excorié, rosacea, mature onset and pyoderma faciale are more likely to manifest in women.1
Aetiology and pathophysiology
Many factors can be implicated in the pathogenesis of acne, including genetic, hormonal, infectious, dietary and environmental elements. In terms of hormonal influence, it is believed that elevated androgen levels increase sebum production and abnormal follicular keratinisation and desquamation, which leads to the blockage of pilosebaceous units and the formation of comedones.1 The peak elevation in androgen, sebum and growth hormone levels during the adolescent period provides some explanation for the increased prevalence of this condition during adolescence.2 Findings from a case study of 34 men and women with acne adds further support to the relationship between androgen levels and acne lesion count.2
The bacterium Propionibacterium acnes is another contributing factor in acne development. This is because the bacterium promotes inflammation by releasing chemotactic factors and proteases while hydrolysing sebum into proinflammatory free fatty acids.1,3 In rosacea, an underlying vascular defect may be responsible,3 although the actual aetiology of this disorder remains unclear. What is apparent is that rosacea can be triggered by a range of exogenous and endogenous stimuli, including cold or hot weather, wind, sun exposure, exercise, hot baths, emotional stress, alcohol, spicy foods, cosmetics and hot drinks.4
Other environmental factors that may be implicated in the pathogenesis of acne include medications (e.g. steroids, anticonvulsants), occlusive objects (e.g. shirt collars, helmets), topical agents (e.g. cosmetics, lotions, creams) and perspiration.4,5
The chronic consumption of foods with a high glycaemic index or glycaemic load also contributes to acne development by promoting hyperinsulinaemia and insulin resistance. This can be followed by elevated free levels of insulin growth factor and androgens, and a subsequent rise in keratinocyte proliferation, sebum production and acne formation.6
Clinical manifestations
Acne can range in severity from mild to severe. In mild cases, acneiform lesions might be limited to open (blackheads) and closed (whiteheads) comedones, and papules. In more severe cases, inflamed papules, pustules, nodules and cysts may develop, which can lead to scarring.1,3 The presence of these lesions, as well as scarring, can impact negatively on the psychological wellbeing of the client and their family. Systemic manifestations of the disease can also present in certain variants, such as acne fulminans, with symptoms that include pyrexia, malaise, arthralgia and weight loss.3 In most cases, acneiform lesions are confined to the face, upper back and chest where pilosebaceous units are most abundant. In rosacea, lesions are generally localised to the face and are often accompanied by facial erythema, oedema and telangiectasia.5
Clinical case
16-year-old male with acne vulgaris to the face and upper back
Rapport
Adopt the practitioner strategies and behaviours highlighted in Table 2.1 (chapter 2) to improve client trust, communication and rapport, as well as the accuracy and comprehensiveness of the clinical assessment.
Medical history
Family history
Illicit drug use
| Diet and fluid intake | |
|---|---|
| Breakfast | Large glass of full-cream milk. |
| Morning tea | Apple, muesli bar. |
| Lunch | Two sandwiches, made with white bread and spread with peanut butter or Vegemite®. |
| Afternoon tea | Sweet biscuits, toasted white bread spread with Vegemite®. |
| Dinner | Spaghetti bolognaise, fettuccine carbonara, lasagne, supreme pizza. |
| Fluid intake | 1 cup of cordial daily, 1 cup of juice daily, 2 cups of water daily, 1 cup of milk daily. |
| Food frequency | |
| Fruit | 1–2 serves daily |
| Vegetables | 1–2 serves daily |
| Dairy | 2 serves daily |
| Cereals | 7 serves daily |
| Red meat | 6 serves a week |
| Chicken | 1 serve a week |
| Fish | 0 serves a week |
| Takeaway/fast food | 2–3 times a week |
Diagnostics
Pathology tests
The use of culture and sensitivity tests to detect the presence of P. acnes is not a reliable diagnostic for acne as this bacterium is a normal resident of human skin. Testing serum androgen levels is also unreliable as most patients with acne demonstrate normal androgen levels.1
Low plasma concentrations of vitamin A are reported in people with acne.7 Assessing hair or serum vitamin A levels may help to determine whether vitamin A deficiency is implicated in this condition.
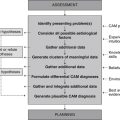
Planning
Expected outcomes
Based on the degree of improvement reported in clinical studies that have used CAM interventions for the management of acne,8–11 the following are anticipated.
Application
Diet
Low glycaemic-load diet (Level II, Strength A, Direction +)
The consumption of foods with a high glycaemic index or glycaemic load may, as discussed earlier, contribute to the pathogenesis of acne. Hence, the substitution of high glycaemic-load foods such as sweetened, refined and highly processed products with foods that have a low glycaemic-load, such as wholegrains and fruits with edible skins or are high in protein, including eggs and lean meat, may be of benefit to those suffering from acne. Indeed, two controlled clinical trials have shown young males who follow a 12-week low glycaemic-load diet demonstrate a significant reduction in insulin resistance, fasting insulin, free androgen levels, BMI, total acneiform lesion counts and inflammatory lesion counts when compared to those consuming a high glycaemic-load diet.9,10
Lifestyle
Relaxation therapy (Level II, Strength B, Direction +)
Psychological stress is positively correlated with the severity of acne.12,13 It is therefore reasonable to assume that successful attempts at reducing stress could lead to clinical improvements in acne. This assumption was tested in a small randomised controlled trial (RCT) of thirty dermatology patients with acne vulgaris, and found 6 weeks of biofeedback-assisted relaxation and cognitive imagery was significantly more effective than attention-comparison and medical control at reducing acne severity.14 Further research is now needed to determine whether similar effects can be observed using other stress reduction techniques, including meditation, yoga and tai chi.
Nutritional supplementation
Ascorbic acid (Level II, Strength C, Direction +)
Vitamin C has the potential to attenuate the pathogenesis of acne by reducing cutaneous lipid peroxidation, P. acnes replication15 and serum C-reactive protein (CRP) levels (a marker of acute inflammation).16 Findings from an open comparative study add some support to this theory, with topically applied five per cent sodium ascorbyl phosphate (SAP) (a vitamin C derivative) twice daily for 12 weeks shown to be more effective than five per cent benzoyl peroxide in reducing the number of inflammatory and non-inflammatory acneiform lesions in 49 subjects.15 A small double-blind RCT (n = 30) also found five per cent SAP lotion (applied twice daily for 8 weeks) to reduce inflammatory lesion count in adults with acne vulgaris, although the difference between the SAP and 0.2 per cent retinol groups was not statistically significant.17 Given that studies have used different active controls, no firm conclusions can yet be made about the efficacy of topical SAP in acne.
Nicotinamide (Level II, Strength B, Direction +)
Vitamin B3 reduces inflammatory mediator release18 and, as such, may play a role in the treatment of inflammatory skin disorders. Several controlled clinical trials have confirmed this, with topically administered nicotinamide gel (four per cent) found to be as effective as antibiotic gel in reducing the number and severity of inflamed acneiform lesions.11,19 Whether this affect can be demonstrated with orally administered vitamin B3 is not yet clear.
Vitamin A (Level II, Strength B, Direction +)
Collagen synthesis, phagocytosis, antibody production and epithelial cell differentiation are key functions of vitamin A.20 Given these actions and the significantly lower plasma concentration of vitamin A reported in people with acne,7 it is not surprising that many studies (albeit methodologically limited studies) have found synthetic vitamin A derivatives to be effective at reducing the severity of acne vulgaris when administered as oral or topical preparations.21–26 Whether these effects translate to betacarotene and/or natural vitamin A is not yet certain.
Zinc (Level II, Strength C, Direction o)
Zinc modulates inflammatory and immune activity27 and demonstrates antimicrobial activity against Propionibacterium strains.28 While low serum and epidermal zinc concentrations have been reported in people with acne vulgaris,29,30 the evidence is not conclusive. Likewise, evidence from trials investigating the clinical efficacy of zinc in acne has not been consistent.31–35
Herbal medicine
Commiphora molmol (Level II, Strength C, Direction o)
Myrrh has long been used as an anti-inflammatory, vulnerary and antimicrobial herb. These effects appear to be of some benefit to patients with nodulocystic acne, with a small RCT of 20 patients finding oral Gugulipid extract (equivalent to 25 mg guggulsterone), administered twice daily for 3 months, to be as effective as oral tetracycline at reducing the number of inflamed acneiform lesions.36 No firm conclusions can be made about the efficacy of myrrh until further evidence from larger studies becomes available.
Vitex agnus-castus (Level III-1, Strength D, Direction +)
Chaste tree may exert a mild antiandrogenic effect by reducing serum prolactin levels37 and thus may attenuate the sequence of events leading to the manifestation of acne. This action may explain why an earlier controlled trial of 161 subjects found chaste tree treatment to be significantly superior to placebo at improving the signs of acne at 12 weeks.38 In view of the paucity of corroborating data and the insufficient details of the study, these results should be interpreted with caution.
Other herbs
Arctium lappa (burdock), Galium aparine (cleavers), Trifolium pratense (red clover), Rumex crispus (yellow dock) and Scrophularia nodosa (figwort) have long been used as treatments for skin complaints because of their depurative action. Experimental data suggest that Glycyrrhiza glabra (licorice) and Echinacea spp. (echinacea) may also be indicated for the treatment of acne because of their anti-inflammatory, immunomodulatory and antimicrobial activity against P. acnes.39,40 Despite the availability of traditional and/or experimental evidence in this area, there is insufficient clinical evidence to support or refute the effectiveness of these herbs in acne.
Other
Acupuncture (Level II, Strength D, Direction +)
Many case reports have been published on the effectiveness of acupuncture in acne;41–43 however, given the methodological limitations of these reports, no firm conclusions can be drawn. A more recent RCT of 52 patients with acne conglobata adds much-needed rigour to this body of evidence. Four weeks of treatment, daily encircling acupuncture, together with twice weekly venesection and cupping, was found to be as effective as orally administered isotretinoin (10 mg three times a day) at reducing the signs of acne, and superior to isotretinoin at lowering serum interleukin-6 levels.44 The risk of placebo bias in this trial and the unknown confounding effect of venesection and cupping makes the translation of these findings into clinical practice difficult.
Melaleuca alternifolia (aromatherapy) (Level II, Strength B, Direction +)
Indigenous Australians traditionally used tea tree for its anti-inflammatory, antimicrobial and antiseptic properties. These effects, as well as the sensitivity of P. acnes to tea tree oil,45 indicate that tea tree may be effective as a treatment for acne. Findings from a systematic review of one RCT (n = 124) support this proposition,46 with the topical application of five per cent tea tree oil gel found to be as effective as five per cent benzoyl peroxide at reducing the number and severity of acneiform lesions, albeit with a relatively slower onset of action.47 Similar outcomes were observed in a more recent RCT (n = 60) that compared five per cent tea tree oil gel to placebo in 60 adolescents and young adults.8
CAM prescription
Primary treatments
Referral
Review
To determine whether pertinent client goals and expected outcomes have been achieved at follow-up, and if any aspects of the client’s care need to be improved, consider the factors listed in Table 8.2 (chapter 8) and the questions listed below.
1. Cargnello J.A. Acne: what’s new? In Marks R., editor: Dermatology, 2nd ed, Sydney: Australasian Medical Publishing Company, 2005.
2. Cappel M., Mauger D., Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Archives of Dermatology. 2005;141:333-338.
3. Buchanan P., Courtenay M. Prescribing in dermatology. Cambridge: Cambridge University Press; 2006.
4. Porter R., et al, editors. The Merck manual. Whitehouse Station: Merck Research Laboratories, 2008.
5. Feldman S., et al. Diagnosis and treatment of acne. American Family Physician. 2004;69:2123-2130.
6. Cordain L. Implications for the role of diet in acne. Seminars in Cutaneous Medicine and Surgery. 2005;24(2):84-91.
7. El-Akawi Z., Abdel-Latif N., Abdul-Razzak K. Does the plasma level of vitamins A and E affect acne condition? Clinical and Experimental Dermatology. 2006;31(3):430-434.
8. Enshaieh S., et al. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: a randomized, double-blind placebo-controlled study. Indian Journal of Dermatology,. Venereology and Leprology. 2007;73(1):22-25.
9. Smith R.N., et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. American Journal of Clinical Nutrition. 2007;86(1):107-115.
10. Smith R., et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Molecular Nutrition and Food Research. 2008;52(6):718-726.
11. Weltert Y., et al. Double-blind clinical assessment of the efficacy of a 4% nicotinamide gel (Exfoliac NC Gel) versus a 4% erythromycin gel in the treatment of moderate acne with a predominant inflammatory component. Nouvelles Dermatologiques. 2004;23(7):385-394.
12. Schulpis K., et al. Psychological and sympatho-adrenal status in patients with cystic acne. Journal of the European Academy of Dermatology and Venereology. 1999;13(1):24-27.
13. Yosipovitch G., et al. Study of psychological stress, sebum production and acne vulgaris in adolescents. Acta Dermato-Venereologica. 2007;87(2):135-139.
14. Hughes H., et al. Treatment of acne vulgaris by biofeedback relaxation and cognitive imagery. Journal of Psychosomatic Research. 1983;27(3):185-191.
15. Klock J., et al. Sodium ascorbyl phosphate shows in vitro and in vitro efficacy in the prevention and treatment of acne vulgaris. International Journal of Cosmetic Science. 2005;27(3):171-176.
16. Wannamethee S.G., et al. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. American Journal of Clinical Nutrition. 2006;83(3):567-574.
17. Ruamrak C., Lourith N., Natakankitkul S. Comparison of clinical efficacies of sodium ascorbyl phosphate, retinol and their combination in acne treatment. International Journal of Cosmetic Science. 2009;31(1):41-46.
18. Namazi M.R. Nicotinamide: a potential addition to the anti-psoriatic weaponry. FASEB Journal. 2003;17:1377-1379.
19. Shalita A.R., et al. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. International Journal of Dermatology. 1995;34(6):434-437.
20. Leach M.J. A critical review of natural therapies in wound management. Ostomy/Wound Management. 2004;50(2):36-51.
21. Fatum B., Hansen H.H.V., Mortensen E. Topical treatment of acne vulgaris with the vitamin A acid derivate motretinide (Tasmaderm®), tretinoin (Airol®) and a placebo cream. Ugeskrift for laeger. 1980;142(51):3364-3366.
22. Gandola M., et al. Topical vitamin A acid in the treatment of acne vulgaris (a controlled multicenter trial). Archives for Dermatological Research. 1976;255(2):129-138.
23. Lucky A.W., et al. Comparative efficacy and safety of two 0.025% tretinoin gels: results from a multicenter double-blind, parallel study. Journal of the American Academy of Dermatology. 1998;38(4):S17-23.
24. Peck G.L., et al. Isotretinoin versus placebo in the treatment of cystic acne. A randomized double–blind study. Journal of the American Academy of Dermatology. 1982;6(4 Suppl. 2):735-745.
25. Schumacher A., Stuttgen G. Vitamin A acid in hyperkeratoses, epithelial tumors and acne. Deutsche Medizinische Wochenschrift. 1971;96:1547-1551.
26. Shalita A.R., et al. Tazarotene gel is safe and effective in the treatment of acne vulgaris: a multicenter, double-blind, vehicle-controlled study. Cutis. 1999;63(6):349-354.
27. Kahmann L., et al. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Research. 2008;11(1):227-237.
28. Fluhr J.W., et al. In-vitro and in-vivo efficacy of zinc acetate against propionibacteria alone and in combination with erythromycin. Zentralblatt fur Bakteriologie. 1999;289(4):445-456.
29. Amer M., et al. Serum zinc in acne vulgaris. International Journal of Dermatology. 1982;21(8):481-484.
30. Michaelsson G., Ljunghall K. Patients with dermatitis herpetiformis, acne, psoriasis and Darier’s disease have low epidermal zinc concentrations. Acta Dermato-Venereologica. 1990;70(4):304-308.
31. Agrawal P., et al. Oral zinc in acne vulgaris (a double blind evaluation). Indian Journal of Dermatology. Venereology and Leprology. 1985;51(1):38-39.
32. Dreno B., et al. Low doses of zinc gluconate for inflammatory acne. Acta Dermato-Venereologica. 1989;69(6):541-543.
33. Goransson K., Liden S., Odsell L. Oral zinc in acne vulgaris: a clinical and methodological study. Acta Dermato-Venereologica. 1978;58(5):443-448.
34. Orris L., et al. Oral zinc therapy of acne. Absorption and clinical effect. Archives of Dermatology. 1978;114(7):1018-1020.
35. Verma K.C., Saini A.S., Dhamija S.K. Oral zinc sulphate therapy in acne vulgaris: a double-blind trial. Acta Dermato-Venereologica. 1980;60(4):337-340.
36. Thappa D.M., Dogra J. Nodulocystic acne: oral gugulipid versus tetracycline. Journal of Dermatology. 1994;21(10):729-731.
37. Bone K. A clinical guide to blending liquid herbs. St Louis: Churchill Livingstone; 2003.
38. Amann W. Improvement of acne vulgaris following therapy with Agnus castus (Agnolyt). Therapie der Gegenwart. 1967;106(1):124-126.
39. Nam C., et al. Anti-acne effects of Oriental herb extracts: a novel screening method to select anti-acne agents. Skin Pharmacology and Applied Skin Physiology. 2003;16(2):84-90.
40. Sharma M., et al. Echinacea extracts contain significant and selective activities against human pathogenic bacteria. Pharmaceutical Biology. 2008;46(1–2):111-116.
41. Ding L.N. 50 cases of acne treated by puncturing acupoint dazhui in combination with cupping. Journal of Traditional Chinese Medicine. 1985;5(2):128.
42. Hou H., Wu T. Fifty-six cases of acne treated by auricular needle-embedding. Journal of Traditional Chinese Medicine. 2002;22(2):115-116.
43. Xu Y.H. Treatment of acne with ear acupuncture – a clinical observation of 80 cases. Journal of Traditional Chinese Medicine. 1989;9(4):238-239.
44. Liu C.Z., Lei B., Zheng J.F. Randomized control study on the treatment of 26 cases of acne conglobata with encircling acupuncture combined with venesection and cupping. Zhen Ci Yan Jiu. 2008;33(6):406-408.
45. Raman A., Weir U., Bloomfield S.F. Antimicrobial effects of tea-tree oil and its major components on Staphylococcus aureus, Staph. epidermidis and Propionibacterium acnes. Letters in Applied Microbiology. 1995;21(4):242-245.
46. Ernst E., Huntley A. Tea tree oil: a systematic review of randomized clinical trials. Forschende Komplementarmedizin und Klassische Naturheilkunde. 2000;7(1):17-20.
47. Bassett I.B., Pannowitz D.L., Barnetson R.S. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Medical Journal of Australia. 1990;153(8):455-458.