Structure and Function of Newborn Skin
Anthony J. Mancini, Leslie P. Lawley
Introduction
The skin of the newborn serves a pivotal role in the transition from the aqueous intrauterine environment to extrauterine terrestrial life and is integral to the vital functions of mechanical protection, thermoregulation, cutaneous immunosurveillance, and maintenance of a barrier that prevents insensible loss of body fluids. The anatomy and function of skin are most easily understood by dissecting the individual compartments (stratum corneum, epidermis, dermoepidermal junction (DEJ), dermis and subcutaneous tissue) and their component cell types. Specialized structures found within these compartments, such as pilosebaceous units, sweat glands, nerves, and vascular networks, play an essential role both anatomically and functionally in cutaneous homeostasis in the neonate. The anatomy of these compartments and structures of the skin, and the physiologic processes involved in their functions, are the focus of this chapter.
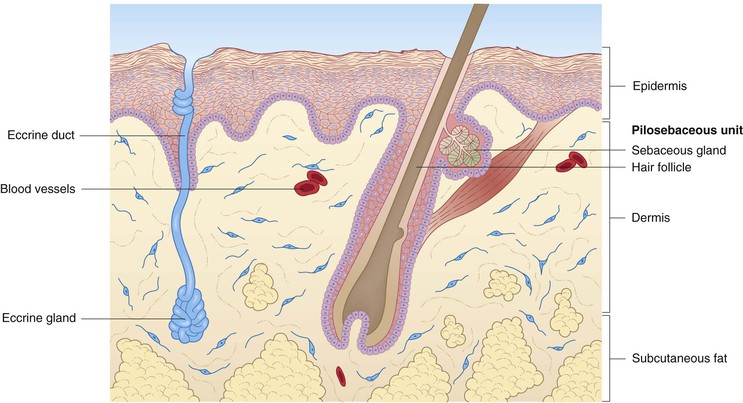
Human skin consists of three layers: epidermis, dermis, and subcutaneous fat (Fig. 2.1). All elements of skin are derived from either ectoderm or mesoderm, the former giving rise to the epidermis and other cutaneous epithelial components.1 A brief description of fetal skin development is helpful in understanding the structure and function of newborn skin, and is incorporated into some of the following discussions of the various compartments and structures. A more thorough review of cutaneous embryology is the focus of Chapter 1.
Stratum corneum and epidermis
The most obvious clinical difference between the skin of the term newborn and that of an adult is the presence of the moist, greasy, yellow-white substance called vernix caseosa, which is a coating comprised of a combination of sebaceous gland secretions, desquamated skin cells, and shed lanugo hairs.2,3 The vernix caseosa has an important role in maintaining hydration and pH balance, and preventing infection during the first few days of life.4,5 Certain components of the innate immune system, termed antimicrobial polypeptides (see ‘Cutaneous immunosurveillance, Langerhans’ cells, and cytokines‘, below), have been isolated in the vernix and probably play an important role in surface defense in the newborn.4,6,7 This coating persists for the first several days of postnatal life, eventually disappearing completely to reveal the more typical, moderately dry newborn skin. Vernix provides water-binding free amino acids, which may help to facilitate the neonate’s adaptation from the amniotic fluid intrauterine milieu to the ambient dryness of the extrauterine environment.8 Vernix-based topical creams have been investigated for treatment of epidermal wounds and augmentation of barrier repair in infants.9
The structure of term newborn skin is histologically similar to that of older individuals, whereas premature infant skin reveals several unique features that have increased our understanding of fetal skin development. The outermost compartment of the skin, the epidermis, arises from surface ectoderm and at about the 3rd week of fetal life, consists of a single layer of undifferentiated cells that becomes two-layered by around 4 weeks.10 The outer layer of cells, the periderm, is found only in developing skin and is transiently present, eventually undergoing a series of apoptotic cellular events as the epidermis becomes multilayered and the stratum corneum, the outermost layer of flattened, non-nucleated skin cells, is forming.11 By 24 weeks’ gestation, the periderm is largely absent,10,11 and the epidermis shows considerable progressive maturation, which is largely complete by 34 weeks.12 A thin, hydrophobic layer of the periderm may persist for several days postnatally and may participate in protective and thermoregulatory functions.13
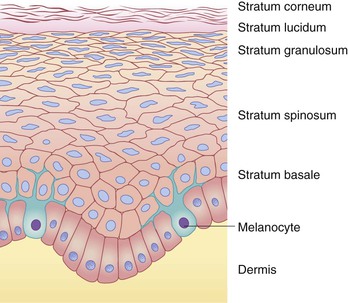
The epidermis is a stratified epithelium, the number of cell layers varying between different body regions. The various layers, from the dermal side toward the skin surface, are termed the stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. In areas of thicker skin, such as the palms and soles, the stratum lucidum is interposed between the granular and corneal layers. These epidermal layers are shown in Figure 2.2.
Individual cells within the epidermis are referred to as keratinocytes, so named for the intermediate-sized filament proteins (keratins) that are synthesized within them. Keratins (K) are the major structural proteins of the epidermis and its appendages, constituting up to 85% of the total protein of fully differentiated epidermal keratinocytes.14 They have been divided into types I and II based on their acidic or basic nature, respectively, and are frequently configured in specific pairs of a type I and a type II protein as obligatory heteropolymers.15 Terminal differentiation of the epidermis involves the sequential expression of different proteins, including the keratins, in the basal and spinal layers.16 An important function of the keratins is imparting mechanical integrity to epithelial cells. Mutations in the genes encoding these proteins have been confirmed as the basis of several inherited skin defects, such as the simplex form of the mechanobullous disease, epidermolysis bullosa.14 The profiles of epithelial cell keratins change during gestation: K5 and K14 (basal cell keratins) are present from 8 weeks’ gestation, K1 and K10 (differentiation-specific keratins) begin to be expressed between 9 and 10 weeks’ gestation, and some keratins (such as K8 and K19) are present in the fetus but not in adult epidermis.17
The stratum basale consists of a single layer of cells, the basal portions of which are in contact with the dermis and contribute to the DEJ. The cells of the basal layer are cuboidal to columnar in shape and are anchored to the underlying dermis by cytoplasmic processes. The stratum basale has an undulating surface inferiorly, forming projections called rete ridges, which lie interposed between the dermal papillae of the superficial (papillary) dermis (Fig. 2.3). The basal cell layer contains cells that eventually replace those continually lost from the epidermis through terminal differentiation, maturation and desquamation. Interspersed among the cells in the basal cell layer are the dendritic, pigment (or melanin)-producing cells (melanocytes), which are discussed in more detail below (‘Melanocytes and pigmentation of the skin’).
The stratum spinosum consists of the cells between the stratum basale and the stratum granulosum and forms the bulk of mammalian epidermis. The keratinocytes in this layer are polyhedral in shape and have numerous tiny, spiny projections spanning the intercellular space between contiguous cells.18 These projections are composed ultrastructurally of desmosomes, which form communication junctions between the cells. Keratinocytes of the spinous layer become larger, flatter, and more desiccated as they progress from the basal layer toward the skin surface. Also present in this layer are Langerhans’ cells, bone marrow-derived cells that are involved in cutaneous immunosurveillance through antigen processing and presentation (see ‘Cutaneous immunosurveillance, Langerhans’ cells and cytokines‘, below).
The stratum granulosum comprises a thin layer of darkly stained keratinocytes at the outermost surface of the stratum spinosum. The dark appearance of these cells is due to the presence of keratohyalin granules, which are composed of an electron-dense protein (profilaggrin) and keratin intermediate filaments.19 Profilaggrin is subsequently converted to filaggrin, a protein involved in the aggregation and disulfide bonding of keratin filaments,20,21 and it has been suggested that keratohyalin serves to form a matrix that provides structural support by linking keratin filaments to one another.18 Filaggrin eventually is degraded into free amino acids, including histidine and glutamine, which are further metabolized into urocanic acid (UCA) and 2-pyrrolidone-5-carboxylic acid (PCA). These free amino acids and their by-products constitute a significant component of natural moisturizing factor (NMF), which is retained in non-nucleated keratized cells (corneocytes) and helps maintain epidermal hydration.22 The granular cell layer is also where lamellar bodies (lamellar granules, Odland bodies, membrane-coating granules) are produced.23 These intracellular organelles participate in the formation of the epidermal permeability barrier through the production and discharge of lipid substances into the intercellular corridors of the stratum corneum. Defective lipid transport in lamellar bodies caused by mutations in ABCA12 underlies the severe skin disorder known as harlequin ichthyosis.24 In areas of thicker skin, such as the palms and soles, the stratum lucidum is present as a layer with a clear hyaline appearance. At this level one can visualize transitional cells that exhibit marked degeneration of the nucleus and other organelles and, ultramicroscopically, keratin filaments and keratohyalin granules, which are abundant but not yet as compact as in the stratum corneum.18
The stratum corneum, or cornified layer, is composed of several layers of flattened corneocytes arranged in an overlapping fashion. The thickness of this layer varies by body region, being thinnest on the face (especially over the eyelids) and genitalia, and thickest on the palms and soles. It is now widely accepted that the epidermal permeability barrier resides in the stratum corneum and serves the vital functions of preventing excessive transepidermal water loss (TEWL) and preventing penetration of a variety of substances.25–29
The formation of the epidermal barrier is accomplished through the lipid secretions of lamellar bodies, which include free fatty acids, ceramides, and cholesterol. These lipids are deposited in the intercellular interstices within the stratum corneum. This arrangement has been likened to ‘bricks and mortar,’ where the corneocytes represent the bricks and the intercellular lipids represent the mortar.30 Although these lipids represent only about 10% of the dry weight of the stratum corneum31 their location and composition are vital, and cutaneous barrier function is dependent on both the generation of sufficient quantities of these lipids and their strategic secretion and organization into lamellar bilayer unit structures.29,30,32–34 In fact, the epidermis is equipped with the necessary machinery to autonomously regulate its lipid-synthesis apparatus in response to specific barrier requirements.35–37 The development of a functional barrier has been shown to be closely correlated with normal ontogenesis and does not appear to be disrupted by somatic growth retardation.38 Hence, more mature infants, even those who are small for gestational age, have a competent epidermal barrier.39
The epidermis and stratum corneum in the full-term infant are well developed, and the barrier properties are excellent.40 Conversely, premature infants have greater skin permeability and a more poorly functioning barrier. Histologically, the term infant has a well-developed epidermis, which is several layers thick, and a well-formed stratum corneum.2,12 This maturity is lacking in preterm infants.40–44 An acceleration of skin maturation may occur postnatally in preterm infants, although in extremely low-birthweight infants (23–25 weeks’ gestational age), complete development of a fully functional barrier may require up to 8 weeks.41,42,45 Studies support the long-held notion that the shift from an aqueous to an air environment, and hence water flux, may be an important factor in this acceleration of barrier formation.46 The nuclear hormone receptors peroxisome proliferator-activated receptor (PPAR-α, -δ, -γ) and their ligands have varied roles in driving the development of the stratum corneum and permeability barrier in the fetus as well as in neonates and adults.47 Functional skin adaptation is an ongoing dynamic process involving acidification, water management and permeability barrier development throughout the first year of life and perhaps beyond. The ability to restore the epidermal barrier declines in adulthood.48,49 During the period of postnatal barrier maturation, large transepidermal water losses contribute to the morbidity of the preterm infant, and therefore a major focus of past studies has been the development of a therapeutic strategy to accelerate epidermal barrier maturation or augment its function, including the use of semipermeable membranes50–53 or topical emollients.54,55 Skin surface pH is another important consideration, as acidification is vital to epidermal barrier maturation, and the ‘acid mantle’ also plays a role in maintaining bacterial and chemical resistance of the skin. While no definitive relationship between gestational age and skin pH has been confirmed, studies have shown that skin pH is higher (more alkaline) immediately after birth, and decreases (becomes more acidic) over the first few weeks of postnatal life.56 Premature infant skin and barrier maturation are discussed in more detail in Chapter 4.
In addition to the prevention of insensible water losses across the skin by the epidermal barrier, the epidermis and stratum corneum of the newborn provide important protection against toxicity from exposure to ultraviolet rays (UVR), and this protective effect may be greater for UVB than for UVA radiation.57 As previously noted, melanin is primarily responsible for UVR protection, although the ‘protein barrier’ of the stratum corneum may augment this cutaneous function.58 Epidermal lipids may also play a role in protection from UVR. Another function of the superficial skin layers is protection against microorganisms, which are blocked from invasion across the skin by an intact stratum corneum. In addition to such physical factors, the antimicrobial qualities of skin may be related to the relative dryness of the stratum corneum, the presence of skin surface lipids, and the degree of epidermal cellular differentiation.58–61 Skin is also a vital participant in the process of neonatal thermoregulation (discussed in more detail later) through regulation of cutaneous blood flow and evaporative water loss.
Percutaneous absorption of substances across neonatal skin requires passage through the stratum corneum and epidermis, diffusion into the dermis, and eventual transfer into the systemic circulation. Transfer across the stratum corneum and epidermis may be through the intercellular corridors (favoring nonpolar or hydrophobic compounds) or via a transcellular route (which favors polar or hydrophilic substances).62 Hair follicles and eccrine sweat ducts may serve as diffusion shunts for certain substances (i.e. ions, polar compounds, very large molecules), which would otherwise traverse the stratum corneum slowly (because of their large molecular weight).63 The rate-limiting step of percutaneous absorption seems to be diffusion through the stratum corneum,63 and hence the effectiveness of the epidermal permeability barrier correlates inversely with percutaneous absorption. Percutaneous absorption, although continuously being explored for therapeutic applications, may contribute to systemic absorption and potential toxicity after topical application of some substances to newborn skin, especially in preterm infants or those with cutaneous damage.41 Importantly, although the barrier function of intact skin in the term infant is usually normal, the surface area-to-weight ratio is greater than in older children and adults. Caution should therefore be exercised in the use of topical agents in any newborn, with extra caution and a thorough risk–benefit analysis being employed in the case of premature infants or any neonate with a compromised skin barrier. Percutaneous absorption is discussed in more detail in Chapter 5.
Dermoepidermal junction
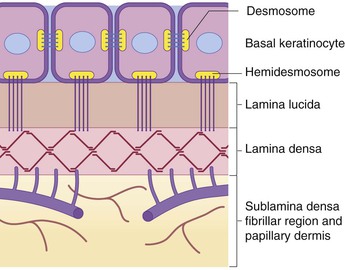
The dermoepidermal junction (DEJ) is an important site of attachment in skin, occurring at the interface between the basal epidermis and the papillary dermis. It appears that the various components of the DEJ are expressed in term newborn skin in a manner similar to that in adults, without apparent differences in their quantity or associations.2 For reasons that are poorly understood, however, skin appears to be more fragile during the newborn period, even in term infants, as evidenced by blisters or erosions developing in situations that do not cause blisters later in life (e.g., erosions due to diapering, sucking blisters on fingers and hands, and disease states such as bullous syphilis).
Specialized structures called ‘hemidesmosomes’ assist in anchoring the basal keratinocytes to the underlying plasma membrane. Ultrastructurally, the DEJ can be broken down into several planes, including (from the epidermal side to the dermal side) the inferior portion of the basal keratinocyte; an empty-appearing, electron-lucent clear plane known as the lamina lucida; a thin, dark, electron-dense layer known as the lamina densa; and the sublamina densa fibrillar region (Fig. 2.4).19,64 Each of these layers contains individual components that function harmoniously in concert to create cohesion between the epidermis and the underlying dermis. Defects in, or antibodies directed against, some of these components have been etiologically linked to cutaneous disease.
Major constituents of the DEJ include bullous pemphigoid (BP) antigens, α6β4 integrin, laminin-5 (laminin-332), type IV collagen, and type VII collagen. The BP antigens are large glycoproteins with both intracellular (BP antigen 1) and transmembrane (BP antigen 2) components. BP antigen 2, also known as collagen type XVII, extends from the basal keratinocyte across the lamina lucida into the lamina densa,65 and autoantibodies directed against it have been found in the sera of patients with BP, pemphigoid gestationis, mucous membrane pemphigoid, linear IgA disease, lichen planus pemphigoides, and pemphigoid nodularis.66,67 Reduced or absent expression of BP antigen 2 is found in patients with a hereditary junctional form of epidermolysis bullosa (EB) termed junctional EB-non-Herlitz, and has been described in a rare variant of EB simplex.67–71
α6β4 integrin is a membrane glycoprotein component of the hemidesmosome, and defects in this integrin have been identified in a subset of patients with junctional EB in combination with pyloric atresia.72–75 Laminin-5 is a glycoprotein localized mainly to the lamina densa and lower lamina lucida,76 and is also associated predominantly with hemidesmosomes.77 Mutations in the genes encoding various chains of laminin-5 have been identified in patients with the lethal (Herlitz) junctional type of EB.78–81
Type IV collagen predominates in the lamina densa region, whereas type VII collagen, which is also known as the epidermolysis bullosa acquisita (EBA) antigen, is situated in the zone beneath the lamina densa. EBA antigen was so named because it was first defined by circulating autoantibodies in the sera of patients with EBA, an acquired autoimmune blistering disease.82 The dystrophic forms of inherited EB have been shown to be a result of defects in the gene encoding type VII collagen.83
Dermis and subcutaneous fat
The dermis of human skin consists primarily of connective tissues, including proteins (collagen and elastic tissue) and ground substance. This compartment lies between the epidermis superiorly and the subcutaneous fat inferiorly and forms a resilient and flexible layer that envelops the entire organism. It is divided into superficial (papillary) and deep (reticular) components, which are anatomically divided by a thin plexus of blood vessels. Although differentiation between these dermal compartments can be ascertained on the basis of the size of the collagen fiber bundles in adult skin, this criterion is less helpful in newborn skin, where there is a more gradual transition in fiber bundle size.2 Structures found within the dermis, which are discussed in different sections of this chapter, include the cutaneous appendages (pilosebaceous units, eccrine and apocrine sweat glands), as well as nerves, blood vessels, and lymphatics.
Collagen is the major constituent of mammalian dermis and accounts for approximately 75% of the dry weight of the skin.19 The collagens are a family of related, yet individually distinct, structural proteins, and in the skin, they provide tensile strength and elasticity. Types I and III collagen are the major collagens found in human dermis, and smaller amounts of types IV (a primary component of the basement membrane as noted above), V, VI, and VII are also present.84 Some 80–90% of dermal collagen is type I. Type III collagen was initially termed ‘fetal collagen’ because of its predominance in fetal tissues, where it accounts for over half of total skin collagen. However, synthesis of type I collagen accelerates during the postnatal period, and eventually, the ratio of type I to type III collagen increases, such that in adult skin it is around 5 : 1–6 : 1.85 Abnormalities in collagen synthesis or post-translational processing may result in clinical disease, including osteogenesis imperfecta and the Ehlers–Danlos syndromes.
Elastic fibers play an important role in the structure and function of skin, providing elasticity and resilience. They consist of two components: elastin, which is a connective tissue protein, and elastic fiber-associated microfibrillar component, a complex of glycoproteins.84 Elastic fibers are distributed in the papillary and reticular dermis. Fibers in the papillary dermis have been subdivided into elaunin fibers, which are oriented parallel to the DEJ, and oxytalan fibers, which connect the elaunin fibers to the DEJ.1 It has been demonstrated that elastic fibers are distributed in the term newborn dermis in a manner similar to that of the adult, albeit with a decreased elastin content in the papillary dermal bundles, and with a finer fiber diameter in the reticular dermis.2 The most widely recognized disease related to abnormalities in elastin production is cutis laxa, a heterogeneous group of disorders featuring lax skin and occasional systemic involvement in the form of hoarseness, emphysema, hernias, and diverticulae.86
The ground substance of the dermis is an amorphous material that surrounds and embeds the fibrous and cellular components found in this compartment. Glycosaminoglycans (GAGs), which are long chains of aminated sugars, and proteoglycans (PGs), which are large molecules consisting of a core polypeptide linked to GAGs, are major constituents of ground substance.1,19 Major GAGs and PGs in the dermis are chondroitin sulfate, dermatan sulfate, heparin/heparin sulfate, chondroitin 6-sulfate, and hyaluronic acid (hyaluronan).1,19,87 These components are capable of retaining large amounts of water and may also play a role in binding growth factors and providing structural support, anticoagulation, and adhesion.1,88,89 Hyaluronic acid has been demonstrated in large amounts in fetal dermis and amniotic fluid and is thought by some to be associated with the rapid wound healing without scarring that has been observed to occur in fetal wounds.90 These observations have been applied to the study of diabetic ulcers, where hyaluronic acid levels have been shown to be decreased, leading to the hypothesis that application of this substance may induce healing.91 Fibronectin is a large glycoprotein also found in the dermis and is associated with a variety of putative functions, including organization of the extracellular matrix, wound healing, attachment, and chemotaxis.1,19 More recent evidence suggests that dermal extracellular matrix components (fibronectin and chondroitin sulfate) and possibly the paucity of elastin compared with adult skin, as well as circulating amniotic stem cells, may play a role in fetal scarless healing.92
The subcutaneous fat is an important layer, playing a role in shock absorption, energy storage, and maintenance of body heat. The individual cells in the subcutaneous fat – adipocytes – form lobules that are separated by fibrous septa. The fibrous septa contain neural and vascular elements and connect deeper with the fascia of underlying skeletal muscle. In contrast, brown adipose tissue (BAT or brown fat) is a distinct type of adipose tissue, traditionally believed to be present only in newborns, that plays a vital role in neonatal thermoregulation (discussed in more detail later) through the oxidation of fatty acids.93 BAT makes up 2–6% of the neonate’s total body weight and is found primarily in the scapular region, the mediastinum, around the kidneys and adrenal glands, and in the axilla.94 The nonshivering thermogenesis that occurs in this tissue appears to be regulated by the enzyme-uncoupling protein thermogenin (more recently known as uncoupling protein 1 or UCP-1), which serves as a protonophore through the mitochondrial membrane, enabling high rates of cellular respiration and proton conductivity.95 BAT is believed to be depleted over time, although recent studies suggest that functionally active BAT is present in at least some adults.96
Pilosebaceous units, apocrine glands, and nails
Hair follicles
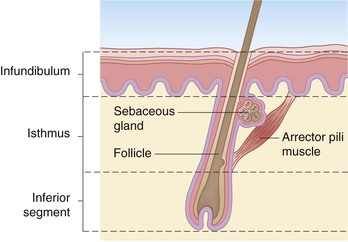
The earliest hair follicles begin to form at 9–12 weeks’ gestation97 primarily in a facial location, and the bulk of the remaining hairs start developing around 16–20 weeks, progressing in a cephalocaudad fashion.97,98 In some full-term infants, and especially in premature infants, the skin surface is covered with lanugo hairs, which are soft, fine hairs with limited growth potential.2 These hairs are usually shed by term, or shortly thereafter, and are replaced by vellus hairs, which are eventually replaced on the scalp by coarse terminal hairs. The growth of a hair follicle is cyclic, the stages being divided into anagen (active growth), catagen (transitional involution), and telogen (resting) phases. The typical length of each of these phases is 2–5 years, 3 days, and 3 months, respectively.98 No new hair follicles are formed after birth. The majority of hairs present at birth are synchronized in their growth phase.3,99 However, the initiation of hair production occurs in waves, such that follicles in the frontal and parietal regions of the neonate are already converting to the telogen phase, whereas occipital scalp hair progresses towards the telogen phase between 8 and 12 weeks’ postnatal age.100 This contributes to the frequent appearance of temporary occipital alopecia in young infants. The hair follicle is organized into a series of concentric cellular compartments, the details of which are beyond the scope of this chapter. The structure of a pilosebaceous unit is depicted in Figure 2.5. Longitudinally, the hair follicle can be divided into three zones: the infundibulum, extending from the opening of the follicle to the entrance of the sebaceous duct; the isthmus, extending from the entrance of the sebaceous duct to the insertion of the arrector pili muscle; and the inferior segment, which forms the remainder of the follicle from the insertion of the pili muscle to the base. A subpopulation of hair follicle keratinocytes has been identified in the upper follicle near the insertion site of the arrector pili.101,102 This area has been termed ‘the bulge’, and these cells may be involved not only in the regeneration of the anagen hair follicle, but also in the long-term maintenance of the epidermis.103 Within the specialized environment of the bulge are multipotent stem cells, Merkel cells, and melanocytes, which are thought to interact, leading to the differentiation of stem cells into the components of the hair follicle, sebaceous gland and epidermis.104–107 The exact signaling and control of these stem cells is not known, but it appears that Lhx2 transcription factor plays an important role in their regulation in addition to adhesion molecules, epidermal growth factor, nerve growth factor, and platelet-derived growth factor.106,107 Lhx2 has been shown to have a role in regulation of these bulge region stem cells in embryonic and postnatal hair follicle growth and in wound healing.107–109 The integrity of the hair shaft is related to its protein constituents, including the intermediate filament hair keratins and high-sulfur proteins, and to the strong disulfide bonding between these proteins.98 In neonates, hair may be a source of valuable clinical information: neonatal hair shaft analysis as a marker for intrauterine exposure to drugs of abuse having emerged as a useful tool over the last decade.103–115
Sebaceous glands
Sebaceous glands begin to develop between 13 and 15 weeks of fetal life.116 They are nearly always associated with hair follicles and are found diffusely in the skin, except on the palms, soles, and dorsal feet.117 The locations of the most prominent glands are the face and scalp, and in term neonates, may be quite evident over the nose, forehead, and cheeks. Modified glands are found in the skin of the nipples and areolae (Montgomery’s tubercles), on the labia minora and prepuce (Tyson’s glands), on the vermillion border of the lips (Fordyce’s condition), and in the eyelids (meibomian glands). Sebaceous glands are well formed at birth and are quite active during the neonatal period, when they are stimulated by transplacentally derived steroid hormones and possibly by endogenous steroid production.3 This sebaceous activity in the newborn is reflected by the common finding of neonatal acne. Sebum, the substance produced by the holocrine sebaceous glands, is a composite of triglycerides, wax esters, squalene, cholesterol, and cholesterol esters and serves a role in lubrication of the follicle and epidermal surface.1 Sebum levels sharply decline over the first year of life,118 putatively in response to diminished levels of circulating hormones. The glands then remain relatively quiescent, producing only small amounts of sebum, until puberty.2
Apocrine glands
The apocrine glands are limited in distribution and are found primarily in the axillae, areolae, mons pubis, labia minora, scrotum, perianal area, external ear canal, and eyelids (Moll’s glands).117 Their function in humans is unclear, although they may serve as scent glands. Apocrine glands remain small until puberty, when they enlarge and begin the process of secreting a milky white fluid. Body odor in postadolescent individuals is related to bacterial action on these secretions.
The nail
The nail acts as a hard, protective covering over the distal end of the digit and may have served a function in evolution to assist in grasping small objects. The nail unit is depicted in Figure 2.6. The nail plate consists of cornified cells with a high protein content (primarily keratin) and is produced by the matrix, a cellular zone situated underneath the proximal nail fold at the base of the nail. The nail plate is situated on top of the nail bed, a highly vascular zone. The lateral nail folds consist of skin that envelops the lateral borders of the nail plate. The average growth rate of the human fingernail is 0.10–0.12 mm/day and appears to be greatest during the second decade of life.119 Toenails, which grow at a slower rate, may appear to be abnormal or ‘ingrown’ in newborns as a result of relative nail plate hypoplasia with a bulbous distal phalanx.120 Despite their abnormal appearance, these nails eventually grow out and take on a more normal appearance.
Eccrine glands and neonatal sweating
Eccrine sweating is a physiologic response to increased body temperature and is the most effective means by which humans regulate their body temperature through evaporative heat loss.121 Gestational age, postnatal age, and body site are all important variables with regard to eccrine glands, and much of what is known about the process of neonatal sweating has been learned from studies of the normal physiologic eccrine gland responses of term and preterm neonates to various sweat-inducing stimuli.
Eccrine sweat glands first appear during fetal development at 14 weeks and are initially limited to the volar surface of the hands and feet.122 They then appear in the axillae and eventually in a generalized distribution, with a full complement of anatomically normal glands present by the 28 weeks’ gestation, although functionally, the glands are immature until 36 weeks’ gestation.123 The total number of eccrine sweat glands is formed before birth112 and is estimated to be between 2 and 4 million.122
The eccrine sweat gland consists of two segments: a secretory coil and a duct. The secretory coil is composed of secretory cells and myoepithelial cells, the latter being contractile cells with smooth muscle-like characteristics.122 The duct is composed of two cell layers, the basal and luminal ductal cells, which are involved in secretion and reabsorption of solutes. Components of eccrine sweat include water, sodium, chloride, potassium, urea, lactate, and ammonia.122 Although newly formed sweat is isotonic, reabsorption of water and solutes occurs in the duct, such that the expelled product is hypotonic. Evaporation of sweat from the surface of skin removes 0.58 calories of heat for each gram of water that evaporates.124
Eccrine sweat glands are innervated anatomically by fibers of the sympathetic nervous system, although functionally, they are under cholinergic influence, and acetylcholine is the major neurotransmitter released from the periglandular nerve endings.122 Circulating catecholamines can also have a stimulatory effect on eccrine sweat production,124 as can a variety of other peptides or neurotransmitters.
Sweating can be induced by pharmacologic stimulation and by emotional or thermal stress, and all mechanisms appear to be developed to some extent at birth in term infants. Levels of sweat production in response to the intradermal injection of pharmacologic agents have been demonstrated to bear a direct relation to gestational age,125–128 as well as to birthweight.125 Thermal stress-induced sweating, although present in infants, appears to require a greater thermal stimulus in neonates than in adults, and this response also appears to be less developed in premature infants,128–132 but increases with postnatal age.130 However, the thermal stimulus of sweating is an important contributor to increased insensible water loss in certain infants at risk, such as those treated with phototherapy for hyperbilirubinemia133 and those under radiant warmers.134,135 The core temperature at which sweating begins in full-term newborns has been estimated at around 37.2°C.136
‘Emotional sweating’ also appears to be well developed at birth in full-term but not premature neonates.123 In one study, skin conductance after heel prick for routine blood testing rose sharply, and to a greater extent, in infants of more advanced gestational ages,137 supporting the role of postconceptual age in maturation of the sweating response to emotional stress. Another study using auditory stimuli revealed that the sympathetic nervous system innervating the eccrine glands developed over the first 10 weeks of life.138
The process of neonatal sweating, therefore, appears to develop early anatomically in fetal life and functionally at later stages, and the sweating response appears to be well developed at birth in term but not preterm infants. Hypotheses on the potential mechanisms for progressive postnatal maturity of the sweating response include anatomic development of the sweat gland, functional development of the gland, or nervous system maturation.130
Nerves, vascular networks, and thermoregulation
The cutaneous neural and vascular networks both develop early in the fetus, and their architecture becomes organized into adult patterns with increasing postnatal age.2 Nerve networks in the skin contain both somatic sensory and sympathetic autonomic fibers and function as innervation for arrector pili muscles, cutaneous blood vessels, and sweat glands, as well as serving as receptors for touch, pain, temperature, itch, and mechanical stimuli. Large myelinated fibers, which are cutaneous branches of musculoskeletal nerves, innervate the skin in a pattern similar to that of vascular supply, whereas sensory nerves follow segmental dermatomes, which often show some overlap. Although cutaneous nerve fibers in the neonate are similar in structure and distribution to those in the adult, ultramicroscopic examination has revealed a higher percentage of unmyelinated fibers with bundling of axons, suggesting cytoarchitectural immaturity or incomplete growth.139
Sensory cutaneous nerves may end freely or in encapsulated terminals. Free nerve endings in skin represent the most important of sensory receptors and include penicillate fibers found in a subepidermal location in hairy skin,140 multiple types of free endings in digital (non-hairy) skin,141 and papillary nerve endings found at the orifice of hair follicles.19 Free nerve endings may also be associated with Merkel cells, neurosecretory cells of uncertain biologic significance that are of epithelial derivation and which become scarce in human skin after fetal development.2,142,143 Studies suggest that Merkel cells may actually be trophic for developing nerves and therefore play an inductive role in the development of the human cutaneous nerve plexus.144 Specialized sensory receptors are present to varying degrees at birth, including Pacinian corpuscles, which are well developed and abundant in palm and sole skin, and Meissner’s corpuscles, which are not fully formed and undergo continued morphologic changes with age.2
The vasculature of human dermis comprises two plexuses that parallel the skin surface: one in the lower dermis (deep plexus) and one just beneath the papillary dermis (superficial plexus).117 These two systems are connected by intercommunicating vessels, and vertical vessel arcades project superiorly from the superficial plexus toward the epidermis to form papillary loops (Fig. 2.7). This subpapillary plexus also gives rise to vessels that infuse the periadnexal structures.117 The cutaneous vascular system also contains arteriovenous shunts, or glomi, which are specialized anastomoses that assist in the regulation of skin blood flow and thermoregulatory shunting.3,129 The cutaneous capillary network is fairly disordered at birth and assumes a more orderly network pattern by the second week of life,145 with continued development until around 3 months.146
Vasomotor tone is under the control of a complex series of neurogenic, myogenic, and pharmacologic mechanisms,3 and the ability to control skin blood flow is now known to be well developed in neonates.147 It was previously suggested that skin blood flow and total peripheral blood flow both correlate inversely (and decrease) with increasing birthweight, gestational maturity, and postnatal age, along with the development of increasing peripheral vascular resistance.148 However, studies of capillary blood cell velocity (CBV) in full-term infants have demonstrated a correlation between CBV and postnatal age, making the significance of previous microvascular findings in the neonate unclear.149
Thermoregulation, which maintains an equilibrium between heat production and heat loss, is a crucial requirement in the neonate for maintenance of optimal core body temperature. It is a complex physiologic process under the control of the nervous (most importantly the hypothalamus) and endocrine systems. Although the thermoregulatory response is present in both term and preterm neonates, it is more pronounced in term infants.150 The primary contributors to thermogenesis are muscles (voluntary and involuntary, or ‘shivering’ thermogenesis), sweat glands, blood vessels, and adipose tissue.151 Heat loss, or thermolysis, is accomplished by flow of heat from the center of the body to the surface and, subsequently, flow of heat from the body surface to the environment.151 Heat transfer to the surroundings can be accomplished through conduction (thermal exchange between the body surface and objects with which it is in contact), convection (heat loss from mass flow of moving air over the body surface), or radiation (electromagnetic heat loss to cool surfaces within the environment). Water evaporation, the fourth mechanism of heat loss, is discussed in the section on neonatal sweating, above.
Thermal stimuli providing information to the hypothalamus are transmitted from skin thermal receptors, as well as from deeper receptors present in the abdominal cavity and central nervous system.151,152 In general, increased environmental temperature results in cutaneous arteriolar vasodilatation and heat dissipation, whereas cold stress leads to vasoconstriction, with resultant decreased skin blood flow and reduced heat loss from the body core. Heat production in the neonate is accomplished primarily through nonshivering thermogenesis, which utilizes the increased number of mitochondria, increased glycogen stores, and abundant blood supply of brown adipose tissue.152 The primary mechanism utilized by the overheated neonate to dissipate heat is evaporative water loss through sweating.
Although temperature regulation is developed to some extent in most infants, they are susceptible to both cold and heat stress. Transition out of the stable thermal environment of the uterus, as well as birth trauma, malformations, drugs, and respiratory deficiency, may predispose the newborn to hypothermia, whereas birth trauma and exogenous sources of heat may lead to hyperthermia.151 Studies of both full-term and preterm infants reveal a decreased ability to vasoconstrict blood vessels in the extremities following exposure to cool temperatures, further predisposing infants to hypothermia.136,153 Extremely low birthweight infants (400–1000 grams, with a corresponding gestational age of around 23–28 weeks) were shown to largely lack the ability to peripherally vasoconstrict in the face of hypothermia during the first 12 h of life. This finding likely reflects immature autoregulation, and may extrapolate to cerebral blood flow and explain the increased susceptibility of this population to brain injury.154 Thermoregulation is a multifaceted process, which at times may be inadequate in the maintenance of the homeothermic state in the neonate. An understanding of these processes is therefore vital for providing appropriate thermal support to such infants.
Melanocytes and pigmentation of the skin
As mentioned, interspersed among the basal layer cells are the dendritic, melanin-producing cells called melanocytes. These cells first appear between a gestational age of 40 and 50 days and migrate to the skin from the neural crest.155 Whereas melanocytes are found in both basal and suprabasal locations during embryogenesis, neonatal skin reveals a more limited distribution restricted to the basal epidermal layer.156,157 Melanin is manufactured within organelles called melanosomes, which are formed in melanocytes and transferred to neighboring keratinocytes via dendritic connections. Each melanocyte is in contact with roughly 36 keratinocytes, an association that is referred to as the epidermal melanin unit. The transfer of melanin from the melanocyte to the keratinocytes within this unit results in pigment being distributed in the basal layer, as well as more superficially, where melanin serves a protective role by absorbing and scattering ultraviolet radiation (UVR).19
Two forms of melanin are present in human skin: eumelanin, which is brown, and pheomelanins, which are red and yellow.130,158 Differences in native skin pigmentation among individuals are related to the concentration, as well as the distribution and retention, of melanin in the basal cell layer, rather than to the absolute number of melanocytes.1,159,160 Although melanocytes in newborn skin are quantitatively comparable with those in older individuals, melanin production, and hence skin pigmentation, is relatively decreased during the neonatal period,2,3 with gradual darkening over several months following birth. Several disorders of either increased or decreased pigmentation, as well as proliferation of melanocytes, are seen in the newborn period. These include disorders such as albinism, piebaldism, café-au-lait macules, congenital nevi, and others. Disorders of pigmentation and melanocytes are discussed in Chapters 23 and 24.
Cutaneous immunosurveillance, Langerhans’ cells, and cytokines
Cutaneous immunosurveillance
While participating in the important roles of physical protection, barrier function, and thermoregulation, the skin also occupies a niche in the immunologic system of the host as a peripheral immune organ. Various models and terms have been used to describe the immunologic capacities of the skin, including skin-associated lymphoid tissues (SALT), skin immune system (SIS), dermal microvascular unit (DMU), and dermal immune system (DIS).161,162 SALT are composed of epidermal Langerhans’ cells and keratinocytes, as well as dermal endothelial cells and the skin-draining lymph nodes, and are an important system in the induction of immunity and tolerance.162 The broader terminology of the SIS refers to the entire complex interplay of immune response-related systems in the skin, including cellular components and humoral factors,162,163 and both dermal and epidermal components.
These immunologic systems in the skin provide cutaneous immunosurveillance, which functions in the prevention of the development of cutaneous neoplasms and mediates against persistent infections with intracellular pathogens.164 Cellular components include keratinocytes, antigen-presenting cells (APCs), monocytes and macrophages, granulocytes, mast cells, lymphocytes, and endothelial cells, whereas humoral constituents include antimicrobial peptides, complement proteins, immunoglobulins, cytokines, and prostaglandins.162 Antimicrobial peptides and proteins are an important innate cutaneous defense mechanism against microbial intruders. They have a broad-spectrum killing activity, and their presence in both amniotic fluid and vernix caseosa has been well documented, suggesting that effective innate immune protection begins during fetal and early neonatal life.4,6,165,166 Human antimicrobial peptides include the cathelicidin, dermacidin and β-defensin families. Regulation of these peptides may involve Ca2+, 1, 25(OH)2VD3, retinoic acids, and kallikreins.167,168
Characterization of lymphocyte populations within normal human skin has revealed that they are predominantly T cells, with 90% of cells clustered around postcapillary venules or adjacent to cutaneous appendages.163,169 Intraepidermal localization of T lymphocytes accounts for less than 2% of skin lymphocytes normally present. B lymphocytes are not present in normal human skin, but may be found in mucosal locations.
Langerhans’ cells
The cell that sets the SIS apart from others is the Langerhans’ cell (LC). This APC resides in the epidermis and is involved in skin allograft rejection, delayed hypersensitivity reactions, and specific T-cell responses.170 LCs are derived from the bone marrow and migrate via a hematogenous route to the skin. They are present in the fetus as early as 16 weeks’ gestation, with early restriction to the basal layer and eventual distribution among suprabasal cells.171
The function of the LC was unclear until the 1970s, when surface Fc receptors, major histocompatibility complex (MHC) class II molecules, and C3 receptors were described on its surface,164 suggesting an immunologic role. It is now well accepted that the epidermal LC is involved in antigen processing and presentation in a variety of skin-induced immune responses against a variety of antigens, including contact allergens, alloantigens, tumor antigens, and microorganisms.172 These cells have been found to have positive staining for other characteristic surface markers, including CD1a and S100 proteins and membrane-bound adenosine triphosphatase (ATPase).172 Although the exact function of the CD1a glycoprotein remains unclear, relatively weak expression of the antigen on LCs from neonatal skin has been demonstrated173 and may partially explain why neonatal donor skin demonstrates extended survival compared to adult donor skin after transplantation in animal models.174–176 Ultrastructurally, LCs are found to contain Birbeck granules, distinctive cytoplasmic organelles with central striations and a characteristic ‘tennis racket’ appearance on thin electron micrograph sections.170 Although the exact function of these granules is unknown, it has been suggested that they may be involved in receptor–ligand interactions and surface antigen trafficking.177
LCs are a member of the dendritic family of cells, which are stellate cells with cytoplasmic extensions, or dendrites. Other dendritic APCs are present in human skin, including dermal dendritic cells, which also contribute to the surveillance function of the immune system and initiation of the primary immune response. These cells were also shown to express high levels of MHC class II molecules, as well as factor XIIIa, and are isolated primarily from the dermis.178 Some dermal dendritic cells may acquire the ultrastructural characteristics of LCs and have been described to express Langerin (CD207), a C-type lectin receptor found on the surface of LCs and a major constituent of Birbeck granules.178,179 There are also Langerin-negative dermal dendritic cells. It appears that these different subtypes of dendritic cells may have independent and specific roles in presenting foreign substances and pathogens to the immune system.180 In neonatal murine skin, LCs are less effective at antigen presentation, corresponding to less T-cell activation and the promotion of immune tolerance.181
Cytokines
In addition to the role of such cellular components in cutaneous immunity, a complex interplay with several humoral factors is also present, including the biologic proteins known as cytokines. These autocrine, paracrine, endocrine, exocrine, and intracrine proteins include the interleukins (ILs), interferons (IFNs), colony-stimulating factors (CSFs), tumor necrosis factors (TNFs), and growth factors (GFs).163,172 They are produced by various cell types, including keratinocytes, which have been demonstrated to be capable of secreting several types of cytokines.163
Cytokines have multiple biologic functions and act on target cells by binding to specific receptors. The result of such binding is signal transduction to the cell interior followed by activation of various second-messenger pathways and eventual altered gene expression and cell function.172 For instance, on exposure to contact allergens, LCs may show enhanced migration after induction of local IL-1β production, ultimately resulting in activation and expansion of allergen-specific T-cell populations,162 whereas IL-10 inhibits the ability of LCs to stimulate T cells.170 Cytokines are involved in many cutaneous processes, both physiologic and pathologic, the details of which are beyond the scope of this chapter. Although not clearly elucidated, the secretion, activity, and effector functions of cytokines in neonates may differ from those in adults. In a study of immunity biomarkers, neonates ≤32 weeks’ EGA had significantly greater levels of proinflammatory cytokines (including IL-1β, IL-6 and IL-8) than full-term infants and adults.182 Another example is the newborn hypothalamic response to IL-1, also known as endogenous pyrogen. The synthesis of prostaglandins in response to this protein normally shifts the thermoregulatory set-point, resulting in fever, but this responsiveness is decreased in the neonate, which may account for the attenuated fever response in the setting of infection.158 Abnormalities in the control of proinflammatory cytokines may lead to a variety of clinical disorders, often grouped under the ‘cryopyrin-associated periodic syndromes’. Deficiency of the interleukin 1-receptor antagonist (DIRA) is one such condition, which presents as fetal distress, a severe pustular exanthem, joint swelling, bone lesions and pain in neonates, and has been successfully treated with anakinra, a recombinant human IL-1 receptor antagonist which blocks the effects of IL-1β.183
Access the full reference list at ExpertConsult.com ![]()