Chapter 25 Spinal Injection Techniques
Spine pain is a common reason for physician visits, as approximately one fourth of Americans report at least 1 day of low back pain in the last 3 months, and 14% have similar reports of neck pain.59 Low back pain is the main reason that patients younger than 45 years limit their activities.39,126 Although 90% of episodes of low back pain resolve within 6 weeks, there is a 60% to 80% rate of recurrence within 2 years.185
Direct costs of low back pain have been estimated to be between $20 and $50 billion per year, with indirect costs reaching more than $100 billion.58,147,185 In addition, there has been a large increase in utilization of invasive interventional procedures.77,200 Within the Medicare population, lumbar epidural injections increased by 271% and lumbar zygapophysial joint injections increased by 231% from 1994 to 2001.77
General Procedural Considerations
The absolute contraindications to spinal injections include pregnancy, infection within the procedural field, and the inability of the patient to give informed consent.25 Informed consent must be obtained from the patient before all spinal procedures. The patient must understand the purpose of the intervention as well as the associated risks.
Preprocedural Risk Assessment
Red Flags
Considering underlying medical conditions such as tumor, infection, inflammatory disease, or trauma as a cause of spinal pain is important before performing spinal procedures.60,151,176,187 Although the incidence of these conditions is low, any delay in diagnosis can worsen the prognosis.60 Red flags are signs associated with an increased likelihood of an underlying medical condition as a cause of pain. These include the following101,180,190:
Bleeding Risk
Coagulopathy
Spinal interventions carry an inherent risk of bleeding complications because of the highly vascular nature of the spine. The reported incidence of a clinically significant epidural hematoma is rather low (1 in 70,000 to 1 in 190,000).3,205 However, severe morbidity such as paraparesis or quadriparesis can be associated with an epidural hematoma.32,202
Identifying patients with an increased bleeding tendency is essential, and several guidelines exist for performing spinal procedures in patients with an increased bleeding tendency.97,121 Information including a medical and family history of coagulation disorders, a careful medication history to identify the use of anticoagulants, and a history of prolonged bleeding after trauma or prior surgery should be obtained from every patient. If a coagulation disorder is diagnosed and managed, spinal procedures may be considered after evaluation and disclosure of the expected risks and benefits.181,196
Antiplatelet Medication
Existing guidelines for spinal procedures in patients receiving antiplatelet and anticoagulant therapy vary by the type of medication.96,97,108,121 Nonsteroidal antiinflammatory drugs (NSAIDs), including aspirin, reduce platelet aggregation, but have not been implicated in increased bleeding risk or complications in lumbar epidural injections.96 In patients with otherwise normal coagulation status, discontinuation of these drugs is not required.
Clopidogrel (Plavix) and ticlopidine (Ticlid) inhibit adenosine diphosphate–induced platelet aggregation, but bleeding risk after spinal injection has not been studied. Based on labeling precautions, surgical literature, and interventional cardiology and radiology data, it is recommended that before spinal intervention, clopidogrel be discontinued for 7 days and ticlopidine for 14 days.108,121
Glycoprotein IIb/IIIa receptor antagonists such as abciximab, eptifibatide, and tirofiban inhibit the binding of thrombocytes to fibrinogen and von Willebrand factor, thereby inhibiting platelet aggregation. This class of medications is an absolute contraindication to spinal procedures. Because these medications are used in the management of acute coronary syndromes, the physician is unlikely to encounter them when assessing for a spinal procedure. Discontinuation of eptifibatide and tirofiban for 8 hours before an intervention, and for 24 to 48 hours for abciximab, is recommended by guidelines for regional anesthesia.97
Anticoagulants
Warfarin has a profound impact on coagulation as a vitamin K antagonist. Active use of warfarin results in an increased risk of spinal hematoma after lumbar puncture.97 The use of warfarin for therapeutic anticoagulation is therefore considered a contraindication for spinal interventions. Warfarin should be discontinued for 4 to 5 days before any spinal intervention to allow the prothrombin time and international normalized ratio to return to the normal range. For invasive procedures such as spinal interventions and surgery, an international normalized ratio of 1.5 or less is considered safe in most guidelines.97,108
Low-molecular-weight heparin (LMWH) is administered either for thrombus prophylaxis or therapeutically at higher doses. After a prophylactic dose of LMWH, it is recommended that spinal procedures be postponed for 12 hours. The medication must be held at least 24 hours before the procedure when a patient is receiving a therapeutic dose of LMWH, and cannot be administered until 24 hours after the procedure.125
Unfractionated heparin is administered either as a prophylactic or therapeutic dose. Prophylactic low-dose unfractionated heparin is not a contraindication for spinal procedures.192 Therapeutic doses of heparin should be discontinued 2 to 4 hours before any spinal procedure and a normal activated partial thromboplastin time documented before the procedure. Heparin may be restarted 1 hour after the procedure.125
Bleeding: Diagnosis and Management
The risk for a spinal hematoma is present regardless of the patient’s hemostasis status. The outcome after spinal hematoma is highly dependent on the time interval between symptom onset and treatment with surgical decompression. The prognosis of spinal hematoma is poor without definitive treatment within 8 to 12 hours after onset of symptoms.161 Warning symptoms include sensory and/or motor block longer than expected, recurrence of sensory and/or motor block after initial resolution, diffuse sensory and/or motor block beyond what was expected, and unexplained spine pain with or without radicular pain.116,161 These symptoms are not specific and can present immediately or be delayed several hours to days.116 Specific postprocedure instructions, including a contact if symptoms are encountered, are essential. Emergent magnetic resonance imaging (MRI) is required for diagnostic evaluation, followed by emergent decompressive surgery if a spinal hematoma is present.
Allergy
Often the first medication administered in the procedure suite is a sedative. One case of a severe anaphylactoid reaction to midazolam has been reported,78 although the risk for an allergic reaction to sedatives is exceedingly low.
Allergy to contrast is more common and is usually an anaphylactoid type of reaction. There are several mistaken beliefs about contrast allergy. Iodine is present in ionic and nonionic contrast agents, and many who believe they are allergic to contrast dye believe incorrectly that they have an allergy to iodine. Iodine is a naturally occurring substance found throughout the body and in table salt, and true iodine allergy has not been cited. Another misconception is that those who are allergic to shellfish are allergic to iodinated contrast. The allergy to shellfish is to the muscle protein tropomyosin, which does not correlate to an iodinated contrast agent allergy.114 Likewise, allergy to topical iodine solutions is not an allergy to iodine, but to other agents within the solution.114 Similarly, allergy to iodinated contrast dye is not actually due to iodine.6 Consequently a patient should not be considered allergic unless the patient has had an allergy to contrast dye.
Anaphylactic food allergies in general (including shellfish, fish, eggs, milk, and chocolate), however, do correlate with an increased risk of contrast allergy, with a 5.7% incidence, whereas persons with allergic asthma have a 7.7% incidence.114 Screening for atopic reactions of any kind can help determine those at higher risk of a contrast allergy.
If patients have a history of a contrast allergy, they can be premedicated with prednisone, 20 to 50 mg orally via several different regimens. Oral administration must be given, however, at least 6 hours before contrast administration. Intravenous dosing immediately before the procedure is not effective.114 Ranitidine, 50 mg orally 2 hours precontrast, and diphenhydramine, 25 to 50 mg orally 2 hours precontrast or intravenously 10 minutes precontrast, can also be helpful. Use of gadolinium is also considered acceptable. However, these measures do not completely prevent an anaphylactoid reaction.134
Anesthetics can also cause an allergic reaction. The ester subgroup is metabolized by plasma pseudocholinesterase into para-aminobenzoic acid (PABA). This can lead to an allergic reaction in persons sensitive to PABA in other products (such as sunscreen). It is important to realize that those with an allergy to esters are not usually allergic to amide anesthetics, and vice versa.
An allergic reaction to corticosteroid is rare, but a case of sustained urticaria after spinal injections has been reported in which the patient skin tested positive to 21 of 26 corticosteroids.154 Also, adjuvants and preservatives commonly added to glucocorticoids could potentially cause an allergic reaction.
Treatment of Allergic Reaction
Allergic reactions might not be avoidable, and preparation including basic monitoring and advanced cardiac life support training is recommended. Unfortunately, many practitioners are unaware of what is considered appropriate treatment.198
Treatment with high–flow rate oxygen delivered by facemask is appropriate when oxygen saturation falls below 92%.198 Fluid is administered regardless of whether hypotension is present, because allergic reactions, especially those caused by contrast agents, are worsened by dehydration.198 Subcutaneous or intravenous epinephrine is indicated, although the intravenous route has a more dependable uptake, particularly when a patient becomes hypotensive.198 Diphenhydramine can be given, but is contraindicated if the patient is hypotensive. Consequently it has limited use in an emergent anaphylactic reaction.7 Corticosteroids do not modify the acute symptoms in an anaphylactoid reaction and should not be given as the sole treatment.198
Procedural Risks
Medication Interactions and Toxicity
Sedatives
The use pattern of sedatives for spinal procedures is variable. Some physicians think sedatives cloud the diagnostic value of injections, while others think they are necessary and decrease anxiety and the incidence of vasovagal reactions.68 Their use should be judicious, in view of the obvious risk of respiratory depression from overdose. Many procedures require a responsive patient to minimize risk, and oversedation could lead to serious complications. Some patients are more susceptible to conscious sedation than others; therefore slow titration is recommended.127
The use of sedatives requires that there are no gastric contents at risk of being aspirated. The recommendation, therefore, is that the patient should have no clear liquids for 2 hours and no solids for 6 hours before the procedure. This guideline is generally followed even when sedation is not planned. Patients who have not followed this recommendation should have their procedures delayed.8
Contrast Agents
Using the lowest possible dose of the nonionic form of contrast in most spinal procedures helps to minimize the risk of developing renal toxicity. In those patients with normal renal function, even in the setting of diabetes mellitus, multiple myeloma, and metformin treatment, the risk of renal toxicity is low.114 Minimizing the dose and ensuring precontrast hydration are recommended to decrease nephrotoxicity.49
Various medications can interact with iodinated contrast agents. Metformin can increase the risk of renal toxicity and precipitate severe lactic acidosis in diabetic patients. It might be prudent to withhold metformin after use of iodinated contrast agents in spinal procedures as well. Combining iodinated contrast administration with any nephrotoxic medication (even NSAIDs) increases the risk of toxicity.134 Neural, cardiac, and osmotic toxicity are also possible but more rare.114
In spinal procedures, 0.2 to 15 mL of contrast containing between 200 and 300 mg/mL of iodine is generally used. Keeping the iodine dose below 3 g minimizes toxicity.114 Gadolinium can be used in place of iodinated contrast and has less toxicity.
Corticosteroids
Corticosteroids are thought to relieve pain by reducing inflammation and by blocking transmission of nociceptive C-fiber input. They have been shown to directly inhibit the activity of phospholipase A2 within the inflammatory cascade.93 In addition to their antiinflammatory actions, corticosteroids also have another pain-relieving mechanism of action via nerve membrane stabilization through inhibition of ectopic impulses, ion conductance, C-fiber transmission, and hyperpolarized spinal neurons.57,88,89,104
Local tissue toxicity has been demonstrated with corticosteroids. Dexamethasone was shown to decrease proteoglycan concentration in human chondrocytes.183 Although there had been some concern about neural toxicity with spinal administration, no concrete evidence of neurotoxicity from intrathecally administered corticosteroids has been reported.94
Corticosteroids have systemic side effects that should be considered when assessing risk. A single epidural steroid injection (ESI) can cause adrenal suppression and hypothalamic-pituitary-adrenal axis imbalances for 1 to 5 weeks.136 Complete adrenal suppression is usually limited to 4 to 7 days, but the level of incomplete suppression can be similar to that of low-dose oral corticosteroids. Some individuals are more susceptible to suppression and long-term effects, but clinical prediction of these individuals is not possible.69 An ESI precipitates more prolonged suppression than does an intraarticular injection.209 Intraarticular injection studies have shown that the duration of both systemic and local effects increased with decreased corticosteroid solubility. The degree of cortisol suppression was dependent on the number of joints injected, independent of the corticosteroid dose.10
Specific guidelines for the acceptable cumulative dose of spinal corticosteroids do not exist. Many physicians advise no more than three ESIs per year or a 6-month limit of 5 mg per kilogram of body weight.182 There are no published data, however, of an evidence-based definitive guideline. Nonetheless, judicious use is recommended because the additive local effect on the spinal structures is unknown, and the systemic effects of prolonged adrenal suppression are detrimental.
Endocrine
Patients with diabetes mellitus have an increased risk from the effects of adrenocortical suppression. After an ESI, persons with diabetes can have elevated blood glucose levels particularly during the first 4 to 7 days postinjection. Some studies have found the severity of this glucose increase related to the hemoglobin A1c level at the time of injection.209 Blood glucose levels can exceed 400 mg/dL, with an attendant risk of ketoacidosis.
Cardiovascular
Corticosteroids increase cardiac output and vascular tone, resulting in increased blood pressure. Another effect can be increased demand on the myocardium.209
Immune System
The general infection risk is elevated in all patients after glucocorticoid injection, secondary to immunosuppression. An almost immediate suppression of T lymphocytes occurs.209 Newly acquired viral infections and reactivation of latent herpes virus are more likely to occur after glucocorticoid administration. Any existing infection is a contraindication to injection of glucocorticoids. Local infection at the site of proposed injection is a contraindication to injection, even when glucocorticoids are not being administered.
Skeletal System
Long-term effects such as osteoporosis can occur with ESIs. No large clinical studies have assessed this risk. However, the fact that systemic absorption can approximate the effects of low oral dosing for at least 30 days suggests that bone density loss is likely after ESIs.182
Gastrointestinal System
Glucocorticoids potentiate the risk of gastrointestinal bleeding, especially when used in conjunction with NSAIDs. Although no data clearly indicate that patients have to discontinue NSAIDs before spinal procedures because of the risk for bleeding at the site of injection,96 several studies have reported approximately a twofold increase in gastrointestinal bleeding risk for those taking NSAIDs and glucocorticoids combined compared with NSAIDs alone.145,195 This may provide enough reason to withhold NSAIDs when performing spinal procedures with glucocorticoids.
Corticosteroid Particles
The particulate size of corticosteroids is another property that can contribute to adverse events during epidural injection.165 Accidental intraarterial injection of particulates can cause serious harm in the cervical, thoracic, and even the lumbosacral spine by causing a spinal cord infarction.182 The cervical transforaminal epidural approach with particulate corticosteroid has been associated with serious neurologic compromise including death, stroke, and quadriplegia in at least 70 cases.165
All corticosteroid preparations used for epidural injection are particulate except dexamethasone and betamethasone sodium phosphate.21 Dexamethasone has not been implicated in any of the embolic events associated with epidural injection. Although no large-scale studies have compared the efficacy of different corticosteroids, one smaller study suggests some slightly longer term effects with particulate corticosteroid in the cervical spine compared with dexamethasone.62
Anesthetics
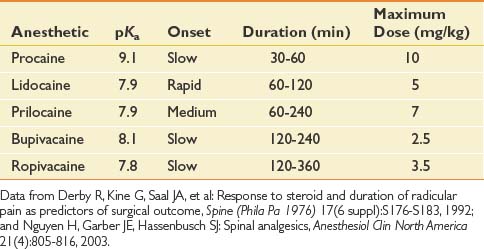
Anesthetics inhibit migration of sodium ions into the axon to slow conduction, and reduce the potassium conductance to slow repolarization. Anesthetics have known systemic toxicity, which limits the total dose (Table 25-1). There are two chemical subgroups of anesthetics, the amides and the esters. The amides are metabolized in the liver and excreted in the urine, whereas the esters are metabolized by plasma pseudocholinesterase into PABA.54,148 Systemic toxicity for all anesthetics affects the central nervous system (CNS). The initial symptoms frequently are uneasiness, tinnitus, metallic taste, and visual auras. These symptoms can progress to shivering and then to seizures. The management of CNS toxicity is airway protection and benzodiazepine administration for control of seizures.148
Cardiac effects of anesthetic toxicity are manifested through decreased cardiac conduction and atrioventricular block. Bupivacaine is 16 times more potent for cardiac QRS prolongation than is lidocaine. Bupivacaine also has a slow “on” and slow “off” binding to the sodium channels, which makes its duration of action longer for the anesthetic affect, but also for its toxicity. Cardiac toxicity with bupivacaine often precedes the CNS toxicity, and once present is rarely reversed, with a high fatality rate despite adequate resuscitation efforts.148
Epidural anesthetics have been shown to locally inhibit platelets, fibrinolysis, and leukocyte function. It has been proposed that reduced granulocyte migration and metabolic activation at surgical sites infiltrated with lidocaine might be a mechanism of decreased pain postsurgically. This could also be a mechanism for the duration of pain relief exceeding the anesthetic action in spinal procedures.148 Lidocaine and bupivacaine have been shown to have an antiinflammatory effect on nucleus pulposus–induced nerve injury, and to increase intraradicular blood flow in an animal nerve root compression model.206,207
Infection
Prevention of infection starts with the proper preparation, including a sterile procedural field and maintaining sterile equipment and medications. Risk of infection cannot be completely eliminated and might occur with any spinal injection. A 1% to 2% infection rate is reported for all spinal injections, but only a 0.01% to 0.1% rate for severe infections.65 The infection can spread through Batson’s plexus. Malposition of the needle, such as into the pelvic cavity, could cause infection with enteric organisms.203
Needle Malpositioning
Placement of the spinal needle outside the target area can result in deleterious consequences. Dural puncture, disk entry, intraabdominal puncture, pelvic puncture, pneumothorax, intravascular injection, nerve trauma, and spinal cord trauma are all possible with spinal interventions. They are best avoided with proper use of fluoroscopy and radiopaque contrast.85
Fluoroscopy
The use of fluoroscopy is essential for spinal procedures. Radiation exposure is inherent and must be considered as part of the risk for both the patient and the interventionalist. Radiation doses to the interventionalist are approximately 0.39 mrem at the glasses per 100 cases, with a mean of 15 seconds per case.38 This is well within radiation exposure safety guidelines, but protective lead aprons, thyroid shields, and glasses are recommended along with increasing distance from the x-ray beam.38 Patients have higher levels of exposure per procedure, and consent for the procedure should include radiation exposure.71 Pregnancy is an absolute contraindication to fluoroscopy. The radiation risk should be taken even more seriously in younger patients.
Epidural Steroid Injections
General
Background
The concept of injecting medication into the epidural space has been around since the early 1950s.123 Initial reports in the United States from the 1960s described treatment for sciatica using the caudal and interlaminar (IL) approaches.20,83 By the mid 1970s and 1980s, nerve root injections were described for the treatment of radicular pain. The transforaminal (TF) route of administering medication to the epidural space has become more popular in the past decade. This was largely prompted by the introduction of fluoroscopic guidance and reviews reporting that epidural corticosteroids administered by the conventional routes were not as effective as claimed in uncontrolled studies.27,109,113 Studies by Derby et al.56 and others in the 1990s described outcome improvements with prospective studies evaluating the TF route for radicular pain.132 The last decade has been characterized by a more detailed look at the efficacy of epidural steroids, with a number of reviews conducted. The proportion of these published studies that were randomized controlled trials, however, remains very small. The findings of many of these studies are described below.
The usefulness of cervical, thoracic, and lumbar epidural injections remains controversial. Underlying this controversy is the dramatic increase in lumbosacral injections in the Medicare population from 1994 to 2001. Friedly et al.77 reported that less than half of these epidural injections were performed for sciatica or radiculopathy, where the greatest evidence of benefit is available.
Epidural Anatomy
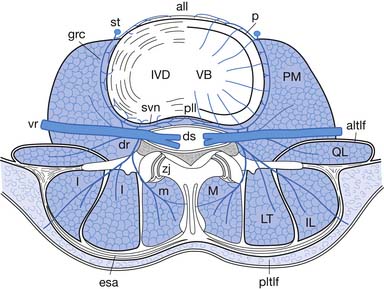
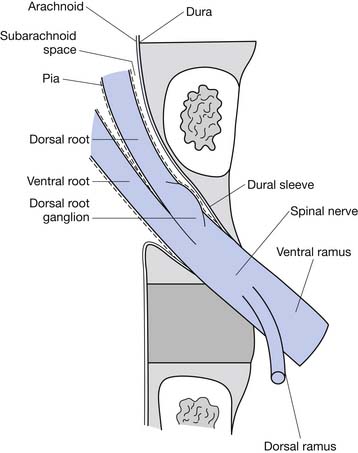
The epidural space is a tissue plane between the dura mater and periosteum and ligaments within the vertebral canal. It is contained anteriorly by the posterior longitudinal ligament and the vertebral bodies, and posteriorly by the laminae and ligamentum flavum. Its lateral borders are the pedicles and intervertebral foramina (Figure 25-1).
The epidural space contains the spinal nerve roots and their dural sleeves, the internal vertebral venous plexus, loose areolar tissue, segmental blood supply, and lymphatics. The epidural veins form an arcuate pattern, positioned laterally at the level of each vertebral body. This is an important consideration for ESIs because venous puncture is more likely to occur laterally than with midline approaches.158
Key anatomic features of the cervical spine include a thin ligamentum flavum (unfused in the midline in approximately half of individuals), absence of the interspinous ligament, and a small posterior epidural space (distance between the ligamentum flavum and dura mater).95,158 At C6–C7 and C7–T1 the epidural space has a mean width of 3 mm (1 to 4 mm).5
The artery of Adamkiewicz is a concern for thoracic and lumbar interventional procedures. It is the largest radiculomedullary artery and the major supplier of the anterior spinal artery in the lumbar region. The artery enters the spinal canal through a single intervertebral foramen in 85% of individuals between T9 and L2,53,98 and is located 63% of the time on the left side.29 This structure has been implicated in paraplegia after lumbar nerve root block.98
Pathophysiology
The primary cause of radicular pain is attributed to the inflammatory effect on the nerve from the nearby disk herniation. During surgery and on myelography, nerve roots appear swollen in patients with radiculopathy.22,163 A herniated nucleus pulposus induces marked inflammatory responses in the dura, nerve roots, and the spinal cord with high levels of phospholipase A2 activity.164 The level of phospholipase A2 activity in extracts from human disk herniations was found to be 20 to 10,000 times more than that in any other human source.164 Herniated disk specimens have also demonstrated increased levels of matrix metalloproteinase activity, nitric oxide, prostaglandin E2, and interleukin-6.82,106
Injectant
Unfortunately, there are no standardized practices for administering an ESI (Table 25-2). Across disciplines and institutions the content and volume of the injectant vary significantly. A Corticosteroid–local anesthetic mixture is the most common medication used for ESIs in both academic institutions and private practices. The results for other medications vary, with a minority of centers adding opioids clonidine, or using local anesthetic alone for their injections.51
Table 25-2 Percentage of Institutions Using Given Medication for Epidural Steroid Injection by Spinal Region
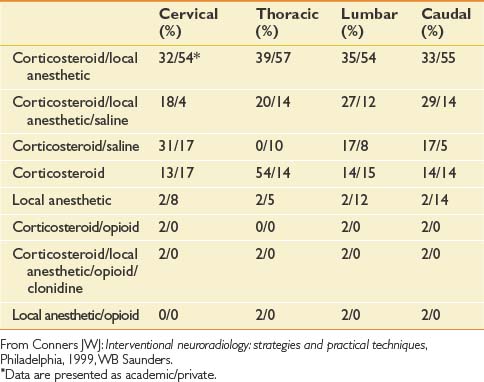
The volume of the injectant varies based on the approach used. In cervical and thoracic epidural injections, a total of up to 3 to 5 mL might be used for ESIs in which the IL approach is used. In cervical and thoracic TF ESIs, however, clinicians generally use a maximum total volume of 1.5 to 2 mL. The volume used for lumbar ESIs is slightly greater, generally not exceeding 3 to 4 mL for TF ESIs, 6 to 10 mL for IL ESIs, and 20 mL for a caudal approach.48
Complications
These procedures are not without risks, and some of the complications can be catastrophic, although infrequent. Transient complications encountered during epidural injections include insomnia, nonpositional headaches, increased back pain, facial flushing, vasovagal reactions, nausea, and increased leg pain.36 Reported complications with lumbar ESIs include infection, hematoma, intravascular injection of medication, direct nerve trauma, subdural injection of medication, air embolism, disk entry, urinary retention, radiation exposure, and hypersensitivity reactions.36 Cervical TF or IL injections are associated with relatively frequent minor adverse events (5% to 20%); however, serious adverse events are very uncommon (<1%).46 In a 2008 review of complications of lumbar IL and TF ESI, Goodman et al.85 concluded that most if not all serious adverse events can be avoided by careful technique, sterile precautions, and a thorough understanding of the relevant anatomy and fluoroscopic contrast patterns.
Approaches, Evidence, and Efficacy
In a 2002 survey of academic and private practices, a significant variance in the use of fluoroscopy for spinal procedures was noted. Private practices used fluoroscopy with more regularity than academic institutions, most notably in the cervical region where 73% of private practices and only 39% of academic institutions polled used fluoroscopy.51
Thoracic Epidural Steroid Injection
A paucity of literature on thoracic epidurals is available. This is most likely due to the significantly smaller incidence of acute herniated disks in the more stable thoracic spine. The incidence of thoracic disk injuries is 1 in 1 million persons per year, and these injuries account for only 0.25% to 0.75% of all disk herniations.9 IL and TF approaches are described for this region. Often a cervical technique is used for the upper thoracic region, and a lumbar technique is used for the lower thoracic region.
Access to the epidural space in the thoracic spine is somewhat more difficult secondary to the unique anatomic properties of the thoracic region. These include the closer proximity of the spinal cord and a narrower epidural space than the lumbar region. The overlapping laminae and spinous processes in this region limit midline access to the epidural space. It is also difficult to view the IL space on radiographs or using fluoroscopy. Botwin et al.35 describe an overall prevalence of complications per injection of 20.5%. All were transient and resolved without any residual morbidity.
Interlaminar Versus Transforaminal Technique
Injectant Flow
Kim et al.111 conducted a recent analysis of epidurography contrast patterns for cervical IL epidural injections using the midline approach. The rate of ventral epidural spread from this posterior approach was 56.7% with 1 mL of injectant and 90% with 2 mL. Botwin et al.37 evaluated the contrast patterns in lumbar IL injections in 2004 and found that approximately one third revealed ventral contrast flow and 16% were bilateral. In contrast, Manchikanti et al.139 evaluated lumbar TF ESI flow patterns and demonstrated ventral epidural filling in 88% of the procedures and nerve root sleeve filling in 97% of procedures. In a prospective evaluation of contrast flow patterns with fluoroscopically guided lumbar ESI via the lateral parasagittal IL approach versus the TF epidural approach, Candido et al.43 found the parasagittal IL approach superior to the TF approach for placing contrast into the ventral epidural space, with reduced fluoroscopy times and improved spread.
Effectiveness
The effectiveness of TF versus IL ESIs has been compared, and the results are mixed. Schaufele et al.166 evaluated the effectiveness of TF versus IL ESI for the treatment of symptomatic lumbar disk herniations and found TF injections resulted in better short-term pain improvement and fewer long-term surgical interventions than the IL ESI. A literature review on the management of cervical radiculitis with IL versus TF ESI concluded that there was insufficient evidence supported by prospective studies to determine whether one technique was more effective or safer than the other.100 Ackerman and Ahmad4 compared TF, IL, and caudal approaches for the treatment of lumbar disk herniations and found that pain relief was significantly more effective with TF injections.4
Interlaminar
Technique
The IL technique achieves access to the epidural space using the paramedian or midline approaches. The route of penetration from superficial to deep includes the skin, subcutaneous tissue, paraspinal muscles (paramedian approach) or interspinous ligament (midline approach), ligamentum flavum, and then entry into the epidural space. The most common technique includes a “loss of resistance approach” used to identify entry into the epidural space. Fluoroscopy with contrast is used to confirm the location (Figure 25-2).
Evidence
The evidence is moderate for short- and long-term relief in managing cervical radiculopathy. In managing lumbar radiculopathy, the evidence is strong for short-term relief and limited for long-term relief.2,33 The evidence is indeterminate in the management of neck pain, low back pain, and lumbar spinal stenosis.
For the treatment of diskogenic pain without radiculitis, the evidence is lacking (level III) for short- and long-term relief.19
Transforaminal
Technique
The lumbar TF approach is considered to be more specific and allows for the smallest aliquot of injectant to be delivered to the site of pathology. The technique involves using fluoroscopy to identify a subpedicular target within the intervertebral foramen. The needle is advanced to achieve a perineural location in the “safe” triangle that lies just below the pedicle and above the dural sleeve of the target nerve root (Figure 25-3). Precautions are taken to avoid advancing the needle tip medial to the “six-o’clock” position of the pedicle in the anteroposterior view (Figure 25-4).
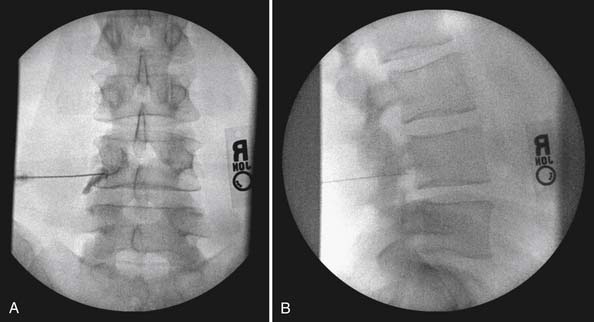
FIGURE 25-4 Lumbar transforaminal epidural steroid injection. A, Anteroposterior view. B, Lateral view.
A slightly different technique is used to deliver medication to the S1 nerve root in its foramen. The approach is analogous to that of the subpedicular approach at lumbar levels. The target point for an S1 TF injection is on the caudal border of the S1 pedicle, just dorsal to the internal opening of the S1 anterior sacral foramen. The needle is inserted into the posterior sacral foramen just short of the floor of the sacral canal.25
The technique for cervical TF injections is similar to that for lumbar TF injections. The correct oblique view of the target foramen is obtained with fluoroscopy. An entry point overlying the posterior half of the target foramen is used for initial needle placement. Care is taken to ensure that the tip of the needle is positioned over the anterior half of the superior articular process to ensure that it is not prematurely inserted too far into the foramen. The needle is advanced to the superior articular process and then readjusted to enter the foramen tangential to its posterior wall, opposite the equator of the foramen. Accurate placement is confirmed in multiple planes with radiopaque contrast and fluoroscopy.159
Selective Nerve Root Block
Epidural injection literature uses the terms selective nerve root block (SNRB) and TF injection interchangeably. The term SNRB implies selectivity of the TF approach, enabling the interventionalist to implicate a specific nerve root as a pain generator by anesthetizing it. It has recently been demonstrated that L4 and L5 SNRBs are often nonselective or at least only partially selective.193 Furman et al.81 observed that with as little as 0.5 mL of contrast, 30% of lumbosacral TF ESIs were no longer selective for the specified root level. The selective nature of the nerve root block applies to where the injectant travels, and the literature demonstrates that this might not be confined to a specific nerve root. The use of the term SNRB should consider these observations.
Evidence
Although the evidence in the literature is often conflicting, two recent randomized controlled trials support the efficacy of TF ESI. The first is a study by Riew et al.160 that considered avoidance of surgery as the outcome measure, comparing patients treated with lumbar TF injections of bupivacaine plus betamethasone with those treated with bupivacaine alone. Significantly fewer (29%) of the patients treated with corticosteroid preparation than those treated with bupivacaine alone (67%) required surgery. The second study compared outcomes of patients treated with lumbar TF injections of corticosteroids with those treated with paraspinal injections of saline. At 12 months’ follow-up, 84% in the TF ESI group reported greater than 50% reduction in pain scores, compared with 48% treated with paraspinal injections.186
The evidence for cervical TF ESI in managing cervical nerve root pain is moderate for short- and long-term improvement. For lumbar TF ESI, the evidence for managing lumbar nerve root pain is strong for short-term and moderate for long-term improvement. The evidence is limited in managing lumbar radicular pain in post–lumbar laminectomy syndrome. The evidence is indeterminate in managing axial low back pain, axial neck pain, and lumbar disk extrusions.2 In a systematic review of TF ESI for low back and lower limb pain, the evidence for TF lumbar ESI is Level II-1 for short-term relief and Level II-2 for long-term improvement (Table 25-3).40
Caudal
Technique
Caudal ESIs have the least risk for dural puncture, and access is relatively easily to achieve. Because the access point for this procedure is more distal to the presumed in most cases, larger volumes of injectant are usually instilled typically to treat the pathologic lesion at L4–L5 or L5–S1 levels. Patients are placed in the prone position, and the caudal space is identified using palpation of the sacral hiatus and fluoroscopy. The spinal needle is introduced through the sacral hiatus, and accurate needle placement is ensured using contrast and fluoroscopy (Figure 25-5). Given that the dural sac typically ends at the S2 level, to avoid dural puncture the needle should not be advanced higher than S3.
Evidence
The evidence for caudal ESIs in managing chronic low back and radicular pain is strong for short-term relief and moderate for long-term relief, whereas the evidence for post–lumbar laminectomy syndrome and spinal stenosis evidence is limited.2 A recent randomized equivalence trial found that between 79% and 91% of patients had significant pain relief and improvement in functional status with caudal injections in managing chronic low back pain for disk herniation with radiculitis.138 Preliminary findings of a randomized, double-blind equivalence trial investigating patients with spinal stenosis who had chronic function-limiting low back and lower limb pain, showed that fluoroscopically guided caudal ESIs were effective in approximately 60% of subjects.140 In a prospective cohort study of patients with bilateral radicular pain from spinal stenosis, caudal ESIs were found to reduce radicular pain and improve standing and walking tolerance.34 The results of this study are limited by the lack of a placebo group. Other studies concluded that the effectiveness of caudal ESIs in managing lumbar radiculopathy was moderate (see Table 25-3).173
Conclusion
Most studies collectively demonstrate ESIs in the lumbar spine to be effective in providing a more rapid resolution of acute to subacute radicular leg pain caused by a herniated nucleus pulposus. Evidence that ESIs have any beneficial effect, however, is limited in patients with acute axial low back pain or in those with chronic axial low back or radicular pain. Furthermore, studies have failed to demonstrate that ESIs reduce long-term pain or obviate the need for surgery.173 The success rates for cervical epidural injections for symptomatic cervical disk herniations appear to be very similar to prior studies of lumbar epidural injections for symptomatic disk herniations.124
Zygapophysial Joint Interventions
General
Background
The zygapophysial joint is a known pain source with distinct innervation and pain referral maps in the cervical, thoracic, and lumbar spine.67,79,80 Although the anatomy is similar throughout the spine, the prevalence of zygapophysial joint–mediated pain and the utility of zygapophysial joint procedures differ between spinal regions.
The prevalence of cervical zygapophysial joint pain has been studied in patients with whiplash injuries and was found to be 54% to 60% using double-blind dual or triple medial branch blocks (MBBs).16,130 Thoracic spine prevalence has been cited at 48%.137 In the lumbar spine, the incidence of zygapophysial joint pain is between 15% and 40% in patients with chronic low back pain.169,171 In younger injured workers with chronic back pain, the prevalence of zygapophysial joint pain is approximately 15%.171 The prevalence increases with age, reaching up to 40% in noninjured individuals with a median age of 59 years.171
Anatomy
Understanding zygapophysial joint anatomy is key to understanding the procedures targeting this structure. It is a synovial joint composed of the inferior articular process of the vertebra above and the superior articular process of the vertebra below with articular cartilage and a capsule. In the cervical spine, a small meniscus is also present.208 Innervation is via the medial branches of the dorsal rami.
The cervical zygapophysial joint innervation has been described by Bogduk.24 For C3–T1, the medial branches of the primary dorsal rami of the corresponding joint travel along the waist of the articular pillars to innervate the corresponding joint (e.g., C5–C6 zygapophysial joint is innervated by C5 and C6 medial branches). The exceptions include the C2–C3 joint, which is innervated by the third occipital nerve (TON), and C0–C2 articulations, which are innervated by branches of the C1 and C2 ventral rami.
In the lumbar spine, as the medial branch courses inferiorly, it lies at the junction of the superior articular process and transverse process under the mamilloaccessory ligament of the vertebra below (e.g., the L3 medial branch lies on the L4 vertebra). Each zygapophysial joint is innervated by two medial branches, one from the level above and one from the corresponding level (e.g., the L4–L5 joint is innervated by the L3 and L4 medial branches) (Figure 25-6). The exception is L5–S1, which is innervated by the L4 medial branch and the L5 dorsal primary ramus, which lies at the junction of the sacral ala and the S1 superior articular process.28
Indications and Principles
No history or physical examination findings, including rotation plus extension, have been shown to predict placebo-controlled or comparative block–established cervical, thoracic, or lumbar zygapophysial joint pain.105 Also, no imaging studies can provide a diagnosis; consequently the first role of zygapophysial injections is for diagnostic purposes. Zygapophysial joint injections are appropriate only after adequate noninterventional care, including medications, a physical therapy program, and patient education, has failed.
Complications
Severe complications of zygapophysial joint injections are rare. Using multiplanar fluoroscopy with little or no sedation nearly eliminates the risk for nerve root injury or entry into the spinal canal, although secondary lumbar radiculopathy, paraspinal abscess, epidural abscess, infective diskitis, and septic zygapophysial joint arthritis have been described.172,179 There are no case reports of complications from MBBs.
Zygapophysial Intraarticular Injection
Technique
The target joint is visualized fluoroscopically. The exact view varies between spinal levels, between individuals, and even from side to side. The needle is advanced to lie at the joint line and within the capsule. The joint capsule is redundant, and capsular flow can be obtained without visualization of the joint line. Confirmation of placement must be made using the smallest amount of contrast adequate to confirm placement within the joint space (0.1 to 0.3 mL). Next a preparation of corticosteroid mixed with anesthetic is administered into the joint until capsular resistance is encountered (0.5 to 1.75 mL). Care is taken not to overdistend and disrupt the capsule.25
Evidence
No studies have compared the diagnostic utility of cervical intraarticular injection with that of cervical MBBs, but there is a theoretical risk of needle placement or capsular leakage into or near the vertebral foramen with intraarticular injection, and consequently less specificity.112 Intraarticular injection with corticosteroid and anesthetic is routinely performed for diagnostic purposes, but has not been validated. In uncontrolled studies, intraarticular injection has shown some therapeutic efficacy.110,175 Only one randomized controlled trial (RCT) of therapeutic cervical zygapophysial intraarticular injections has been published, and interestingly, corticosteroid was not shown to be superior to the injection of bupivacaine alone.15
Data regarding thoracic intraarticular injections are not sufficient to make any conclusions on their efficacy.12 In a recent review of lumbar zygapophysial joint injection procedures, there were no intraarticular studies of sufficient quality to be entered into the analysis.44,55
Diagnostic Zygapophysial Joint Intervention
Background
MBBs are considered the primary means to test a hypothesis that pain is zygapophysial joint mediated by delivering anesthetic specifically to the medial branches. Modified dual comparative blocks are considered the criterion standard for diagnosing zygapophysial joint–mediated pain, giving the highest positive predictive value (PPV) and the lowest false-positive (FP) rate. This technique uses two separate injections with anesthetics of differing lengths of action. A positive test requires that a minimum length of relief is obtained with each (e.g., 1 to 2 hours of relief with lidocaine and ≥3 hours’ relief with bupivacaine), in addition to a high level of relief (>80%).63 Diagnostic blocks are generally a precursor to more definitive treatment with medial branch radiofrequency neurotomy.
Technique
After proper preparation the target medial branch at its osseous landmarks is viewed fluoroscopically. The spinal needle is advanced in the oblique view (Figure 25-7, A), and placement is confirmed in multiple planes with radiopaque contrast and fluoroscopy (Figure 25-7, B). Vascular uptake is ruled out, because limitation to the procedure exists if venous uptake occurs (even if needle position is corrected with lack of subsequent venous uptake), incurring an associated 50% false-negative rate.107 Once placement is confirmed and without vascular uptake, a small amount (0.3 to 0.5 mL) of anesthetic is injected.
In the cervical spine, anesthetic blockade of the TON is used for diagnosing C2–C3 joint pain, or anesthetic blockade of the two corresponding medial branches is used for diagnosng C3–C4 to C7–T1 pain. No validated MBB technique exists for the thoracic spine. Current technique is based on principles from validated cervical and lumbar techniques.12 In the lumbar spine, anesthetic blockade of the two corresponding medial branches (or the L5 primary dorsal ramus) is performed for each joint.25
Evidence
In the cervical spine, with the use of comparative blocks as the criterion standard, the PPV of a single cervical MBB has been cited at 60% to 73%, with an FP rate of 27% to 40%.13,14,129,137 With placebo injections used as the criterion standard, the PPV increases dramatically for comparative MBBs to 81%, with a FP occurrence rate of 19%.129
The FP rate in the thoracic spine is also high. By using comparative or modified comparative blocks and by requiring at least 75% pain relief, the single block FP rate is between 42% and 58%.12
The specificity of lumbar MBBs for achieving the targeted nerve has been established using computed tomography (CT) confirmation of contrast flow postprocedure. With fluoroscopically guided precise needle position, injected contrast incorporates the lumbar medial branches and the L5 dorsal ramus without spread to alternative structures that are also potential pain generators.65
Quantifying the amount and length of relief is essential in evaluating lumbar zygapophysial joint blocks. Schwarzer et al.170 found that the PPV was 31%, with a 69% occurrence of FP responses without the use of standard modified comparative blocks. Dreyfuss,65 however, found that with the use of modified comparative blocks, the PPV was 68%, with a 32% occurrence of FP responses. Requiring a duration of improvement coincident with the type of anesthetic used and requiring high-grade relief substantially improves the PPV and decreases the occurrence of FP responses.
Sacroiliac Joint Interventions
Background
The sacroiliac joint (SIJ) is a synovial joint with documented, clinical nociceptive capacity. Nociception from the articular cartilage, as well as from the posterior joint capsule and ligaments, has been supported.168 The prevalence of SIJ-mediated pain in those with suspected SIJ pain by physical examination is only about 15%.135,141,168 The incidence of the SIJ as the primary pain generator of lumbopelvic pain is lower than that of radicular or zygapophysial origin. The incidence, however, might increase after trauma as well as in the peripartum period.156
The SIJ is innervated by the posterior rami of primarily S1 and S2 via the lateral branches. The L5 dorsal ramus and the S3 lateral branch have a documented contribution, but to a lesser extent. S4 has been shown to contribute in some studies, but not in others.72,201 The lateral branches were found to innervate the posterior joint capsule and ligaments.
Indications
No historical features or physical examination tests for SIJ pain have been validated with confirmatory SIJ blocks. SIJ-mediated pain also cannot be diagnosed by imaging. Bone scintigraphy has the best specificity of 90% to 100%, but a sensitivity of only 12% to 46%.177 Consequently diagnostic blocks are the only means of diagnosis.∗
If the patient has pain above the level of L5 and does not have pain over the sacral sulcus (SIJ) sulcus or point to the posterior superior iliac spine as the primary pain source, then the likelihood of SIJ-mediated pain is low (<10%).64 It was noted, however, that SIJ pain can refer into the lower limb. If none of the six provocation tests (distraction, compression; thigh thrust; Gaenslen’s; flexion, abduction, external rotation [FABERs]; sacral thrust) are positive, the SIJ can be ruled out as a pain source.118 If maximal pain is below the level of L5 and three or more of these six provocation tests are positive, the PPV of having SIJ pain is 77%.188
Technique
Fluoroscopy is used to identify the inferior border of the joint at a point where the anterior and posterior joint lines overlap. The exact angles to find this hyperlucent area vary from patient to patient. Nonfluoroscopically guided SIJ injections have been shown to be intraarticular only 22% of the time, with undesired epidural flow 24% of the time. Consequently fluoroscopy is required to perform an SIJ intraarticular injection.162 SIJ injections done without fluoroscopy are properly described as ligamentous or trigger point injections.
Once the spinal needle is advanced to the inferior border of the joint and intraarticular placement is expected, needle placement is confirmed with radiopaque contrast and fluoroscopy (Figure 25-8, A). Views are taken in multiple planes to be certain flow is intraarticular and nonvascular. A lateral view (Figure 25-8, B) is taken to show whether there is a ventral defect in the capsule that could allow spread of the medication to the lumbosacral plexus. Extraarticular extravasation has been cited at 61%.73 A corticosteroid and anesthetic mixture is usually injected until capsular resistance is encountered (0.8 to 2.5 mL).73
Evidence
In those with true sacroiliitis, three of four RCTs show benefit from intraarticular corticosteroids.31,133,143 Evidence of therapeutic efficacy of intraarticualr injection for other causes for SIJ pain is not conclusive, although one study suggests additional extraarticular pain generators such as the posterior interosseous ligaments. This study showed a modest benefit of a combined intraarticular and extraarticular technique.31 There is conjecture that the posterior ligaments are a major contributor to SIJ pain.
The ability to reliably block the innervation of the SIJ has not been demonstrated. Controlled studies have shown that the diagnosis of SIJ pain cannot be made via attempted blockade of the lateral branch divisions of the L5–S3 dorsal rami.61 Intraarticular injection is used as the diagnostic block for the SIJs until a reliable lateral branch block technique can be established.
Zygapophysial and Sacroiliac Joint Summary
Radiofrequency Neurotomy
Background
Radiofrequency neurotomy (RFN) of the medial branches of the dorsal primary rami aims to destroy the afferent nerve supply to the zygapophysial joints via heat lesioning. It uses radiofrequency current to create heat. The lesion is performed at 85°C for 60 to 90 seconds.194 Nerve regeneration is assumed to occur in 9 to 12 months, and pain might recur. The same lesion can be repeated, however, and again pain relief is usually obtained.99,167
Thermal RFN uses a noninsulated electrode tip delivering electrical current to the tissue. Heat is generated as a result of the current flow through the tissues, which causes charged molecules to oscillate rapidly, producing friction. The lesion produced is an ellipse along the length of the noninsulated tip with a diameter 1 to 1.5 times the size of the tip. Accordingly, needle placement parallel to the target nerve is required.120
Pulsed Radiofrequency
A potential risk of thermal RFN is that it might cause a painful neuritis from coagulation of the dorsal root ganglion. Some practitioners have proposed that spinal instability results from denervation of the multifidi muscles.146 Pulsed RF, performed at temperatures not exceeding 42° C, has been proposed as a method to achieve pain interruption with less risk. A study of pulsed RF in the cervical and lumbar spine reported a 60% success rate (pain reduction >50%), with pain relief lasting approximately 6 months.146 Consequently the routine use of pulsed RFN is not recommended because thermal RFN has shown much better outcomes with little risk.
Technique
Proper patient selection is a prerequisite for RFN as discussed below. Fluoroscopic guidance with the use of radiopaque contrast is required. RFN is often more painful than other spinal procedures, and conscious sedation is typically administered. Physiologic monitoring is mandatory. The needle electrode is positioned over the proper osseous anatomy, parallel with the nerve, using fluoroscopic guidance (Figure 25-9). Motor and sensory stimulation is often used to rule out placement near the nerve root or dorsal root ganglion. The nerve is anesthetized, and finally several overlapping lesions are performed.

FIGURE 25-9 Third occipital nerve radiofrequency neurotomy. A, Anteroposterior view. B, Lateral view.
A cadaveric study of 30 nerve locations in six cadavers determined precise placement for RFN, and it was recommended that needles larger than 22 gauge with a 10-mm active tip would be the most effective.120
Complications
Serious complications of RFN are rare. Inadvertent placement of the probe near the dorsal root ganglion or nerve root, however, can cause serious injury.1 Proper needle placement as established by multiplanar imaging, motor stimulation prelesioning, and a responsive patient provide for safe lesioning.
Excluding treatment of the TON, review of 83 cervical RFN procedures documented postoperative pain in 97%, ataxia in 23%, numbness in 80% at C3–C4 and in 19% at lower levels, and dysesthesias in 30% at C3–C4 and in 17% at lower levels.128 In 51 RFN procedures to the TON, side effects included numbness in 97%, ataxia in 95%, dysesthesias in 55%, hypersensitivities in 15%, and pruritus in 10%. Most of these side effects were limited to 2 weeks postprocedure and lasted up to 4 weeks in one patient.86 Complications of lumbar RFN have been studied, and in 116 procedures, the incidence of local pain lasting more than 2 weeks was 0.5%, and the incidence of neuritic pain lasting more than 2 weeks was 0.5%.115
Conjecture that RFN could cause multifidus atrophy and therefore spinal instability146 is unfounded. A recent observational study, although small, did not show identifiable segmental multifidus atrophy by MRI 17 to 24 months after successful lumbar medial branch denervation with RFN despite segmental denervation documented by electromyography (EMG) 6 weeks after RFN. Patients had sustained pain relief.66
Evidence
Evidence supporting cervical RFN is substantial. Lord et al.131 studied 24 patients with chronic neck pain selected from comparative MBBs and lack of relief with saline injections under double-blind conditions. With a 22-gauge needle, RFN with four to six lesions per medial branch was performed to accommodate for variations in nerve position. Eight percent of the control group and 58% of the active group had greater than 90% relief, and the median time until pain returned to a level of at least 50% of the pretreatment level was 263 days (8.8 months) in the active group and 8 days in the placebo group (p = 0.04). Stovner et al.184 compared sham and active RFN in patients with cervicogenic headache, but did not use any diagnostic blocks for selection. Results did not show statistical significance, highlighting the need for proper patient selection.
A second RFN is successful for patients who have relief from the initial RFN. If patients had at least 90 days of relief from the first RFN, then the chance of a second RFN being successful RF was 82%. If pain relief from the first RF was for less than 90 days, then the success rate for a second RFN was only 33%.144
Neurotomy of the TON for pain mediated by C2–C3 had poor outcomes initially. However, with a new technique using a larger 16-gauge needle and multiple parallel lesions to account for variability of nerve location, the success rate was doubled from 40% to 80%. Govind et al.86 treated 49 patients who had been selected with comparative TON blocks, and achieved complete relief of pain for at least 90 days in 88% of the patients. The median duration of relief was 297 days.
Unfortunately, much of the literature available on the efficacy of RFN has used a single-block technique to identify patients for study inclusion, particularly in the lumbar spine. By using a single MBB technique with only 50% relief required, the best results are greater than 50% pain relief in 46% of the active group versus 12% of the sham group at 1 year.189 Other studies that have used single intraarticular blocks without high-grade relief for patient selection fare worse, with no active patients having obtained benefit after RFN.122,191 When substantially more stringent selection criteria are used, however, favorable results have been documented. In a study by Dreyfuss et al.,63 of 41 patients who met the initial inclusion criteria, only 15 were included for lumbar RFN after modified comparative blocks. After RFN the mean visual analog scale (VAS) score dropped 88% (VAS score, 0.5) at 6 weeks and 76% (VAS score, 1) at 1 year. Eighty-seven percent of patients had at least 60% relief at 1-year follow-up and did not seek ancillary treatment for their pain. The small number of patients extracted (15 of 138 total patients with back pain [11%]) for lumbar RFN from the initial population with nonradicular low back pain reflects the low prevalence of pure zygapophysial joint pain in the lumbar spine. A double-blind RCT was performed by Nath et al.149 comparing lumbar RFN with sham treatment in patients with modified comparative block–proven zygapophysial-mediated pain, establishing that lumbar RFN is superior to placebo.
Appropriate RFN technique is also relevant to its efficacy. Lesion size sufficient to denervate the medial branch within its osseous territory is far more likely by using larger radiofrequency probes (16 to 18 gauge) and with a longer (e.g.,10 mm) active tip. The lumbar RF study by Dreyfuss et al.63 used a 16-gauge, 5-mm tip needle, but two contiguous lesions were performed to ensure a 10-mm-long lesion. Technical success, as measured by presegmental- and postsegmental-specific multifidus EMG, was 90%. The study by Nath et al.149 used a 22-gauge electrode needle with a 5-mm active tip, and two contiguous lesions (equating to a 10-mm lesion) were made at three different locations for each medial branch. The inaccuracies of the RFN technique used in other studies could have had a negative effect on the outcomes.
Sacroiliac Joint Radiofrequency Neurotomy
Background
Because intraarticular sacroiliac joint (SIJ) injections have not offered long-term relief of SIJ-mediated pain, RFN has been postulated for longer term pain management because of its effectiveness in the cervical and lumbar spine. Retrospective studies of CT or fluoroscopically guided “denervation” along the posterior SIJ line or in the interosseous ligament did not show benefit. This technique is flawed, however, because the lateral branches are not accessible at these locations.70,84
Evidence
Cohen and Abdi52 reported retrospectively in 2003 on 18 subjects who had greater than 80% relief with SIJ intraarticular blocks. Nine of the 18 subjects had greater than 50% relief from L4 medial branch, L5 dorsal ramus, and S1–S3 lateral branch blocks. Eight of these 9 patients who subsequently underwent SIJ RFN had greater than 50% pain relief that was persistent at 9 months’ follow-up. A prospective cohort study of bipolar lesioning showed little result for decreasing pain.41 Consequently the evidence for RFN to manage SIJ pain is still quite limited.
Discography
The differential diagnosis of axial low back pain is presented in Chapter 40. Interventional strategies are used for both the diagnosis and management of diskogenic pain. Patients with degenerative disk disease diagnosed via imaging, such as MRI or CT, can be asymptomatic.23,102 No physical examination finding is diagnostic for diskogenic pain, and it is difficult to attribute a specific disk level to these symptoms. Abnormal-appearing disks have also been seen in both symptomatic and asymptomatic subjects, leading to uncertainty about whether a specific disk can be precisely pinpointed as a focal pain generator.11,76,87 Lumbar provocation discography is a technique designed to attempt to diagnose a “symptomatic” disk.
Discography was originally described by Lindblom to diagnose a herniated lumbar intervertebral disk, and has been used clinically since the 1940s. During early discography procedures, patients were noted to commonly report reproduction of their typical pain25 with stimulation, and abnormal disks were rarely painful in asymptomatic controls.142
Risks, contraindications, and potential complications are similar to those common to all interventional spine procedures. Unique to discography is a higher risk for infection.
Antibiotic prophylaxis was not routinely used for lumbar discography in the past. At that time the rate of diskitis was reported to be 0.1% to 2.3% per patient, and 0.05% to 1.3% per disk.26 Typically infections were caused by Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli. With current techniques, including routine prophylactic antibiotics, the rate of diskitis has been lowered.75
The validity of discography has been evaluated in several studies, but still remains controversial. Discography has been performed in both symptomatic and asymptomatic subjects, to analyze its validity and concordance. Walsh et al.197 in 1990 reported a FP rate of 0% in asymptomatic controls, and a true-positive rate of 89% in symptomatic subjects. Conversely, Carragee et al.47 in 2006 reported that the best-case PPV for discography was 50% to 60%. More recently Wolfer et al.,204 in a metaanalysis of 11 studies, reported FP rates of 9.3% per patient and 6.0% per disk. In data pooled from asymptomatic patients, the FP rate was 3.0% per patient and 2.1% per disk. They reported an improvement in the FP rate with low-pressure positive criteria (≤15 psi). They calculated an overall specificity of 94% (95% confidence interval, 0.88 to 0.98) and a FP rate of 6%. Another study has suggested discography as a potential cause of accelerated disk degeneration changes.45
Intrathecal Delivery Systems
Intrathecal drug delivery systems such as implantable subcutaneous pumps are being used for patients who have chronic pain that is resistant to management with oral or parenteral medications. As early as 1979, both epidural and intradural opioid delivery systems had been reported in the literature.17,199 Delivery of agents directly to opioid receptors was noted to decrease the systemic effects of the medications and also to decrease the dosage needed to achieve analgesia.
For pain treatment, three classes of medications are used for intrathecal delivery: opioids, local anesthetics, and nonopioid agents such as adrenergic receptor agonists or NMDA (N-methyl-D-aspartate) receptor antagonists. Infusion of opioids is the most frequently used.157 Intrathecal morphine infusion has been shown to be safe at moderate concentrations, with favorable efficacy data.18,150,153 There is, however, increasing interest in using hydromorphone because it is five times as potent as morphine with a better side effect profile.150 Bupivacaine and clonidine also demonstrate favorable efficacy and toxicology.90,91
According to Prager,155 indications for intraspinal drug delivery include chronic pain with known pathophysiology, sensitivity of pain to the medication being used, failure of more conservative therapy, a favorable psychosocial evaluation, and a favorable response to a screening trial. Contraindications include systemic infection, coagulopathy, allergy to the medication being used, inappropriate drug habituation (untreated), failure to obtain pain relief in a screening trial, unusual observed behavior during the screening trial, poor personal hygiene, and poor patient compliance. Although rare, granuloma formation is a potentially severe complication of intrathecal delivery systems.
Spinal Cord Stimulation
Spinal cord stimulation is a procedure using electrodes placed within the epidural space adjacent to the area of the spinal cord or root presumed to be the source of pain. An electric current is then applied to achieve sympatholytic and other neuromodulatory effects.50 The premise of spinal cord stimulation is based on the gate control theory of pain.42 The “gates” that control nociceptive input can be “closed” via stimulation of low-threshold primary afferent fibers.
Studies evaluating spinal cord stimulation in patients who have failed back syndrome with persistent radicular pain have demonstrated positive outcomes, including decreased opioid use and increased pain relief as compared with reoperation.117,152 Randomized trials for the treatment of low back pain using spinal cord stimulators in nonfailed back syndrome, or failed back surgery syndrome without radicular pain, are not found in the current literature.50
Sympathetic Blocks
The study of the sympathetic nervous system began with Galen in the second century AD. Much of the work on sympathetic nerve blocks blossomed from necessity of treatment of traumatic casualties during World War II. In 1953 Bonica103 proposed guidelines for neural blockade.
The ganglion impar, also called the ganglion of Walther, is located in the sacrococcygeal region. The pelvic portion of each sympathetic trunk runs anterior to the sacrum, medial to the anterior sacral foramina. The two pelvic sympathetic trunks converge at the distal sacrum and terminate on the anterior surface of the coccyx in a small ganglion called the ganglion impar. Foye et al.74 reported that a single local nerve block of the ganglion of impar can provide 100% relief of coccydynia.
Additional Procedures and Emerging Interventions
With a growing population that is living longer, the prevalence and incidence of low back pain are certain to increase. Spinal procedures will need to adapt, and new technology must emerge to improve both evaluation and treatment. In addition to the most common interventions described previously in this chapter, a number of other procedures are being used including chemonucleolysis, intradiskal corticosteroid injection, coblation nucleoplasty, intradiskal electrothermal therapy (IDET), percutaneous intradiskal radiofrequency therapy, and biacuplasty.50 These procedures have not been shown to be effective in randomized, placebo-controlled trials, but are mentioned for completeness.
Chemonucleolysis is the treatment of a herniated disk with intradiskal injection of proteolytic enzymes. One such enzyme is chymopapain, extracted from papaya, which works to digest the nucleus pulposus but without damaging the annulus fibrosis. Intradiskal corticosteroid injections have been reported in the literature, but are not widely used because they have not been found to be efficacious. Coblation nucleoplasty involves using a bipolar radiofrequency current to decrease the volume of disk tissue. IDET is performed by placing an electrode or catheter into the annulus of the intervertebral disk to apply electrothermal energy that destroys adjacent pain receptors. Percutaneous intradiskal radiofrequency therapy is similar to IDET, as it requires placement of an electrode or catheter into the intervertebral disk, but an alternating radiofrequency current is applied. Biacuplasty is a new technique that is currently under investigation for disk-mediated pain. Early studies seemed promising, but follow-up studies have not proven to be supportive of initial findings.92 More outcomes-based research of these newer techniques needs to be completed in human subjects.
1. Abbott Z., Smuck M., Haig A., et al. Irreversible spinal nerve injury from dorsal ramus radiofrequency neurotomy: a case report. Arch Phys Med Rehabil. 2007;88(10):1350-1352.
2. Abdi S., Datta S., Lucas L.F. Role of epidural steroids in the management of chronic spinal pain: a systematic review of effectiveness and complications. Pain Physician. 2005;8(1):127-143.
3. Abram S.E., O’Connor T.C. Complications associated with epidural steroid injections. Reg Anesth. 1996;21(2):149-162.
4. Ackerman W.E.III, Ahmad M. The efficacy of lumbar epidural steroid injections in patients with lumbar disc herniations. Anesth Analg. 2007;104(5):1217-1222,.
5. Aldrete J.A., Mushin A.U., Zapata J.C., et al. Skin to cervical epidural space distances as read from magnetic resonance imaging films: consideration of the “hump pad,” J Clin Anesth. 1998;10(4):309-313.
6. American Academy of Allergy and Immunology. Available at: http://www.aaaai.org. Accessed July 11, 2009.
7. American College of Radiology Committee on Drugs and Contrast Media and Committee on General and Pediatric Radiology: Manual on contrast media, Reston, V.A., 2004, Committee on Drugs and Contrast Media and Committee on General and Pediatric Radiology
8. American Society of Anesthesiologist Task Force on Preoperative Fasting. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90(3):896-905.
9. Arce C.A., Dohrmann G.J. Herniated thoracic disks. Neurol Clin. 1985;3(2):383-392.
10. Armstrong R.D., English J., Gibson T., et al. Serum methylprednisolone levels following intra-articular injection of methylprednisolone acetate. Ann Rheum Dis. 1981;40(6):571-574.
11. Ashton I.K., Walsh D.A., Polak J.M., et al. Substance P in intervertebral discs: binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 1994;65(6):635-639.
12. Atluri S., Datta S., Falco F.J., et al. Systematic review of diagnostic utility and therapeutic effectiveness of thoracic facet joint interventions. Pain Physician. 2008;11(5):611-629.
13. Barnsley L., Lord S., Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain. 1993;55(1):99-106.
14. Barnsley L., Lord S., Wallis B., et al. False-positive rates of cervical zygapophysial joint blocks. Clin J Pain. 1993;9(2):124-130.
15. Barnsley L., Lord S.M., Wallis B.J., et al. Lack of effect of intraarticular corticosteroids for chronic pain in the cervical zygapophyseal joints. N Engl J Med. 1994;330(15):1047-1050.
16. Barnsley L., Lord S.M., Wallis B.J., et al. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine (Phila Pa 1976). 1995;20(1):20-25. discussion 26
17. Behar M., Magora F., Olshwang D., et al. Epidural morphine in treatment of pain. Lancet. 1979;1(8115):527-529.
18. Bennett G., Serafini M., Burchiel K., et al. Evidence-based review of the literature on intrathecal delivery of pain medication. J Pain Symptom Manage. 2000;20(2):S12-S36.
19. Benyamin R.M., Singh V., Parr A.T., et al. Systematic review of the effectiveness of cervical epidurals in the management of chronic neck pain. Pain Physician. 2009;12(1):137-157.
20. Benzon H.T. Epidural steroid injections for low back pain and lumbosacral radiculopathy. Pain. 1986;24(3):277-295.
21. Benzon H.T., Chew T.L., McCarthy R.J., et al. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 2007;106(2):331-338.
22. Berg A. Clinical and myelographic studies of conservatively treated cases of lumbar intervertebral disk protrusion. Acta Chir Scand. 1952;104(2-3):124-129.
23. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(3):403-408.
24. Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine (Phila Pa 1976). 1982;7(4):319-330.
25. Bogduk N., editor. International Spine Intervention Society practice guidelines for spinal diagnostic and treatment procedures. San Refael, CA: International spine Intervention Society, 2004.
26. Bogduk N., Aprill C., Derby R. Discography. St Louis: Mosby; 1995.
27. Bogduk N., Christophidis N., Cherry D., et al. Epidural use of steroids in the management of back pain and sciatica of spinal origin. Report of the working party on epidural use of steroids in the management of back pain. Canberra: National Health and Medical Research Council; 1993.
28. Bogduk N., Wilson A.S., Tynan W. The human lumbar dorsal rami. J Anat. 1982;134(pt 2):383-397.
29. Boll D.T., Bulow H., Blackham K.A., et al. MDCT angiography of the spinal vasculature and the artery of Adamkiewicz. AJR Am J Roentgenol. 2006;187(4):1054-1060.
30. Bollow M., Braun J., Taupitz M., et al. CT-guided intraarticular corticosteroid injection into the sacroiliac joints in patients with spondyloarthropathy: indication and follow-up with contrast-enhanced MRI. J Comput Assist Tomogr. 1996;20(4):512-521.
31. Borowsky C.D., Fagen G. Sources of sacroiliac region pain: insights gained from a study comparing standard intra-articular injection with a technique combining intra- and peri-articular injection. Arch Phys Med Rehabil. 2008;89(11):2048-2056.
32. Bose B. Quadriparesis following cervical epidural steroid injections: case report and review of the literature. Spine J. 2005;5(5):558-563.
33. Boswell M.V., Trescot A.M., Datta S., et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10(1):7-111.
34. Botwin K., Brown L.A., Fishman M., et al. Fluoroscopically guided caudal epidural steroid injections in degenerative lumbar spine stenosis. Pain Physician. 2007;10(4):547-558.
35. Botwin K.P., Baskin M., Rao S. Adverse effects of fluoroscopically guided interlaminar thoracic epidural steroid injections. Am J Phys Med Rehabil. 2006;85(1):14-23.
36. Botwin K.P., Gruber R.D., Bouchlas C.G., et al. Complications of fluoroscopically guided caudal epidural injections. Am J Phys Med Rehabil. 2001;80(6):416-424.
37. Botwin K.P., Natalicchio J., Hanna A. Fluoroscopic guided lumbar interlaminarepidural injections: a prospective evaluation of epidurography contrast patterns and anatomical review of the epidural space. Pain Physician. 2004;7(1):77-80.
38. Botwin K.P., Thomas S., Gruber R.D., et al. Radiation exposure of the spinal interventionalist performing fluoroscopically guided lumbar transforaminal epidural steroid injections. Arch Phys Med Rehabil. 2002;83(5):697-701.
39. Bratton R.L. Assessment and management of acute low back pain. Am Fam Physician. 1999;60(8):2299-2308.
40. Buenaventura R.M., Datta S., Abdi S., et al. Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician. 2009;12(1):233-251.
41. Burnham R.S., Yasui Y. An alternate method of radiofrequency neurotomy of the sacroiliac joint: a pilot study of the effect on pain, function, and satisfaction. Reg Anesth Pain Med. 2007;32(1):12-19.
42. Cahana A., Mavrocordatos P., Geurts J.W., et al. Do minimally invasive procedures have a place in the treatment of chronic low back pain? Expert Rev Neurother. 2004;4(3):479-490.
43. Candido K.D., Raghavendra M.S., Chinthagada M., et al. A prospective evaluation of iodinated contrast flow patterns with fluoroscopically guided lumbar epidural steroid injections: the lateral parasagittal interlaminar epidural approach versus the transforaminal epidural approach. Anesth Analg. 2008;106(2):638-644.
44. Carette S., Marcoux S., Truchon R., et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med. 1991;325(14):1002-1007.
45. Carragee E.J., Don A.S., Hurwitz E.L., et al. 2009 ISSLS prize winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine (Phila Pa 1976). 2009;34(21):2338-2345.
46. Carragee E.J., Hurwitz E.L., Cheng I., et al. Treatment of neck pain: injections and surgical interventions: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 suppl):S153-S169.
47. Carragee E.J., Lincoln T., Parmar V.S., et al. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine (Phila Pa 1976). 2006;31(18):2115-2123.
48. Chen B: Epidural steroid injections. Emedicine. Updated February 18, 2009. Available at: http://emedicine.medscape.com/article/325733-overview. Accessed June 2, 2009.
49. Chopra P., Smith H. Use of radiopaque contrast agents for the interventional pain physician. Pain Physician. 2004;7(4):459-463.
50. Chou R., Atlas S.J., Stanos S.P., et al. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976). 2009;34(10):1078-1093.
51. Cluff R., Mehio A.K., Cohen S.P., et al. The technical aspects of epidural steroid injections: a national survey. Anesth Analg. 2002;95(2):403-408,.
52. Cohen S.P., Abdi S. Lateral branch blocks as a treatment for sacroiliac joint pain: a pilot study. Reg Anesth Pain Med. 2003;28(2):113-119.
53. Conners J.W.J. Interventional neuroradiology: strategies and practical techniques. Philadelphia: WB Saunders; 1999.
54. Cousins M.J., Bridenbaugh P.O. Neural blockade in clinical anesthesia and management of pain, ed 3. Philadelphia: Lippincott-Raven; 1998.
55. Datta S., Lee M., Falco F.J., et al. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician. 2009;12(2):437-460.
56. Derby R., Kine G., Saal J.A., et al. Response to steroid and duration of radicular pain as predictors of surgical outcome. Spine (Phila Pa 1976). 1992;17(6 suppl):S176-S183.
57. Devor M., Govrin-Lippmann R., Raber P. Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain. 1985;22(2):127-137.
58. Deyo R.A. Low-back pain. Sci Am. 1998;279(2):48-53.
59. Deyo R.A., Mirza S.K., Martin B.I. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976). 2006;31(23):2724-2727.
60. Deyo R.A., Rainville J., Kent D.L. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
61. Dreyfuss P. Proceedings of the 8th Annual Scientific Meeting of the International Spine Intervention Society. San Francisco. 2000.
62. Dreyfuss P., Baker R., Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med. 2006;7(3):237-242.
63. Dreyfuss P., Halbrook B., Pauza K., et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine (Phila Pa 1976). 2000;25(10):1270-1277.
64. Dreyfuss P., Michaelsen M., Pauza K., et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976). 1996;21(22):2594-2602.
65. Dreyfuss P., Schwarzer A.C., Lau P., et al. Specificity of lumbar medial branch and L5 dorsal ramus blocks: a computed tomography study. Spine (Phila Pa 1976). 1997;22(8):895-902.
66. Dreyfuss P., Stout A., Aprill C., et al. The significance of multifidus atrophy after successful radiofrequency neurotomy for low back pain. Phys Med Rehabil. 2009;1(8):719-722.
67. Dreyfuss P., Tibiletti C., Dreyer S.J. Thoracic zygapophyseal joint pain patterns. A study in normal volunteers. Spine (Phila Pa 1976). 1994;19(7):807-811.
68. Dreyfuss P Yin W: Conscious sedation pros and cons, Proceedings of the 15th Annual Scientific Meeting of the International Spine Intervention Society, Baltimore, July 2007.
69. Dubois E.F., Wagemans M.F., Verdouw B.C., et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
70. Ferrante F.M., King L.F., Roche E.A., et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26(2):137-142.
71. Fishman S.M., Smith H., Meleger A., et al. Radiation safety in pain medicine. Reg Anesth Pain Med. 2002;27(3):296-305.
72. Fortin J.D., Kissling R.O., O’Connor B.L., et al. Sacroiliac joint innervation and pain. Am J Orthop. 1999;28(12):687-690.
73. Fortin J.D., Washington W.J., Falco F.J. Three pathways between the sacroiliac joint and neural structures. AJNR Am J Neuroradiol. 1999;20(8):1429-1434.
74. Foye P.M., Buttaci C.J., Stitik T.P., et al. Successful injection for coccyx pain. Am J Phys Med Rehabil. 2006;85(9):783-784.
75. Fraser R.D., Osti O.L., Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br. 1987;69(1):26-35.
76. Freemont A.J., Peacock T.E., Goupille P., et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350(9072):178-181.
77. Friedly J., Chan L., Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine (Phila Pa 1976). 2007;32(16):1754-1760.
78. Fujita Y., Ishikawa H., Yokota K. Anaphylactoid reaction to midazolam. Anesth Analg. 1994;79(4):811-812.
79. Fukui S., Ohseto K., Shiotani M., et al. Referred pain distribution of the cervical zygapophyseal joints and cervical dorsal rami. Pain. 1996;68(1):79-83.
80. Fukui S., Ohseto K., Shiotani M., et al. Distribution of referred pain from the lumbar zygapophyseal joints and dorsal rami. Clin J Pain. 1997;13(4):303-307.
81. Furman M.B., Lee T.S., Mehta A., et al. Contrast flow selectivity during transforaminal lumbosacral epidural steroid injections. Pain Physician. 2008;11(6):855-861.
82. Furusawa N., Baba H., Miyoshi N., et al. Herniation of cervical intervertebral disc: immunohistochemical examination and measurement of nitric oxide production. Spine (Phila Pa 1976). 2001;26(10):1110-1116.
83. Gardner W.J., Goebert H.W.Jr., Sehgal A.D. Intraspinal corticosteroids in the treatment of sciatica. Trans Am Neurol Assoc. 1961;86:214-215.
84. Gevargez A., Groenemeyer D., Schirp S., et al. CT-guided percutaneous radiofrequency denervation of the sacroiliac joint. Eur Radiol. 2002;12(6):1360-1365.
85. Goodman B.S., Posecion L.W., Mallempati S., et al. Complications and pitfalls of lumbar interlaminar and transforaminal epidural injections. Curr Rev Musculoskelet Med. 2008;1(3-4):212-222.
86. Govind J., King W., Bailey B., et al. Radiofrequency neurotomy for the treatment of third occipital headache. J Neurol Neurosurg Psychiatry. 2003;74(1):88-93.
87. Guyer R.D., Ohnmeiss D.D. Lumbar discography. Position statement from the North American Spine Society Diagnostic and Therapeutic Committee. Spine (Phila Pa 1976). 1995;20(18):2048-2059.
88. Hall E.D. Acute effects of intravenous glucocorticoid on cat spinal motor neuron electrical properties. Brain Res. 1982;240(1):186-190.
89. Hall E.D. Glucocorticoid effects on central nervous excitability and synaptic transmission. Int Rev Neurobiol. 1982;23:165-195.
90. Hassenbusch S.J., Gunes S., Wachsman S., et al. Intrathecal clonidine in the treatment of intractable pain: a phase I/II study. Pain Med. 2002;3(2):85-91.
91. Hassenbusch S.J., Portenoy R.K., Cousins M., et al. Polyanalgesic Consensus Conference 2003: an update on the management of pain by intraspinal drug delivery—report of an expert panel. J Pain Symptom Manage. 2004;27(6):540-563.
92. Helm S., Hayek S.M., Benyamin R.M., et al. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009;12(1):207-232.
93. Hirata F., Schiffmann E., Venkatasubramanian K., et al. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980;77(5):2533-2536.
94. Hodgson P.S., Neal J.M., Pollock J.E., et al. The neurotoxicity of drugs given intrathecally (spinal). Anesth Analg. 1999;88(4):797-809.
95. Hogan Q.H. Epidural anatomy examined by cryomicrotome section. Influence of age, vertebral level, and disease. Reg Anesth. 1996;21(5):395-406.
96. Horlocker T.T., Bajwa Z.H., Ashraf Z., et al. Risk assessment of hemorrhagic complications associated with nonsteroidal antiinflammatory medications in ambulatory pain clinic patients undergoing epidural steroid injection. Anesth Analg. 2002;95(6):1691-1697.
97. Horlocker T.T., Wedel D.J., Benzon H., et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003;28(3):172-197.
98. Houten J.K., Errico T.J. Paraplegia after lumbosacral nerve root block: report of three cases. Spine J. 2002;2(1):70-75.
99. Husted D.S., Orton D., Schofferman J., et al. Effectiveness of repeated radiofrequency neurotomy for cervical facet joint pain. J Spinal Disord Tech. 2008;21(6):406-408.
100. Huston C.W. Cervical epidural steroid injections in the management of cervical radiculitis: interlaminar versus transforaminal: a review. Curr Rev Musculoskelet Med. 2009;2(1):30-42.
101. Hutchinson A., Waddel G., Feder G., et al. Clinical guidelines for the management of acute low back pain. London: Royal College of General Practitioners; 1996.
102. Jarvik J.G., Deyo R.A. Imaging of lumbar intervertebral disk degeneration and aging, excluding disk herniations. Radiol Clin North Am. 2000;38(6):1255-1266,. vi
103. Bonica J.J. The management of pain. Philadelphia: Lea & Febinger; 1953.
104. Johansson A., Hao J., Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34(5):335-338.
105. Jull G., Bogduk N., Marsland A. The accuracy of manual diagnosis for cervical zygapophysial joint pain syndromes. Med J Aust. 1988;148(5):233-236.
106. Kang J.D., Georgescu H.I., McIntyre-Larkin L., et al. Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976). 1995;20(22):2373-2378.
107. Kaplan M., Dreyfuss P., Halbrook B., et al. The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint. A physiologic challenge. Spine (Phila Pa 1976). 1998;23(17):1847-1852.
108. Kearon C., Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336(21):1506-1511.
109. Kepes E.R., Duncalf D. Treatment of backache with spinal injections of local anesthetics, spinal and systemic steroids: a review. Pain. 1985;22(1):33-47.
110. Kim K.H., Choi S.H., Kim T.K., et al. Cervical facet joint injections in the neck and shoulder pain. J Korean Med Sci. 2005;20(4):659-662.
111. Kim K.S., Shin S.S., Kim T.S., et al. Fluoroscopically guided cervical interlaminar epidural injections using the midline approach: an analysis of epidurography contrast patterns. Anesth Analg. 2009;108(5):1658-1661.
112. Kirpalani D., Mitra R. Cervical facet joint dysfunction: a review. Arch Phys Med Rehabil. 2008;89(4):770-774.
113. Koes B.W., Scholten R.J., Mens J.M., et al. Efficacy of epidural steroid injections for low-back pain and sciatica: a systematic review of randomized clinical trials. Pain. 1995;63(3):279-288.
114. Konen E., Konen O., Katz M., et al. Are referring clinicians aware of patients at risk from intravenous injection of iodinated contrast media? Clin Radiol. 2002;57(2):132-135.
115. Kornick C., Kramarich S.S., Lamer T.J., et al. Complications of lumbar facet radiofrequency denervation. Spine (Phila Pa 1976). 2004;29(12):1352-1354.
116. Kreppel D., Antoniadis G., Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003;26(1):1-49.
117. Kumar K., Taylor R.S., Jacques L., et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188.
118. Laslett M., Aprill C.N., McDonald B., et al. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
119. Laslett M., Young S.B., Aprill C.N., et al. Diagnosing painful sacroiliac joints: a validity study of a McKenzie evaluation and sacroiliac provocation tests. Aust J Physiother. 2003;49(2):89-97.
120. Lau P., Mercer S., Govind J., et al. The surgical anatomy of lumbar medial branch neurotomy (facet denervation). Pain Med. 2004;5(3):289-298.
121. Layton K.F., Kallmes D.F., Horlocker T.T. Recommendations for anticoagulated patients undergoing image-guided spinal procedures. AJNR Am J Neuroradiol. 2006;27(3):468-470.
122. Leclaire R., Fortin L., Lambert R., et al. Radiofrequency facet joint denervation in the treatment of low back pain: a placebo-controlled clinical trial to assess efficacy. Spine (Phila Pa 1976). 2001;26(13):1411-1416. discussion 1417
123. Lievre J.A., Bloch-Michel H., Attali P. [Epidural hydrocortisone in the treatment of sciatica.] [French]. Rev Rhum Mal Osteoartic. 1955;22(9-10):696-697.
124. Lin E.L., Lieu V., Halevi L., et al. Cervical epidural steroid injections for symptomatic disc herniations. J Spinal Disord Tech. 2006;19(3):183-186.
125. Liu S.S., Mulroy M.F. Neuraxial anesthesia and analgesia in the presence of standard heparin. Reg Anesth Pain Med. 1998;23(6 suppl 2):157-163.
126. Lively M.W. Sports medicine approach to low back pain. South Med J. 2002;95(6):642-646.
127. Lofsky A. Sleep apnea and narcotic postoperative pain medication: a mortality and morbidity risk. Anesth Patient Saf Newslett. 17(39), 2002.
128. Lord S., McDonald G., Bogduk N. Percutaneous radiofrequency neurotomy of the cervical medial branches: a validated treatment for cervical zygapophysial joint pain. Neurosurg Q. 1998;8:288-308.
129. Lord S.M., Barnsley L., Bogduk N. The utility of comparative local anesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophysial joint pain. Clin J Pain. 1995;11(3):208-213.
130. Lord S.M., Barnsley L., Wallis B.J., et al. Chronic cervical zygapophysial joint pain after whiplash: a placebo-controlled prevalence study. Spine (Phila Pa 1976). 1996;21(15):1737-1744. discussion 1744–1745
131. Lord S.M., Barnsley L., Wallis B.J., et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335(23):1721-1726.
132. Lutz G.E., Vad V.B., Wisneski R.J. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil. 1998;79(11):1362-1366.
133. Luukkainen R., Nissila M., Asikainen E., et al. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondylarthropathy. Clin Exp Rheumatol. 1999;17(1):88-90.
134. Maddox T.G. Adverse reactions to contrast material: recognition, prevention, and treatment. Am Fam Physician. 2002;66(7):1229-1234.
135. Maigne J.Y., Aivaliklis A., Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain, Spine (Phila Pa 1976). 1996;21(16):1889-1892.
136. Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician. 2002;5(2):182-199.
137. Manchikanti L., Boswell M.V., Singh V., et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15.
138. Manchikanti L., Cash K.A., McManus C.D., et al. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain. 4. Spinal stenosis. Pain Physician. 2008;11(6):833-848.
139. Manchikanti L., Cash K.A., Pampati V., et al. Evaluation of lumbar transforaminal epidural injections with needle placement and contrast flow patterns: a prospective, descriptive report. Pain Physician. 2004;7(2):217-223.
140. Manchikanti L., Singh V., Cash K.A., et al. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain. 2. Disc herniation and radiculitis. Pain Physician. 2008;11(6):801-815.
141. Manchikanti L., Singh V., Pampati V., et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001;4(4):308-316.
142. Massey W.K., Stevens D.B. A critical evaluation of discography. J Bone Joint Surg Br. 1967;49:1243-1244.
143. Maugars Y., Mathis C., Vilon P., et al. Corticosteroid injection of the sacroiliac joint in patients with seronegative spondylarthropathy. Arthritis Rheum. 1992;35(5):564-568.
144. McDonald G.J., Lord S.M., Bogduk N. Long-term follow-up of patients treated with cervical radiofrequency neurotomy for chronic neck pain. Neurosurgery. 1999;45(1):61-67. discussion 67–68
145. Mellemkjaer L., Blot W.J., Sorensen H.T., et al. Upper gastrointestinal bleeding among users of NSAIDs: a population-based cohort study in Denmark. Br J Clin Pharmacol. 2002;53(2):173-181.
146. Mikeladze G., Espinal R., Finnegan R., et al. Pulsed radiofrequency application in treatment of chronic zygapophyseal joint pain. Spine J. 2003;3(5):360-362.
147. Nachemson A.L. Newest knowledge of low back pain: a critical look. Clin Orthop Relat Res. 1992;279:8-20.
148. Naguib M., Magboul M.M., Samarkandi A.H., et al. Adverse effects and drug interactions associated with local and regional anaesthesia. Drug Saf. 1998;18(4):221-250.
149. Nath S., Nath C.A., Pettersson K. Percutaneous lumbar zygapophysial (facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain: a randomized double-blind trial. Spine (Phila Pa 1976). 2008;33(12):1291-1297. discussion 1298
150. Nguyen H., Garber J.E., Hassenbusch S.J. Spinal analgesics. Anesthesiol Clin North America. 2003;21(4):805-816.
151. Nicholson T.R., Cutter W., Hotopf M. Assessing mental capacity: the Mental Capacity Act. BMJ. 2008;336(7639):322-325.
152. North R.B., Kidd D.H., Farrokhi F., et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-106. discussion 107
153. Penn R.D. Intrathecal medication delivery. Neurosurg Clin N Am. 2003;14(3):381-387.
154. Pollock B., Wilkinson S.M., MacDonald Hull S.P. Chronic urticaria associated with intra-articular methylprednisolone. Br J Dermatol. 2001;144(6):1228-1230.
155. Prager J.P. Neuraxial medication delivery: the development and maturity of a concept for treating chronic pain of spinal origin. Spine (Phila Pa 1976). 2002;27(22):2593-2605. discussion 2606
156. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
157. Rainov N.G., Heidecke V. Management of chronic back and leg pain by intrathecal drug delivery. Acta Neurochir Suppl. 2007;97(Pt 1):49-56.
158. Raj P. Pain medicine: a comprehensive review. St Louis: Mosby; 1996.
159. Rathmell J.P., Aprill C., Bogduk N. Cervical transforaminal injection of steroids. Anesthesiology. 2004;100(6):1595-1600.
160. Riew K.D., Yin Y., Gilula L., et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain: a prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am. 2000;82(11):1589-1593.
161. Rodriguez Y., Baena R., Gaetani P., et al. Spinal epidural hematoma during anticoagulant therapy: a case report and review of the literature. J Neurosurg Sci. 1995;39(1):87-94.
162. Rosenberg J.M., Quint T.J., de Rosayro A.M. Computerized tomographic localization of clinically-guided sacroiliac joint injections. Clin J Pain. 2000;16(1):18-21.
163. Saal J.A., Saal J.S. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy: an outcome study. Spine (Phila Pa 1976). 1989;14(4):431-437.
164. Saal J.S., Franson R.C., Dobrow R., et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine (Phila Pa 1976). 1990;15(7):674-678.
165. Scanlon G.C., Moeller-Bertram T., Romanowsky S.M., et al. Cervical transforaminal epidural steroid injections: more dangerous than we think? Spine (Phila Pa 1976). 2007;32(11):1249-1256.
166. Schaufele M.K., Hatch L., Jones W. Interlaminar versus transforaminal epidural injections for the treatment of symptomatic lumbar intervertebral disc herniations. Pain Physician. 2006;9(4):361-366.
167. Schofferman J., Kine G. Effectiveness of repeated radiofrequency neurotomy for lumbar facet pain. Spine (Phila Pa 1976). 2004;29(21):2471-2473.
168. Schwarzer A.C., Aprill C.N., Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
169. Schwarzer A.C., Aprill C.N., Derby R., et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints: is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976). 1994;19(10):1132-1137.
170. Schwarzer A.C., Derby R., Aprill C.N., et al. The value of the provocation response in lumbar zygapophyseal joint injections. Clin J Pain. 1994;10(4):309-313.
171. Schwarzer A.C., Wang S.C., Bogduk N., et al. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54(2):100-106.
172. Sehgal A., Valentine J.M. Lumbar radiculopathy after zygapophyseal joint injection. Br J Anaesth. 2007;99(3):412-414.
173. Sethee J., Rathmell J.P. Epidural steroid injections are useful for the treatment of low back pain and radicular symptoms: pro. Curr Pain Headache Rep. 2009;13(1):31-34.
174. Slipman C.W., Jackson H.B., Lipetz J.S., et al. Sacroiliac joint pain referral zones. Arch Phys Med Rehabil. 2000;81(3):334-338.
175. Slipman C.W., Lipetz J.S., Plastaras C.T., et al. Therapeutic zygapophyseal joint injections for headaches emanating from the C2-3 joint. Am J Phys Med Rehabil. 2001;80(3):182-188.
176. Slipman C.W., Patel R.K., Botwin K., et al. Epidemiology of spine tumors presenting to musculoskeletal physiatrists. Arch Phys Med Rehabil. 2003;84(4):492-495.
177. Slipman C.W., Sterenfeld E.B., Chou L.H., et al. The value of radionuclide imaging in the diagnosis of sacroiliac joint syndrome. Spine (Phila Pa 1976). 1996;21(19):2251-2254.
178. Slipman C.W., Sterenfeld E.B., Chou L.H., et al. The predictive value of provocative sacroiliac joint stress maneuvers in the diagnosis of sacroiliac joint syndrome. Arch Phys Med Rehabil. 1998;79(3):288-292.
179. Smith H.S., Chopra P., Patel V.B., et al. Systematic review of the role of sedation in diagnostic spinal interventional techniques. Pain Physician. 2009;12(1):195-206.
180. Spitzer W.O., LeBlanc F.E., Dupuis M. Scientific approach to the assessment and management of activity-related spinal disorders. A monographe for clinicians. Report of the Quebec Task Force on Spinal Disorders. Spine. 1987;12(7 suppl):51-59.
181. Stedeford J.C., Pittman J.A. Von Willebrand’s disease and neuroaxial anaesthesia. Anaesthesia. 2000;55(12):1228-1229.
182. Stout A: Risks and side effects of steroids and anesthetics in spinal procedures, Proceedings of the 15th Annual Scientific Meeting of the International Spine Intervention Society, Baltimore, July 2007.
183. Stove J., Schoniger R., Huch K., et al. Effects of dexamethasone on proteoglycan content and gene expression of IL-1beta-stimulated osteoarthrotic chondrocytes in vitro. Acta Orthop Scand. 2002;73(5):562-567.
184. Stovner L.J., Kolstad F., Helde G. Radiofrequency denervation of facet joints C2- C6 in cervicogenic headache: a randomized, double-blind, sham-controlled study. Cephalalgia. 2004;24(10):821-830.
185. UMHS. UMHS low back pain guideline update 2003. Available at: http://cme.med.umich.edu/pdf/guideline/backpain03.pdf. Accessed May 14, 2009.
186. Vad V.B., Bhat A.L., Lutz G.E., et al. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine (Phila Pa 1976). 2002;27(1):11-16.
187. van den Hoogen H.M., Koes B.W., van Eijk J.T., et al. On the accuracy of history, physical examination, and erythrocyte sedimentation rate in diagnosing low back pain in general practice: a criteria-based review of the literature. Spine (Phila Pa 1976). 1995;20(3):318-327.
188. van der Wurff P., Buijs E.J., Groen G.J. A multitest regimen of pain provocation tests as an aid to reduce unnecessary minimally invasive sacroiliac joint procedures. Arch Phys Med Rehabil. 2006;87(1):10-14.
189. van Kleef M., Barendse G.A., Kessels A., et al. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine (Phila Pa 1976). 1999;24(18):1937-1942.
190. van Tulder M., Becker A., Bekkering T., et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15(suppl 2):S169-S191.
191. van Wijk R.M., Geurts J.W., Wynne H.J., et al. Radiofrequency denervation of lumbar facet joints in the treatment of chronic low back pain: a randomized, double-blind, sham lesion-controlled trial. Clin J Pain. 2005;21(4):335-344.
192. Vandermeulen E.P., Van Aken H., Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79(6):1165-1177.
193. Vassiliev D. Spread of contrast during L4 and L5 nerve root infiltration under fluoroscopic guidance. Pain Physician. 2007;10(3):461-466.
194. Vinas F.C., Zamorano L., Dujovny M., et al. In vivo and in vitro study of the lesions produced with a computerized radiofrequency system. Stereotact Funct Neurosurg. 1992;58(1-4):121-133.
195. Vonbach P., Reich R., Moll F., et al. Risk factors for gastrointestinal bleeding: a hospital-based case-control study. Swiss Med Wkly. 2007;137(49-50):705-710.
196. Waldman S.D., Feldstein G.S., Waldman H.J., et al. Caudal administration of morphine sulfate in anticoagulated and thrombocytopenic patients. Anesth Analg. 1987;66(3):267-268.
197. Walsh T.R., Weinstein J.N., Spratt K.F., et al. Lumbar discography in normal subjects. A controlled, prospective study. J Bone Joint Surg Am. 1990;72(7):1081-1088.
198. Wang C.L., Cohan R.H., Ellis J.H., et al. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2008;191(2):409-415.
199. Wang J.K., Nauss L.A., Thomas J.E. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50(2):149-151.
200. Weinstein J.N., Lurie J.D., Olson P.R., et al. United States’ trends and regional variations in lumbar spine surgery: 1992-2003. Spine (Phila Pa 1976). 2006;31(23):2707-2714.
201. Willard F: Proceedings of the Third World Congress on Low Back and Pelvic Pain, Vienna, Austria, 1998. Available at: http://www.fasciare search.com/WCLBP/WCLBP.htm. Accessed May 2, 2009.
202. Williams K.N., Jackowski A., Evans P.J. Epidural haematoma requiring surgical decompression following repeated cervical epidural steroid injections for chronic pain. Pain. 1990;42(2):197-199.
203. Windsor R.E., Storm S., Sugar R. Prevention and management of complications resulting from common spinal injections. Pain Physician. 2003;6(4):473-483.
204. Wolfer L.R., Derby R., Lee J.E., et al. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false-positive rates. Pain Physician. 2008;11(4):513-538.
205. Wulf H. Epidural anaesthesia and spinal haematoma. Can J Anaesth. 1996;43(12):1260-1271.
206. Yabuki S., Kawaguchi Y., Nordborg C., et al. Effects of lidocaine on nucleus pulposus-induced nerve root injury: a neurophysiologic and histologic study of the pig cauda equina. Spine (Phila Pa 1976). 1998;23(22):2383-2389. discussion 2389–2390
207. Yabuki S., Kikuchi S. Nerve root infiltration and sympathetic block: an experimental study of intraradicular blood flow. Spine (Phila Pa 1976). 1995;20(8):901-906.
208. Yoganandan N., Knowles S.A., Maiman D.J., et al. Anatomic study of the morphology of human cervical facet joint. Spine (Phila Pa 1976). 2003;28(20):2317-2323.
209. Younes M., Neffati F., Touzi M., et al. Systemic effects of epidural and intra-articular glucocorticoid injections in diabetic and non-diabetic patients. Joint Bone Spine. 2007;74(5):472-476.