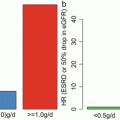
Fig. 6.1
The number of urinary podocytes in patients with IgAN with segmental sclerosis was higher than those in patients without (Modified from Ref. [12])
To more easily interpret the current histological results of patients suspected of having IgAN, it is necessary to develop new noninvasive biomarkers.
6.2 Urinary Podocalyxin
Podocalyxin (PCX) is a sialomucin that is most closely related to CD34 and endoglycan and is expressed by podocytes, hematopoietic progenitors, vascular endothelia, and a subset of neurons [13]. PCX is usually located on the apical cell membrane of podocytes and is shed into urine from injured podocytes [14]. Human urinary PCX (u-PCX) originates not from podocyte exosomes but from the tip vesiculation of glomerular podocyte microvilli [15]. Kanno et al. measured the levels of u-PCX in children with glomerular diseases such as IgAN and concluded that u-PCX was a useful biomarker for estimating the severity of active glomerular injury and could serve as a urinary index of acute extracapillary abnormalities in children [16]. Levels of u-PCX in adults with various forms of active glomerulonephritis were reported to be significantly higher than those in patients with chronic glomerulonephritis in long-term remission [17]. We used the Shigematsu classification to determine acute and chronic extracapillary abnormalities [18]. We found a positive correlation between levels of u-PCX and acute extracapillary abnormalities in adults with IgAN (Fig. 6.2) [12]. Conversely, levels of urinary protein excretion did not correlate with acute glomerular abnormalities. Acute extracapillary abnormalities can be followed by the detachment of podocytes and glomerular basement membrane (GBM) denudation, which are the initial steps in the development of glomerulosclerosis [19]. Possibly, u-PCX reflects acute extracapillary abnormalities and is an index of ongoing podocyte injury leading to glomerulosclerosis.
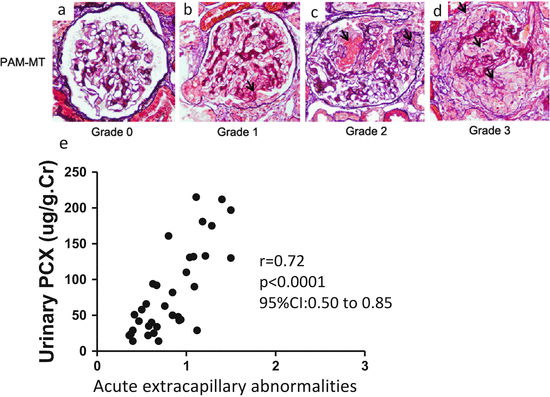

Fig. 6.2
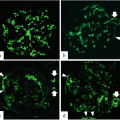
Representative histological findings of acute extracapillary abnormalities stained with periodic acid methenamine silver-Masson trichrome. (a) In grade 0, no acute extracapillary abnormality was observed. (b) In grade 1, a small cellular crescent formation was observed (arrow). (c) In grade 2, exudates that had escaped into the urinary space and a cellular crescent were observed (arrows). (d) In grade 3, three cellular crescents were observed (arrows). (e) Urinary podocalyxin was significantly correlated with the severity of acute extracapillary abnormalities in adult patients with IgAN (Modified from Ref. [12])
Several studies have shown that corticosteroid treatment decreases the levels of urinary protein excretion and improves renal outcomes in patients with IgAN [20–22]. Shoji et al. reported that early corticosteroid treatment for active and proliferative IgAN in adults was effective for reducing renal injury [23]. Immunosuppressive treatment, including corticosteroids, is recommended for crescentic IgAN with active glomerular inflammation [24]. These results suggest that the measurement of u-PCX may be a useful biomarker to determine the effectiveness of corticosteroid treatment.
6.3 Urinary Megalin (uMegalin)
Megalin is a large (600 kDa) glycoprotein that is a member of the low-density lipoprotein receptor family [25, 26]. It is highly expressed at the apical membranes of proximal tubular epithelial cells (PTECs) [27]. Megalin plays a central role in the endocytotic functions of PTECs [27]. Low-molecular-weight protein markers of PTEC injury, such as α1-microglobulin (α1-MG) and β2-microglobulin (β2-MG), are filtered by glomeruli and reabsorbed by PTECs via megalin [28, 29]. Megalin is detected in human urine, and increases in urinary megalin (uMegalin) excretion appear in microalbuminuric patients with type 1 diabetes [30–33]. The levels of uMegalin have been linked to the severity of diabetic nephropathy in type 2 diabetes [34].
To understand the relationship between the levels of uMegalin and renal histological findings in adult IgAN patients, we focused on uMegalin. The levels of uMegalin, α1-MG, β2-MG, and N-acetyl-β-D-glucosaminidase (NAG) are not correlated with tubular atrophy and interstitial fibrosis in the Oxford classification. Surprisingly, however, the levels of uMegalin are correlated with chronic extracapillary abnormalities, showing a distinctive significance compared to preexisting urinary biomarkers (β2-MG, α1-MG, NAG, and urinary total protein) (Fig. 6.3). In addition, the levels of uMegalin are correlated with mesangial hypercellularity in the Oxford classification. Megalin is localized in the brush border of proximal tubules, but not in the glomerulus in the human kidney [35, 36]. There are two proposed mechanisms for uMegalin levels that reflect glomerular abnormalities. The first theory is that glomerular abnormalities trigger PTEC dysfunction, leading to the excretion of megalin in the urine. Although it remains unclear how glomerular abnormalities lead to tubulointerstitial injury in IgAN, a possible pathogenetic mechanism is glomerulopodocytic-tubular communication [37]. The second theory involves the possibility that PTEC dysfunction aggravates chronic glomerular abnormalities. Selective PTEC injury reportedly drives the formation of interstitial fibrosis and potentially glomerulosclerosis [38]. The progression of PTEC injury may aggravate chronic extracapillary abnormalities and lead to glomerulosclerosis.
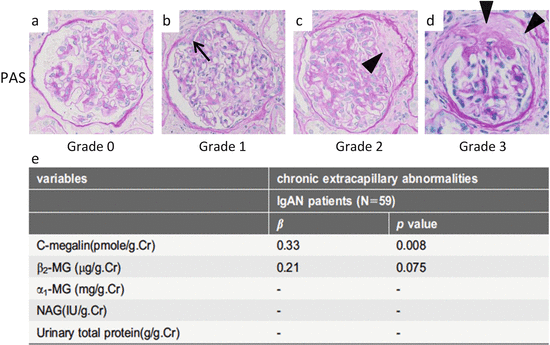

Fig. 6.3
Representative histological findings of chronic extracapillary abnormalities stained with periodic acid-Schiff (PAS). (a) In grade 0, no chronic extracapillary abnormalities were observed. (b) In grade 1, a small adhesion with Bowman’s capsule was observed (arrow). c In grade 2, a moderate fibrous crescent was observed (arrowhead). (d) In grade 3, a severe fibrous crescent was observed (arrowheads). (e) There was a significant correlation between the levels of urinary megalin and the severity of chronic extracapillary abnormalities (Modified from Ref. [35])
Supportive therapy with the cautious use of either angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) should be continued with IgAN patients in order to slow the process, although an eGFR that is persistently less than 30 mL/min/1.73 m2 poses a substantial risk of progression to end-stage renal disease (ESRD) [39]. ACEI or ARB is effective for long-term renal survival of advanced IgAN patients whose histological findings contain chronic glomerular abnormalities [40]. In future studies, we aim to clarify whether uMegalin is a useful biomarker to determine the effectiveness of ACEI or ARB treatment. We also must examine the levels of uMegalin before and after the treatment of IgAN patients.
In our study, levels of uMegalin were associated with dialysis that required risk levels from the Clinical Guidelines for IgAN in Japan, third version [35, 41] (Fig. 6.4). The Oxford classification of IgAN identifies four pathologic abnormalities that independently determine the risk of developing progressive renal disease: mesangial hypercellularity, endocapillary hypercellularity, segmental glomerulosclerosis, and tubular atrophy/interstitial fibrosis [7, 42]. Levels of uMegalin were correlated with only mesangial hypercellularity, according to the Oxford classification. Although levels of urinary β2-MG were correlated with segmental glomerulosclerosis, its urinary excretion offered no advantage over proteinuria to predict the prognosis in IgAN patients [43]. Thus, there is a possibility that uMegalin is an independent predictor of disease progression with IgAN. In future studies, it will be necessary to perform a longitudinal study to follow up with IgAN patients to determine whether uMegalin is a predictor.
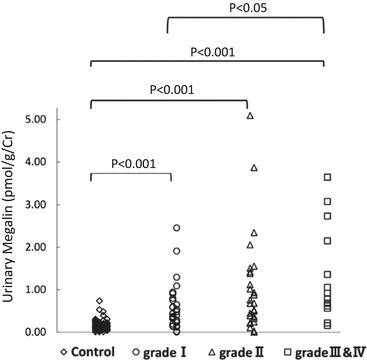

Fig. 6.4
Relationship of risk levels requiring dialysis to levels of urinary megalin. I, low-risk group; II, medium-risk group; and, III and IV, high- and superhigh-risk groups (Modified from Ref. [35])
The number of glomeruli obtained by renal biopsy was sometimes insufficient in order to evaluate the severity of glomerular changes. However, because u-PCX and uMegalin originate primarily from all nephrons in the kidney, the urinary biomarkers seem to reflect the histological findings of all nephrons. Urinary biomarkers might help us to speculate on the histological results of renal biopsy.
In conclusion, the biomarkers u-PCX and uMegalin have a potential role as an adjunct to the diagnosis of renal histology in IgAN patients.
Conflict of Interest
The author declares that he has no conflict of interest.
References
1.
2.
Rychlik I, Andrassy K, Waldherr R, Zuna I, Tesar V, Jancova E, et al. Clinical features and natural history of IgA nephropathy. Ann Med Interne. 1999;150(2):117–26.
3.
Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol. 1968;74(9):694–5.
4.
5.
6.
7.
8.
9.
10.
11.
12.
Asao R, Asanuma K, Kodama F, Akiba-Takagi M, Nagai-Hosoe Y, Seki T, et al. Relationships between levels of urinary podocalyxin, number of urinary podocytes, and histologic injury in adult patients with IgA nephropathy. Clin J Am Soc Nephrol. 2012;7(9):1385–93.CrossRefPubMedPubMedCentral
13.
14.
15.
16.
17.
Habara P, Mareckova H, Sopkova Z, Malickova K, Zivorova D, Zima T, et al. A novel method for the estimation of podocyte injury: podocalyxin-positive elements in urine. Folia Biol. 2008;54(5):162–7.
18.
19.
20.
21.
22.
23.
24.
25.
Saito A, Pietromonaco S, Loo AK, Farquhar MG. Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A. 1994;91(21):9725–9.CrossRefPubMedPubMedCentral
26.
Hjalm G, Murray E, Crumley G, Harazim W, Lundgren S, Onyango I, et al. Cloning and sequencing of human gp330, a Ca(2+)-binding receptor with potential intracellular signaling properties. Eur J Biochem FEBS. 1996;239(1):132–7.CrossRef
27.
Saito A, Sato H, Iino N, Takeda T. Molecular mechanisms of receptor-mediated endocytosis in the renal proximal tubular epithelium. J Biomed Biotechnol. 2010;2010:403272.CrossRefPubMedPubMedCentral
28.
Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol. 1999;155(4):1361–70.CrossRefPubMedPubMedCentral
29.
30.
31.
Bachinsky DR, Zheng G, Niles JL, McLaughlin M, Abbate M, Andres G, et al. Detection of two forms of GP330. Their role in Heymann nephritis. Am J Pathol. 1993;143(2):598–611.PubMedPubMedCentral
32.
Kounnas MZ, Chappell DA, Strickland DK, Argraves WS. Glycoprotein 330, a member of the low density lipoprotein receptor family, binds lipoprotein lipase in vitro. J Biol Chem. 1993;268(19):14176–81.PubMed
33.
Thrailkill KM, Nimmo T, Bunn RC, Cockrell GE, Moreau CS, Mackintosh S, et al. Microalbuminuria in type 1 diabetes is associated with enhanced excretion of the endocytic multiligand receptors megalin and cubilin. Diabetes Care. 2009;32(7):1266–8.CrossRefPubMedPubMedCentral
34.
Ogasawara S, Hosojima M, Kaseda R, Kabasawa H, Yamamoto-Kabasawa K, Kurosawa H, et al. Significance of urinary full-length and ectodomain forms of megalin in patients with type 2 diabetes. Diabetes Care. 2012;35(5):1112–8.CrossRefPubMedPubMedCentral
35.
Seki T, Asanuma K, Asao R, Nonaka K, Sasaki Y, Oliva Trejo JA, et al. Significance of urinary full-length megalin in patients with IgA nephropathy. PLoS One. 2014;9(12):e114400.CrossRefPubMedPubMedCentral
36.
Kerjaschki D, Horvat R, Binder S, Susani M, Dekan G, Ojha PP, et al. Identification of a 400-kd protein in the brush borders of human kidney tubules that is similar to gp330, the nephritogenic antigen of rat Heymann nephritis. Am J Pathol. 1987;129(1):183–91.PubMedPubMedCentral
37.
Lai KN, Tang SC, Leung JC. Recent advances in IgA nephropathy – the glomerulopodocytic-tubular communication. Adv Otorhinolaryngol. 2011;72:40–4.PubMed
38.
Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82(2):172–83.CrossRefPubMedPubMedCentral
40.
Moriyama T, Amamiya N, Ochi A, Tsuruta Y, Shimizu A, Kojima C, et al. Long-term beneficial effects of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy for patients with advanced immunoglobulin A nephropathy and impaired renal function. Clin Exp Nephrol. 2011;15(5):700–7.CrossRefPubMed
41.
42.
43.
Peters HP, van den Brand JA, Wetzels JF. Urinary excretion of low-molecular-weight proteins as prognostic markers in IgA nephropathy. Neth J Med. 2009;67(2):54–61.PubMed