Weaning from Mechanical Ventilation
Mechanical ventilation is often lifesaving, but it is associated with numerous complications.1,2 Accordingly, it is imperative to disconnect patients from the ventilator at the earliest feasible time. Deciding the right time to initiate this disconnection process, usually referred to as weaning, is one of the greatest challenges in critical care medicine.3 If a physician is too conservative and postpones the initiation of weaning, the patient is placed at an increased risk of life-threatening, ventilator-associated complications. Conversely, if weaning is begun prematurely, the patient may suffer cardiopulmonary or psychological decompensation of sufficient severity to set back a patient’s clinical course.4
Pathophysiology of Weaning Failure
After patients have been disconnected from the ventilator, up to 25% experience respiratory distress severe enough to necessitate the reinstitution of mechanical ventilation.5,6 The pathophysiologic mechanisms of weaning failure can be divided into those occurring at the level of the respiratory control system, mechanics of the lung and chest wall, the respiratory muscles, the cardiovascular system, and gas-exchange properties of the lung.7
Control of Breathing
Many weaning-failure patients develop hypercapnia. Accordingly, it had been thought that these patients experience an acute decrease in minute ventilation consequent to a decrease in respiratory center output.3 Measurements of respiratory motor output, using mean inspiratory flow or airway occlusion pressure (P0.1), have consistently revealed an increase, not a decrease, in respiratory drive in weaning-failure patients.4,8,9
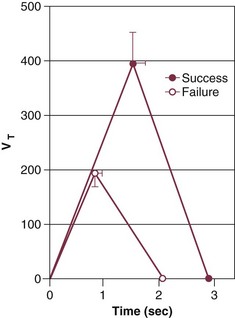
Weaning-failure patients, however, exhibit marked abnormalities in respiratory timing, specifically marked shortening of inspiratory time (TI), which is coupled with shortening of expiratory time (TE). The decrease in both TI and TE means that respiratory frequency (f) is markedly elevated. The shortening of TI combined with a normal mean inspiratory flow (VT/TI) results in a marked decrease of tidal volume (VT).8 This combination (elevated f and decreased VT) is referred to as rapid shallow breathing—now recognized as the physiologic hallmark of weaning failure (Fig. 43.1).3
Respiratory Mechanics
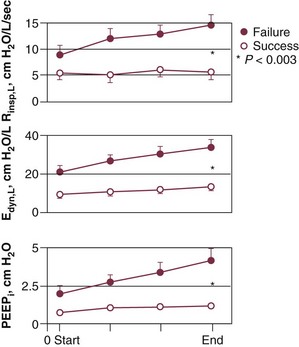
The most detailed study of respiratory mechanics during weaning trials was carried out by Jubran and Tobin.10,11 Immediately before commencement of a trial of spontaneous breathing, patients who went on to tolerate or fail the trial showed little or no difference in detailed measurements of passive respiratory mechanics.11 Resistance, elastance, and intrinsic positive end-expiratory pressure (PEEPi) were equivalent in the two groups.
Over the course of the trial, all of these variables became more abnormal in the weaning-failure patients than in the weaning-success patients (Fig. 43.2).10 Respiratory resistance increased progressively, reaching about seven times the normal value at the end of the trial. Pulmonary elastance increased, reaching five times the normal value. Intrinsic PEEP more than doubled over the course of the trial. A similar pattern has been observed by other investigators.12 The observation that respiratory mechanics were equivalent in weaning-success and weaning-failure patients immediately before a weaning trial but deteriorated immediately in the weaning-failure patients as soon as they began to breathe spontaneously indicates that some mechanism associated with the act of spontaneous breathing causes the worsening of respiratory mechanics that leads to weaning failure.
Patient Effort
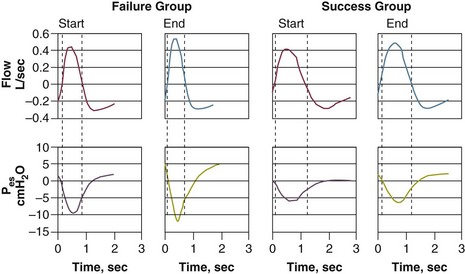
To compensate for the marked worsening of respiratory mechanics, patients need to make a greater inspiratory effort. It had been thought that weaning-failure patients make weaker inspiratory efforts than do weaning-success patients.7 On the contrary, direct measurements of work of breathing and pressure-time product13,14 show that weaning-failure patients consistently make a greater inspiratory effort than do weaning-success patients (Fig. 43.3).10,15
Respiratory Muscles
Numerous research groups have shown that maximal inspiratory pressure (PImax), a measure of respiratory muscle strength, does not discriminate between weaning-success and weaning-failure patients.7 These findings led to the belief that respiratory muscle weakness is not an important determinant of weaning outcome. PImax, however, can misrepresent respiratory muscle strength because the values are heavily influenced by patient motivation and cooperation.13 A more objective measure of diaphragmatic strength is obtained by stimulation of the phrenic nerves and recording the resulting transdiaphragmatic pressure (Pdi). Weaning-failure patients have twitch Pdi values below 10 cm H2O, whereas values of 35 to 39 cm H2O are observed in healthy subjects.16 These data suggest that weaning-failure patients may have considerable muscle weakness.
Stimulation of the phrenic nerves and recording of the resulting Pdi also provides the most direct measure of diaphragmatic fatigue.13 Laghi and coworkers17 employed this technique in 11 weaning-failure and 8 weaning-success patients before and after a T-tube trial. No patient in either group exhibited a fall in twitch pressure. This result was surprising. Related analyses disclosed why. Failure patients became progressively distressed during the trial, leading clinicians to reinstate ventilator support before patients had breathed long enough to develop fatigue (Fig. 43.4).17,18 In other words, monitoring clinical signs of distress provides sufficient warning to avoid respiratory muscle fatigue.
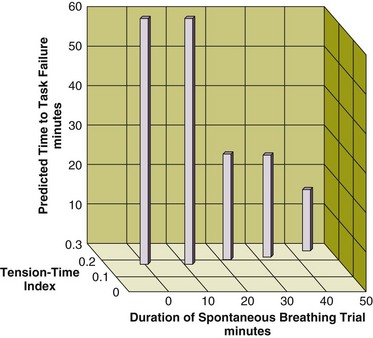
Figure 43.4 Interrelationship between the duration of a spontaneous breathing trial, tension-time index of the diaphragm, and predicted time to task failure in nine patients who failed a trial of weaning from mechanical ventilation. The patients breathed spontaneously for an average of 44 minutes before a physician terminated the trial. At the start of the trial, the tension-time index was 0.17, and the formula of Bellemare and Grassino18 predicted that patients could sustain spontaneous breathing for another 59 minutes before developing task failure. As the trial progressed, the tension-time index increased and the predicted time to development of task failure decreased. At the end of the trial, the tension-time index reached 0.26. That patients were predicted to sustain spontaneous breathing for another 13 minutes before developing task failure clarifies why patients did not develop a decrease in diaphragmatic twitch pressure. In other words, physicians interrupted the trial on the basis of clinical manifestations of respiratory distress, before patients had sufficient time to develop contractile fatigue. (Redrawn from Laghi F, Tobin MJ: Disorders of the respiratory muscles. Am J Respir Crit Care Med 2003;168:10-48.)
Cardiovascular Performance
During a weaning trial, patients can experience substantial increases in right and left ventricular afterload.19,20 These afterload increases most likely result from associated increases in negative swings of intrathoracic pressure. At the completion of a weaning trial, the level of oxygen consumption is equivalent in weaning-success and weaning-failure patients. How the cardiovascular system meets the oxygen demand differs in the two groups of patients. In weaning-success patients, oxygen demand is met through an increase in oxygen delivery, mediated by the expected increase in cardiac output on discontinuation of positive-pressure ventilation.20 In weaning-failure patients, oxygen demand is met through an increase in oxygen extraction; these patients have a relative decrease in oxygen delivery.20 The greater oxygen extraction causes a substantial decrease in mixed venous oxygen saturation, contributing to the arterial hypoxemia that occurs in some patients.20
Gas Exchange
Studies employing the multiple inert-gas technique have revealed that the ventilation-perfusion maldistribution and acute hypercapnia observed in weaning-failure patients is produced primarily by shallow breathing (low VT).7 About half of weaning-failure patients experience an increase in PaCO2 of 10 mm Hg or more over the course of a spontaneous breathing trial.10 The hypercapnia is not usually a consequence of a decrease in minute ventilation. Instead, it results from rapid, shallow breathing, which causes an increase in dead space ventilation. In a small proportion of weaning-failure patients, primary depression of respiratory motor output may be responsible for the hypercapnia.10
Weaning-Predictor Testing
In randomized controlled trials (RCTs) of different weaning techniques, most patients who had received mechanical ventilation for a week or longer were able to tolerate ventilator discontinuation on the first day that weaning-predictor tests were measured.5,6 Many of these patients probably would have tolerated extubation a day or so earlier. As such, one of the main sources of weaning delay is the failure of the physician to think that the patient just might come off the ventilator. Psychological research suggests that much of this delay in ventilator weaning results from clinicians being overconfident in their intuition that a patient is not ready for a weaning trial.4