Chapter 51D Vascular Diseases of the Nervous System
Stroke in Children
Epidemiology
Full-Term and Near-Term Neonates
Neonates appear to be at higher risk for stroke than older children. Asymptomatic subdural hemorrhage affects almost half of term neonates and can occur in infants delivered by both vaginal and cesarean delivery (Rooks et al., 2008). Symptomatic intracranial hemorrhage affects 1 in 100 full-term neonates (Gradnitzer et al., 2002). Most estimates place the rate of arterial ischemic stroke around 1 in 4000 neonates (Lynch and Nelson, 2001), but Gunther and colleagues (2000) found a much lower rate of 1.35 per 100,000 full-term births. Laugesaar et al. (2007) looked at all prospectively and retrospectively diagnosed perinatal stroke in Estonia and found a rate of 1 in 1587 live births. DeVeber and colleagues (2001) found a rate of 0.67 cases of cerebral venous thrombosis (CVT) per 100,000 children per year, with neonates making up 43% of cases; rates were not described in relation to the number of term births.
Chapter 80 discusses cerebral vascular injury in the premature neonate.
The General Population of Children
Estimates of the incidence of all pediatric stroke in the United States and France have ranged from 2.6 to 13 cases per 100,000 children per year (Giroud et al., 1995), with some variation among studies on the inclusion of neonates, traumatic strokes, and meningitis, and whether to use 16 or 18 as the age cutoff for pediatric stroke. Disagreement exists about whether hemorrhagic or ischemic strokes predominate. Estimates of the rate of hemorrhagic stroke ranged between 1.5 and 5 per 100,000 children per year (Giroud et al., 1995), and estimates of the rates of ischemic stroke ranged between 1.2 and 8 per 100,000 children per year (Agrawal et al., 2009; deVeber, 2002; Giroud et al., 1995). A Saudi Arabian study estimated the hospital frequency rate of all pediatric strokes as 27.1 per 100,000 pediatric admissions (Salih et al., 2006a).
High-Risk Subgroups
Vascular malformations may present with intracranial hemorrhage. At the Hospital for Sick Children in Toronto, 80% of children diagnosed with arteriovenous malformations (AVMs) presented with spontaneous intracranial hemorrhage (Humphreys et al., 1996), but it is not clear what percentage of children with AVMs never have hemorrhage or other neurological signs, since neuroimaging is generally prompted by neurological symptoms. Cavernous malformations and aneurysms may be genetic and can present in childhood. Risk varies depending on the mutation involved (Kato et al., 2001; Labauge et al., 2000, 2001). Intracranial hemorrhage may lead to vasospasm and resultant ischemic stroke, but this is less common in children than adults (Menkes et al., 2000).
Children with bleeding disorders are at high risk for intracerebral hemorrhage. Estimates of the rate of intracranial hemorrhage in children with hemophilia are up to 10% (Revel-Vilk et al., 2004); approximately 3% of neonates with hemophilia experience intracranial hemorrhage (Tarantino et al., 2007). Some 4% of patients with hemophilia have intracranial bleeds, and 48% of these bleeds are intracerebral (Klinge et al., 1999).
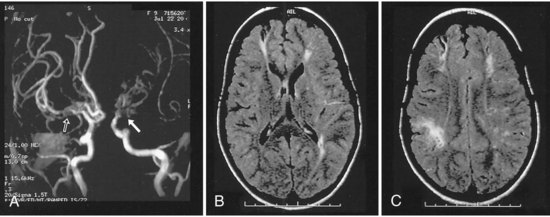
Children with sickle cell anemia are at risk for ischemic stroke because sickling red blood cells may lead to thrombosis or endothelial injury and in some patients are associated with moyamoya syndrome (Pegelow, 2001; Pegelow et al., 2002) (Fig. 51D.1). The rate of stroke in children with sickle cell anemia has dropped since the institution of transfusion therapy (see Therapies, later). In California, the incidence of first stroke in children with sickle cell anemia dropped from 0.88 per 100 person-years to 0.17 per 100 person-years (Fullerton et al., 2004); however, 17% have clinically silent infarcts (Pegelow, 2001). Kwiatkowski et al. (2009) found that 28% of asymptomatic young children (<6 years old) with sickle cell disease had silent infarcts on magnetic resonance imaging (MRI). Children with sickle cell anemia may also develop aneurysms and resultant intracranial hemorrhage, but this is more common in adults with sickle cell anemia (Pegelow, 2001).
Children treated with mechanical circulatory support are at increased risk for both intracranial hemorrhage and embolic ischemic stroke. The rates of infarction after extracorporeal membrane oxygenation (ECMO) vary dramatically among series, ranging from 0% to 26%; one study examined the brains of 44 patients who died while on ECMO and found evidence of focal ischemic infarct in 50% and intracranial hemorrhage in 52%. A recent large study of ECMO survivors found cerebral infarction in 7% and intracranial hemorrhage in 7% (Barrett et al., 2009). Stroke occurred in 7 of 17 treated children (41%) at one center that started to use the Berlin Heart EXCOR ventricular assist device (Rockett et al., 2008).
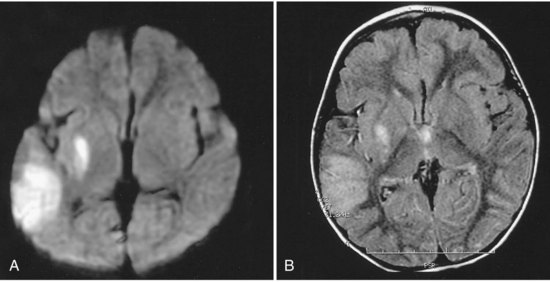
Children with complex congenital heart disease are at risk for cardioembolic stroke, thrombotic stroke, watershed infarcts from drops in perfusion pressure, and cerebral venous thrombosis (CVT) (Fig. 51D.2). The rates of stroke in children with complex congenital heart disease also vary among series, with some of the variation due to the severity of the malformation, the number of corrective surgeries required, anesthetic techniques during surgery, patient selection, and length of follow-up. A large Canadian study looked at 5526 children who underwent cardiac surgery and found a stroke rate of 5 per 1000 children (Domi et al., 2008). Mayer and associates (2002) found evidence of cerebrovascular complications in 5 of 77 (6.5%) pediatric patients with heart transplants and an average of 59.2 months of follow-up; 25 of the 77 patients (32.5%) died. The greatest risk for children with congenital heart disease occurs at the time of surgery or cardiac catheterization.
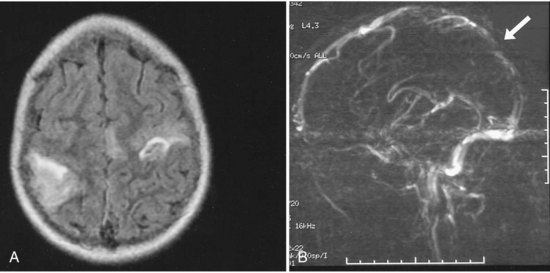
Children with cancer are at risk for both intracranial hemorrhage and ischemic infarction. Intracranial hemorrhage occurs secondary to thrombocytopenia from bone marrow suppression or bleeding within the tumors. Children with cancer may develop ischemic infarction or CVT due to leukostasis in the setting of leukemia; complications of chemotherapy such as l-asparaginase, which cause decreases in antithrombin, fibrinogen, and plasminogen; fungal or bacterial meningitis leading to arteritis; vasculopathy secondary to radiation; or complications of intracranial surgery (Fig. 51D.3). Bowers and colleagues (2002) observed 807 pediatric brain tumor patients for a total of 3224 non-perioperative years and found that 1.6% had a non-perioperative stroke. Children receiving mantle radiation for Hodgkin lymphoma may develop strokes years after treatment, possibly due to radiation-induced vascular changes (Bowers et al., 2005). The Children’s Oncology Group reviewed the literature and found that children surviving central nervous system tumors, Hodgkin lymphoma, or acute lymphoblastic leukemia treated with radiation to the head and/or neck were at increased risk of stroke from childhood into adulthood (Morris et al., 2009).
Presentations
Seizures are the most common manifestation of intraventricular hemorrhages (IVH), arterial ischemic strokes, and CVT in term neonates. Seizures are a presenting sign for 65% of term neonates with IVH, at least 80% of term neonates with arterial ischemic stroke (Volpe, 2001), and over 50% of neonates with CVT (Fitzgerald et al., 2006). Other presenting signs include apnea, irritability, jitteriness, lethargy, and bulging fontanel. The immature central nervous system may not demonstrate focal signs, and hemiparesis may not be apparent until a child is older than 6 months of age.
Children older than 6 months of age may present with seizures or focal signs similar to those seen in adult stroke, with hemiparesis, ataxia, or aphasia. Children younger than 1 year of age are more likely to present with seizures and altered mental status, whereas children older than 1 year of age are more likely to present with focal motor signs (Zimmer et al., 2007). Although severe headaches such as those in intracranial hemorrhages often prompt parents to seek medical attention immediately, most children arrive at the emergency room more than 6 hours after the event (Gabis et al., 2002). Parents may not detect focal motor weakness in a young child who has not yet started to walk or aphasia in a young child who is just starting to speak. Parents may interpret the sudden onset of focal neurological signs as behavioral rather than neurological. It can be easier to detect focal neurological signs and symptoms in older children, but these are also often missed in the first minutes to hours, possibly because many parents and children do not realize children can have strokes. Some children may have no symptoms or only gradual onset of developmental delay. While silent strokes are recognizable in the sickle cell population, and several studies have screened for them, the rates of silent infarction in other cerebrovascular disorders are unclear because radiological investigations are lacking in the absence of clinical manifestations.
Etiology
Cardiac
Complex congenital heart disease may lead to thrombosis and ischemic stroke through several mechanisms. Abnormal cardiac anatomy or associated cardiac arrhythmias lead to abnormal flow and may predispose to the formation of intracardiac thrombi. Septal defects may lead to right-to-left shunts that allow venous thrombi to cross to the arterial side and cause cerebral infarction. Surgery and cardiac catheterization can disrupt the endothelium and lead to thrombosis. Cardiac surgery itself may lead to a temporary prothrombotic state (Petaja et al., 1996). An abnormal heart valve can serve as a nidus for bacterial or fungal vegetations that may cause cardioembolic stroke. Chronic hypoxemia in severe cases of congenital heart disease may lead to polycythemia, and the increased blood viscosity may promote thrombosis. Patent foramen ovale is associated with arterial infarction in both children and adults because of presumed right-to-left shunting.
Hematological
Any hematological disorder that disrupts coagulation can place a child at risk for hemorrhagic stroke. Newborns have lower levels of coagulation factors and have a drop in the vitamin K–dependent factors in the first days of life. Bleeding due to vitamin K deficiency was more common before intramuscular or oral administration to neonates became widespread; Cornelissen and colleagues (1996) found that administering vitamin K lowered the incidence of vitamin K deficiency bleeding from 7 to 1.1 per 100,000 births per year. However, late-onset vitamin K deficiency bleeding can occur in children with undiagnosed cholestatic jaundice who received oral vitamin K and are exclusively breastfed, because they cannot absorb the vitamin, and breast milk is low in vitamin K (Ijland et al., 2008). Accidental ingestion or overdose of warfarin has the same effect as a vitamin K deficiency and may occur when a young child finds an older family member’s medications. The most common congenital coagulation factor deficiencies are deficiencies of coagulation factor VIII (hemophilia A), coagulation factor IX (hemophilia B), and von Willebrand factor. Intracranial hemorrhage is the most common cause of death from bleeding in patients with hemophilia.
A deficiency or imbalance of factors involved in regulating coagulation may place the child at risk for thrombosis. Pediatric ischemic stroke has been associated with deficiencies of protein C, protein S, and antithrombin; activated protein C resistance due to the factor V Leiden mutation; the prothrombin gene 20210A mutation; the methylene tetrahydrofolate reductase (MTHFR) gene variants; the ATG haplotype of the protein Z gene (Nowak-Gottl et al., 2009); elevated lipoprotein (a); elevated antiphospholipid antibodies (Kenet et al., 2010) and lupus anticoagulant; with elevated factor VIII levels; and with low plasminogen or high fibrinogen. The importance of the plasminogen activator inhibitor promoter polymorphism (PAI-1) in childhood stroke is controversial. Temporary hematological abnormalities and resultant thrombosis may result from intercurrent illness; for example, idiopathic nephrotic syndrome is associated with decreased antithrombin levels and CVT and/or arterial thromboembolic events (Fluss et al., 2006).
Several authors have noted the presence of multiple prothrombotic abnormalities in some children with ischemic stroke (Kenet et al., 2010). The combination of MTHFR and endothelial nitric oxide synthase gene variants may raise stroke risk more than MTHFR alone (Djordjevic et al., 2009). Children with congenital heart disease or leukemia may be at higher risk for developing thrombotic complications during hospitalization if they also have a prothrombotic abnormality.
Trauma
Trauma can injure vessels directly, leading to hemorrhage from torn vessels and thrombosis in damaged intima. Subdural hemorrhages, subarachnoid hemorrhages, and ischemic infarctions occur in head injury. Ascertaining the cause of the trauma is important; 95% of serious intracranial injuries in children younger than 1 year of age are due to abuse, and 5% of children returned to abusive parents without any intervention are killed (Johnson, 2000). Most subdural hemorrhages in infants are due to abuse (Matschke et al., 2009). In older children and adults, low admission Glasgow Coma Scale score, low systolic blood pressure, brain herniation and requirement for decompressive craniotomy are all predictors of posttraumatic cerebral infarction (Tian, 2008). Bony abnormalities of the vertebrae or abnormalities of vessel walls due to collagen-vascular disease or metabolic disease may predispose to cervicocephalic arterial dissection after mild trauma. Arterial dissection may be idiopathic in otherwise apparently normal children (Rafay et al., 2006). A prothrombotic state associated with trauma can promote thrombosis and worsen outcome; trauma patients who go into disseminated intravascular coagulation have worse outcomes than those who do not. Trauma is the most common precipitant of hemorrhages in children with hemophilia.
Infection
The consequences of bacterial meningitis are disseminated intravascular coagulopathy and vascular inflammation, and subsequent arterial or venous thrombosis and infarction. Group B streptococcal meningitis is an important cause of stroke in neonates and transmitted vertically from the mother or horizontally by nursery staff. During the first 2 months of life, infants are susceptible to bacteria found in maternal flora or in the local environment, including group B Streptococcus, gram-negative enteric bacilli, and Listeria monocytogenes. After 2 months of age, Streptococcus pneumoniae and Neisseria meningitides are the most common causes of bacterial meningitis. The institution of Haemophilus influenzae type b vaccination at 2 months of age has led to a dramatic drop in H. influenzae-b meningitis. In immunosuppressed patients such as those with cancer or acquired immune deficiency syndrome (AIDS), Aspergillus species may lead to vasculitis and infarction. Patients with AIDS may also develop arteriopathy of medium and small vessels or aneurysms, and although the presumed cause for most cases is direct or secondary infection, the exact pathophysiology is not always clear. Antiretroviral therapy for AIDS may itself cause vascular injury (Mondal et al., 2004). Tuberculosis leads to meningitis in 1% to 2% of cases, which may cause vasculitis and infarction (Starke, 1999). Several cases of stroke occurred in children with neurobrucellosis (Salih et al., 2006c). Lyme disease is a rare cause of infectious vasculitis and aneurysm formation. Varicella-zoster virus (VZV) may cause vasculitis by direct infection of the arterial wall or by a postinfectious inflammatory reaction that manifests weeks to months after the primary infection. In some cases, arteriopathy is associated with recent upper respiratory infection (Amlie-Lefond et al., 2009a).
Vascular Malformations/Vasculopathy/Migraine
As discussed previously, vascular malformations may present with intracerebral hemorrhage, and resulting vasospasm may lead to ischemic infarction. Arterial abnormalities such as large-artery stenosis are common in otherwise healthy children with arterial ischemic stroke (Ganesan et al., 2003). At least 10% of hemorrhagic strokes and most subarachnoid hemorrhages in children are caused by ruptured aneurysms (Jordan et al., 2009).
Primary angiitis of the central nervous system may occur in children and can be fatal if not treated with aggressive immune suppression. There are more frequently reported cases of less virulent vasculopathies in children, often occurring after varicella infection, which respond to aspirin alone and do not require immune suppression (Chabrier et al., 1998; Lanthier et al., 2001). Stroke can result from vasculopathy outside the brain. Childhood stroke can be a first manifestation of Takayasu arteritis, an inflammatory large-vessel vasculitis that affects the aorta and its branches (Brunner, 2008). A growing body of literature exists examining the role of inflammatory factors in stroke in children and adults.
Metabolic
The mitochondrial diseases may lead to metabolic strokes, particularly during times of metabolic stress. MRI can demonstrate infarction in non-vascular territories (Fig. 51D.4). However, in rare cases, mitochondrial mutations have been associated with moyamoya vasculopathy (Longo et al., 2008; Papavasiliou et al., 2007).