Use of Blood Components in the Intensive Care Unit
BLOOD COMPONENTS AND INDICATIONS FOR TRANSFUSION
ADVERSE EFFECTS OF BLOOD COMPONENT TRANSFUSION
Delayed Nonhemolytic Transfusion Reactions
Transfusion-Associated Circulatory Overload
Transfusion-Related Acute Lung Injury
Transfusion-Associated Graft-Versus-Host Disease
SPECIAL TRANSFUSION SITUATIONS IN THE CRITICAL CARE SETTING
Necessary Transfusion of Incompatible Blood
Transfusion in Patients with Disseminated Intravascular Coagulation
ALTERNATIVES TO TRANSFUSION OF BLOOD COMPONENTS
Transfusion of blood components is a frequent intervention in hospitalized patients, particularly in critically ill patients. An estimated 22,628,000 units of red blood cells (RBCs), platelets, plasma, and cryoprecipitate were transfused in 2008 in the United States.1 RBC transfusion is often utilized to optimize oxygen-carrying capacity and tissue perfusion that may be due to blood loss, inadequate marrow function, and RBC destruction. Additionally, hemostatic disorders may necessitate the administration of other blood components such as plasma, platelet concentrates, or cryoprecipitate.
Blood components should be considered therapeutic agents with potential benefits as well as adverse effects. Unlike pharmaceutical agents, however, blood components have fewer objective indications for use and no therapeutic index relating dose to safety. Although infectious risks of blood component transfusion have diminished, recognition of risks such as immunomodulation and transfusion-related acute lung injury (TRALI) has increased. Programs of patient blood management are proposed to determine appropriate evidence-based use of blood components and to minimize use of blood products.2 Although more quality evidence has become available to guide clinical decisions in transfusion, many questions remain to be explored through clinical trials, particularly in critically ill patients.
Blood Components and Indications for Transfusion
Blood component therapy is used to optimize management of the blood supply. The basic principle of blood component therapy is to use the specific blood product that meets the patient’s need. Up to four components (RBCs, plasma, platelets, cryoprecipitate) can be derived from a single whole blood (WB) donation and then distributed to several recipients with differing physiologic needs. Component therapy thus meets the clinical requirements of increased safety, efficacy, and conservation of limited resources. As the variety of available blood product components increases, however, the complexity of transfusion medicine also increases. A WB donation is typically separated into RBCs, a platelet concentrate, and fresh frozen plasma (FFP). The plasma may be further processed into cryoprecipitate and supernatant (cryopoor) plasma. The characteristics of more commonly transfused blood products are described in Table 79.1.
Table 79.1
Characteristics of Blood Components
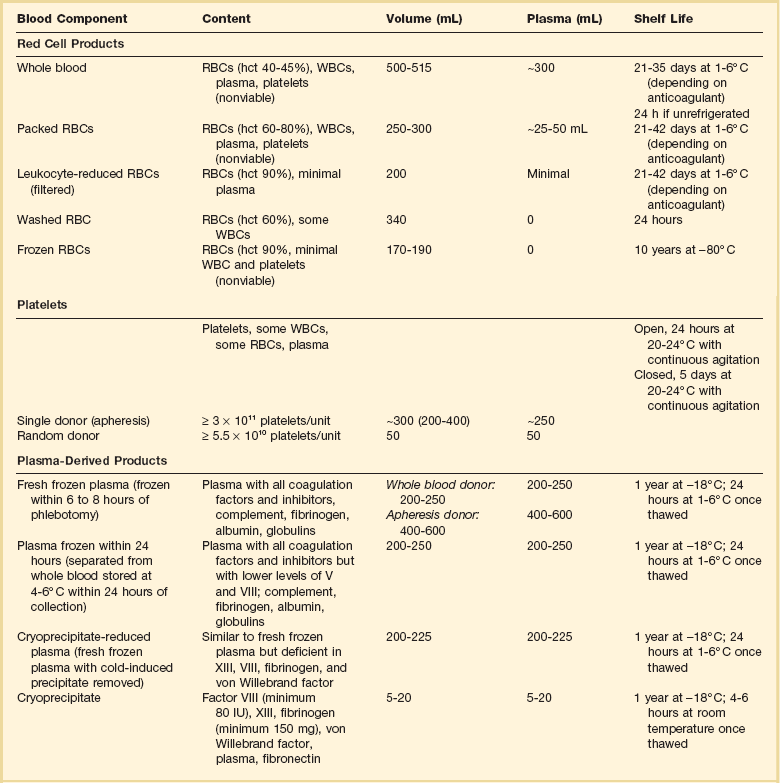
RBCs, red blood cells; WBCs, white blood cells; hct, hematocrit.
Whole Blood and Red Blood Cells
Unseparated venous donor blood with a preservative solution constitutes a WB unit. It contains all blood components, but after less than 24 hours of refrigerated storage, platelet and leukocyte function is lost. With further storage, levels of the labile coagulation factors V and VIII markedly decrease.3 The growing need for specialized blood components has resulted in processing the majority of blood donations into components, thus limiting the availability of WB. Only 0.03% of total blood transfusions in the United States in 2008 were WB units.1
RBCs, commonly known as packed red blood cells (PRBCs), are the blood component most commonly transfused to increase RBC mass. PRBCs are derived from the centrifugation or sedimentation of WB and removal of most of the plasma/anticoagulant solution. PRBCs may be further modified to meet the specific needs of patients or blood bank regulations. Leukocyte-reduced PRBCs are the most commonly transfused modified RBC product. Transfusion of blood components containing leukocytes may lead to nonhemolytic febrile transfusion reactions, a greater propensity for platelet alloimmunization, and transmission of pathogens carried by leukocytes, such as cytomegalovirus (CMV). Leukocyte reduction requires filtration of the blood component by a special filter at the time of blood donation and processing or later at the time of transfusion (“bedside filtration”). Leukocyte-reduced RBC units must contain less than 5.0 × 106 leukocytes. Filtration before storage conveys the benefit of removing white blood cells (WBCs) before they can deteriorate and elaborate cytokines and other unwanted substances during storage.4 Because of proven and theoretical benefits of leukocyte reduction of blood components (discussed later in the section covering the adverse effects of transfusion), many European countries and Canada require that all PRBCs be leukocyte reduced, a process called universal leukoreduction (ULR). Some institutions in the United States have also made that decision, but either method of leukocyte reduction adds significantly to the cost of each transfusion, and the benefits of this measure when applied globally have yet to be quantified.5 Almost 70% of transfused PRBCs are leukoreduced in the United States.1
PRBCs can also be modified by irradiation to inactivate lymphocytes. Transfusion of irradiated blood is indicated in severely immunocompromised patients at risk of graft-versus-host disease (GVHD), such as transplant recipients, those with aggressively treated malignancies, and those with congenital immunodeficiencies. The transfusion of irradiated PRBCs in the United States is increasing and accounted for 10% of blood transfusions in 2008.1 Irradiation of RBCs reduces RBC viability and increases release of intracellular potassium.
RBC components suffer some cell loss during storage. The current technology with preservative solutions attempts to optimize cell quality and quantity by using strict criteria to determine the allowable storage time. Nonetheless, as RBC metabolism decreases progressively, a “storage lesion” results, with accumulation of a variety of undesirable substances and loss of cellular function.6 During storage, a slow rise in the concentration of potassium, lactate, aspartate aminotransferase, lactate dehydrogenase, ammonia, phosphate, and free hemoglobin and a slow decrease in pH and bicarbonate concentration occur. Cytokines and inflammatory mediators such as interleukin 1, interleukin 6, and tumor necrosis factor also accumulate. The pH of freshly stored blood in citrate solution is 7.16, which declines to approximately 6.73 at the end of the unit’s shelf life. As potassium leaks from RBCs during storage, levels as high as 25 mEq/L may result. However, each unit transfused supplies at most 7 mEq of potassium, which is usually well tolerated.
During the storage period there is also a progressive decrease in RBC-associated 2,3-diphosphoglycerate (2,3-DPG) and adenosine triphosphate (ATP).6 A decrease in 2,3-DPG increases the affinity of hemoglobin for oxygen, which shifts the oxygen dissociation curve to the left and decreases oxygen delivery to tissues. There is little evidence, however, that this transient increase in oxygen affinity has clinical importance. After infusion, 2,3-DPG gradually increases as the transfused RBCs circulate, with 25% recovery in 8 hours and full replacement by 24 hours.7 Decreased ATP during storage diminishes the viability of RBCs after transfusion and is one of the chief factors limiting storage time. There is no currently available storage or rejuvenation solution that optimizes these cellular constituents.
Indications for Red Blood Cell Transfusion
Despite a long tradition of transfusion of RBCs in critically ill patients, the precise indications for transfusion remain a source of debate, and transfusion practices may vary widely among clinicians, ICUs, institutions, and geographic regions. Multiple observational studies document transfusion rates in ICU patients that vary from 17% to 53%,8–12 and the rate of transfusion increases with longer ICU length of stay.8,13 There has been a trend over time for use of a lower transfusion threshold in critically ill patients.14,15
Compensatory mechanisms for acute and chronic anemia are complex and work in concert to maintain oxygenation within the microcirculation.16,17 Cardiovascular adjustments leading to increased cardiac output include decreased afterload and increased preload resulting from changes in vascular tone, increased myocardial contractility, and elevated heart rate. Lowered blood viscosity permits improved flow of RBCs within capillaries. Blood flow is redistributed to favor critical organs with higher oxygen extraction such as the heart and brain. Pulmonary mechanisms, though contributing relatively little to short-term oxygenation demands, exert potent effects on related metabolic variables. Finally, the hemoglobin molecule can undergo biochemical and conformational changes to enhance the unloading of oxygen at the capillary level. Increased synthesis of RBC 2,3-DPG in anemia results in a rightward shift of the oxyhemoglobin saturation curve and facilitates the release of oxygen to tissues. A rightward shift of the oxyhemoglobin curve can also occur with a decrease in pH (Bohr effect) but the clinical significance is small.16 All these mechanisms contribute to an oxygen reserve capacity that exceeds baseline requirements by approximately fourfold. Unfortunately, acute illness and chronic morbidities may limit these compensatory mechanisms in critically ill patients. Animal studies and case reports in patients refusing transfusion indicate that an extremely low hematocrit is tolerated if tissue perfusion is adequate.17–19
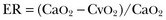
where the arterial content of oxygen (CaO2) equals 1.36 × hemoglobin × SaO2 + 0.003 × PaO2. The venous oxygen content (CvO2) can be calculated by the same formula, replacing the values with mixed venous oxygen saturation (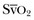 ) and venous partial pressure of oxygen (PvO2). Because the contribution of dissolved oxygen in plasma to the oxygen-carrying capacity is negligible, ER can be estimated by: 1 – (
) and venous partial pressure of oxygen (PvO2). Because the contribution of dissolved oxygen in plasma to the oxygen-carrying capacity is negligible, ER can be estimated by: 1 – (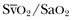 ). The total body ER at baseline is about 25%. A falling CvO2 and an ER increasing to greater than 50% have been proposed as indicators of the need for RBC transfusion, but have never been validated in clinical studies.20
). The total body ER at baseline is about 25%. A falling CvO2 and an ER increasing to greater than 50% have been proposed as indicators of the need for RBC transfusion, but have never been validated in clinical studies.20
Although RBC transfusion can increase oxygen-carrying capacity and thus oxygen delivery, it may not improve tissue oxygen consumption. Multiple case series evaluating the effects of RBC transfusion in critically ill patients have failed to document increased oxygen consumption or improvement in lactate level.21–25 Hypotheses to explain this discrepancy include an increase in blood viscosity limiting microvascular flow and impaired tissue and cellular oxygen utilization.
In practice, clinicians usually rely on the hemoglobin to determine when oxygen-carrying capacity is potentially compromised, despite the limitations noted earlier. Prior transfusion strategies often targeted a hemoglobin goal of greater than 10 g/dL with support of reports that anemia, defined by various criteria, is associated with increased mortality rate in critically ill patients,26 mechanically ventilated COPD patients,27 surgical patients who refuse transfusion,28 and patients undergoing major noncardiac surgery.29,30 However, the first large multicenter randomized trial of RBC transfusion strategies in the critically ill showed this liberal transfusion strategy may actually be unnecessary and potentially detrimental.31 The Transfusion Requirement in Critical Care (TRICC) trial compared a liberal (target hemoglobin, 10 to 12 g/dL) with a restrictive (target hemoglobin, 7 to 9 g/dL) RBC transfusion policy in 838 euvolemic patients with hemoglobin less than 9 g/dL within 72 hours of ICU admission. The primary outcome measure of 30-day all-cause mortality rate was not statistically different between the restrictive strategy and the liberal strategy (p = 0.11). A secondary outcome measure of overall hospital mortality rate was significantly lower in the restrictive strategy group (p = 0.05). The restrictive strategy was superior for subgroups of patients younger than 55 years and patients with lower (<20) APACHE (Acute Physiology, Age, and Chronic Health Evaluation) II scores. In addition, liberal transfusion was not associated with shorter ICU or hospital stays or less organ failure; longer mechanical ventilation times and cardiac events were more frequent in the liberal strategy group. A separate analysis of 713 patients in the study who received mechanical ventilation did not find any significant differences between treatment groups for the duration of mechanical ventilation or extubation success.32 Similarly, a subgroup analysis of 357 patients from the TRICC study with cardiovascular disease did not find any differences in mortality rates between the restrictive and liberal strategies.33
The results of the TRICC study were replicated in a study of transfusion strategy in critically ill pediatric patients.34 A liberal transfusion threshold of 9.5 g/dL was compared with a restrictive transfusion threshold of 7.0 g/dL. The primary outcomes of death and new or progressive multiple organ dysfunction as well as adverse events were similar in both treatment groups, but 54% of patients in the restrictive group did not receive transfusion as compared to only 2% in the liberal group (p < 0.001). Subsequent prospective randomized trials have validated the use of a restrictive transfusion strategy in other adult patient populations.35–39 Although a small study of liberal (hemoglobin threshold of 10 g/dL) versus restrictive (hemoglobin threshold of 8 g/dL) transfusion in 120 hip fracture surgery patients raised concern for increased cardiovascular complications and mortality rate with a restrictive strategy,40 a larger trial in 2016 high-risk patients undergoing surgery for hip fracture found no increase in mortality rate and no difference in functional recovery.35 The Transfusion Requirements After Cardiac Surgery (TRACS) study compared transfusion thresholds of hematocrit less than 30% with hematocrit less than 24% from the start of surgery through the ICU stay.36 There was no difference in the composite outcome of 30-day all-cause mortality rate and severe morbidity between the liberal and restrictive strategies, and fewer blood products were administered in the restrictive group. These results are similar to those in an earlier study in coronary artery bypass surgery patients that evaluated liberal and restrictive transfusion strategies in the postoperative period.41
The TRACS study and the analysis of patients with cardiovascular disease from the TRICC study suggest that RBC transfusion using a hemoglobin threshold of 7 to 8 g/dL in stable patients at risk for myocardial ischemia is well tolerated. However, these studies do not answer the question of whether patients with acute coronary syndromes (current or recent ischemia) would benefit from a liberal transfusion strategy. Results from retrospective and prospective observational studies of anemia and transfusion in acute coronary syndrome patients have yielded conflicting results.42–45 A recent prospective, randomized pilot study of 45 patients with acute myocardial infarction (MI) and hematocrit 30% or less compared a transfusion threshold of hematocrit less than 24% with hematocrit less than 30%.46 The composite safety end point of in-hospital death, new MI, or new or worsening heart failure occurred in 38% of the liberal strategy patients versus 13% in the conservative strategy patients (p = 0.046). These results suggest that even in acute MI moderate anemia may not be as harmful as the risks involved with transfusions. Larger studies in the future should help determine whether a restrictive transfusion strategy should be adopted in patients with acute coronary syndromes.
Prior guidelines for transfusion of RBCs did not specifically address critically ill patients and were primarily based on consensus rather than evidence.47–51 Transfusion was usually recommended if hemoglobin was less than 6 or 7 g/dL and not indicated when hemoglobin was greater than 10 g/dL.48,52 Guidelines of the American Association of Blood Banks46 and the Society of Critical Care Medicine/Eastern Association for Surgery of Trauma53 and a Cochrane review54 provide an evaluation of clinical evidence and more specific recommendations that are applicable to the critically ill. A summary of the guideline recommendations is presented in Box 79.1. Transfusion of single units of RBCs is recommended except in the setting of acute hemorrhage.53 Implementation of a restrictive transfusion practice as recommended by the guidelines could decrease patient RBC exposure by an estimated 40%.46 The threshold for administration of RBCs should also be considered as part of a comprehensive, multidisciplinary patient blood management program to detect and treat anemia, reduce surgical blood loss, and optimize hemostasis.2
Platelets
Platelet components are available as random donor units or single donor apheresis units. Because of the limited storage time and the increasing demand for this component, platelets are often subject to supply shortages. A random donor platelet concentrate is obtained by centrifugation from a unit of WB. This type of platelet concentrate contains up to 50% of the leukocytes from the WB unit. The average transfused dose of random donor platelets has been decreasing over time and is now 5 units in the United States.1 If bags are entered for pooling before transfusion, the platelets must be administered within 4 hours. A single donor apheresis platelet unit contains the equivalent of 4 to 6 units of random donor platelet concentrates. This type of platelet concentrate is considered to be leukoreduced and no additional filtration is needed. Single-donor platelets offer the benefit of reducing the risk of multiple-donor exposure to the recipient, and may also be the only available alternative for recipients who have been alloimmunized by previous platelet transfusions. Apheresis platelets now account for 87% of all platelets transfused in the United States.1
A CCI of 10 × 109/L or higher can be considered a good response, whereas a CCI of 5 × 109/L or lower indicates a poor response to transfusion.55 The increment in platelet count is higher with single-donor apheresis units, ABO-identical platelets, and platelets stored no longer than 3 days.56,57 However, there is no advantage of these platelet characteristics on prevention of clinical bleeding.56
Indications for Platelet Transfusion
Although the prevalence of thrombocytopenia in the critically ill varies with the definition used and clinical setting, thrombocytopenia is associated with platelet transfusions and increased mortality rate in ICU patients.58,59 Guidelines for transfusion of platelets are derived from consensus opinion and experience primarily in patients with chemotherapy-induced thrombocytopenia rather than critically ill patients.48,60–63 Extrapolation of these guidelines to critically ill patients is problematic because the cause, risks, and consequences of thrombocytopenia may be different. Indications for platelet transfusions include active bleeding due to thrombocytopenia or functional platelet defects (therapeutic transfusion) or prevention of bleeding due to thrombocytopenia (prophylactic transfusion). The majority of platelet transfusions in the ICU are performed for prophylactic indications and often do not result in an increase in platelet count.64
Suggested indications for platelet transfusion are summarized in Table 79.2. There is good evidence that medical or surgical patients with active bleeding and platelet counts of 50 × 109/L or above will not benefit from transfusion if thrombocytopenia is the only abnormality. Prophylactic platelet transfusions are administered to prevent spontaneous bleeding or bleeding with invasive procedures. The threshold for platelet transfusion prior to invasive procedures is usually recommended as less than 50 × 109/L, but transfusion decisions should take into account the type of procedure, bleeding risks associated with the procedure, consequences of bleeding, and concomitant factors affecting hemostasis. For critical invasive procedures in which even a small amount of bleeding could lead to loss of vital organ function or death, maintaining the platelet count greater than 50 × 109/L is typically preferred. The presence of factors that diminish platelet function, such as certain drugs, foreign intravascular devices (e.g., intra-aortic balloon pump or membrane oxygenator), infection, or uremia, alter this requirement upward. Patients at risk for small but strategically important hemorrhage, such as neurosurgical patients, may need to be maintained at platelet counts of 80 to 100 × 109/L.
Table 79.2
Indications for Platelet Transfusion
| Clinical Situation | Platelet Count: × 109/L |
| Therapeutic | |
| Active bleeding | <50 |
| Prophylactic | |
| Spontaneous bleeding risk very high | 0-10 |
| Spontaneous bleeding risk high with concomitant coagulation abnormality, anticoagulant therapy, sepsis, fever, concurrent antibiotic use, rapidly decreasing count or planned invasive procedure | 11-20 |
| Planned invasive procedure | 21-50 |
| Consider with platelet dysfunction (uremia, antiplatelet drugs) and planned invasive procedure when other therapies are ineffective | >50 |
The most appropriate platelet count for procedures that may be performed in critically ill patients, such as placement of central venous catheters and arterial catheters, thoracentesis, and paracentesis, has not been defined. A retrospective study suggests that central venous catheters can be placed safely when the platelet count is greater than or equal to 20 × 109/L.65
Patients undergoing cardiac bypass surgery experience a drop in platelet count and often acquire a transient platelet functional defect from damage associated with the bypass apparatus.66 Most patients do not experience platelet-associated bleeding, however, and prophylactic transfusion in the absence of bleeding is not warranted. In a patient who continues to bleed postoperatively, more likely causes are a localized, surgically correctable lesion or failure to reverse the effects of heparin. If these conditions are excluded, empiric transfusion of platelets may be justified.
Patients without hemorrhage who have platelet counts of 5 × 109/L or lower are at increased risk for significant spontaneous bleeding, and the majority of guidelines propose prophylactic platelet transfusion to prevent hemorrhage at a threshold of 10 × 109/L or less. The recommendations are based on experience in patients with hematologic malignancies and chemotherapy-induced underproduction of platelets. The prior practice of transfusion to maintain the platelet count above 20 × 109/L derives from data published in 1962, which demonstrated an increase in spontaneous bleeding in leukemic patients at that level.67 However, critical evaluation of the data reveals that serious hemorrhage was not greatly increased until counts fell to 5 × 109/L or lower and that these patients received aspirin for fever, which might have compromised platelet function and enhanced bleeding.
A prospective study of a more conservative platelet transfusion protocol found that major bleeding episodes occurred on 1.9% of days with counts of less than 10 × 109/L and on only 0.07% of days with counts of 10 to 20 × 109/L.68 Additional studies have confirmed the safety of using less than or equal to 10 × 109/L as a prophylactic platelet transfusion threshold in patients with hematologic malignancies or stem cell transplants.69–72 The trigger for prophylactic platelet transfusion of less than or equal to 10 × 109/L, however, applies primarily to a specific population of stable thrombocytopenic patients. Factors such as fever, use of anticoagulant or antiplatelet drugs, and invasive procedures must be considered when generating a treatment plan for individual patients. Patients experiencing rapid drops in platelet count may be at greater risk than those at steady state and thus may benefit from transfusion at higher counts. Prospective studies in critically ill patients have not been reported. Benefits to the patient of more conservative use of platelet transfusion include decreased donor exposure, which lessens the risk of transfusion-transmitted disease; fewer febrile and allergic reactions that may complicate the hospital course; and the potential delay or prevention of alloimmunization to HLA and platelet antigens.73
Patients thrombocytopenic by virtue of immunologic destructive processes such as idiopathic thrombocytopenic purpura (ITP) receive little benefit from platelet transfusions because transfused platelets are rapidly removed from the circulation. In the event of life-threatening hemorrhage or an extensive surgical procedure, transfusion may prove beneficial for its short-term effect but may require higher doses of platelets. Transfusion may be accomplished effectively by pretreatment with high-dose immunoglobulin or high-dose anti-D antiserum.74,75 Platelet transfusion is contraindicated in thrombotic thrombocytopenic purpura (TTP),76 hemolytic-uremic syndrome, and heparin-induced thrombocytopenia. Cautious administration of platelets may be considered in cases of life-threatening thrombocytopenic bleeding.
The development of refractoriness to platelet transfusions due to alloimmunization is a serious event. Poor response to platelet transfusions due to increased platelet consumption also occurs with splenomegaly, fever, trauma and crush injury, burns, disseminated intravascular coagulation (DIC), concomitant drugs, and transfusion of platelets of substandard quality.77 These factors should be identified and corrected if possible. Alloimmunization is characterized by the development of anti-HLA or platelet-specific antibodies, with resultant immune platelet destruction. As many as 70% of patients receiving multiple RBC or platelet transfusions become immunized.73 Leukocyte depletion of transfused components can prevent or delay this phenomenon, but it is important to use leukoreduced components early in the course of transfusion therapy.73,78 When patients fail to achieve expected increments after platelet transfusion, provision of ABO-specific platelet concentrates that are less than 48 hours old may improve the response. If no improvement is seen, the patient should be screened for HLA antibodies or be HLA typed and provided with HLA-compatible single-donor platelets. Alternatively, platelet crossmatching with the patient’s serum can be carried out. There is no advantage to unmatched single-donor platelets in this situation.
Plasma-Derived Components
Plasma
Standard FFP is prepared by centrifugation of WB or single-donor apheresis and is frozen within 8 hours of blood donation. Standard FFP contains all coagulation factors (including the labile factors V and VIII) and inhibitors, approximately 400 mg fibrinogen, complement, albumin, and globulins. By convention, the coagulation factors are present in concentrations of 1 U/mL. Plasma that is separated and frozen from refrigerated WB more than 8 hours but within 24 hours of phlebotomy is referred to as PF24. PF24 differs from standard FFP by having lower levels (approximately 15% to 25% reduction) of factors V and VIII, but the decrease in labile factor levels is not considered to be clinically significant. The processing technique for PF24 allows for clinical utilization of plasma collected at distant sites and increases the plasma supply. Cryoprecipitate-reduced plasma (also called cryopoor plasma) refers to FFP with the cold-induced precipitate removed. Cryopoor plasma will thus be deficient in factors VIII and XIII, fibrinogen, and von Willebrand factor. In the United States, FFP accounted for 54% of plasma transfusions and PF24 accounted for 39%.1 The most common method of thawing FFP requires about 30 to 45 minutes in a 37° C water bath. Crossmatching to the recipient is not performed, but FFP must be ABO compatible. Standard FFP is as likely to transmit hepatitis, HIV, and most other transfusion-related infections as cellular components. The following types of pathogen-reduced plasma products are available in some countries outside the United States: solvent/detergent-treated plasma, methylene blue–treated plasma, psoralen- and ultraviolet light–treated plasma, and riboflavin- and ultraviolet light–treated plasma.79
Indications for Fresh Frozen Plasma
FFP is frequently transfused inappropriately in critically ill patients who are not bleeding or in whom the international normalized ratio (INR) is less than 1.5.80,81 Guidelines for transfusion of FFP have been primarily based on expert opinion rather than clinical evidence (Box 79.2).60,82,83 A summary of practice recommendations for specific clinical circumstances based on a systematic review is presented in Box 79.3. The review emphasized the lack of high-quality evidence on plasma infusion and the need for well-designed trials to address relevant knowledge gaps.84
FFP should be administered only to provide coagulation factors or plasma proteins that cannot be obtained from safer sources. FFP is commonly used to treat bleeding patients with acquired deficiency of multiple coagulation factors, as in liver disease, DIC, or dilutional coagulopathy. However, changes in INR after FFP transfusion are usually minimal and not clinically significant when the pretransfusion INR is less than 2.0.81,85 FFP may be indicated for the provision of protein C or S in patients who are deficient and suffering acute thrombosis. FFP should be administered as boluses as rapidly as feasible so that the resulting factor levels achieve hemostasis. The use of FFP infusions is not helpful. Variable doses of FFP have been recommended including 2 units initially (probably underdosage) up to 10 to 15 mL/kg. However, some studies suggest that doses as high as 30 mL/kg may be needed to achieve adequate factor levels.86 Due to the short half-life of factor VII, FFP should be infused every 6 to 8 hours if bleeding continues. FFP should not be used for volume expansion or as a nutritional source of protein. Anticoagulation induced by heparin, direct thrombin inhibitors (e.g., dabigatran), or direct factor Xa inhibitors (e.g., rivoraxaban) is not reversed by FFP.
Patients do not usually bleed as a result of coagulation factor deficiency when the INR is less than about 2.0, and even then the results are not always predictable.87 The partial thromboplastin time (PTT) is also not useful in predicting procedural bleeding risk.88 Prophylactic administration of FFP does not improve patient outcome in the setting of cardiac surgery unless there is bleeding with an associated documented coagulation abnormality.89 FFP is often requested prophylactically before an invasive procedure when the patient exhibits mild prolongation in coagulation studies. Most of these procedures may be carried out safely without transfusing FFP.87,90 A randomized trial of FFP versus no FFP in critically ill nonbleeding patients with INR between 1.5 and 3.0 scheduled to undergo central venous catheter placement, thoracentesis, percutaneous tracheostomy, or drainage of abscess or fluid is under way.91
Coagulation factors are normally present in the blood far in excess of the minimum levels required for hemostasis. As little as 10% of the normal plasma concentration of several factors will effect hemostasis. Conversely, FFP treatment of acquired multiple deficiencies, as in hepatic failure, is often ineffective because many patients cannot tolerate the infusion volumes required to achieve hemostatic levels of coagulation factors, even transiently.92 The plasma half-life of transfused factor VII is only 2 to 6 hours. It may be impossible to administer sufficient FFP every few hours without encountering intravascular volume overload. Finally, in some instances, transfusion of seemingly adequate volumes may still fail to correct the coagulopathy.93 Careful documentation of both the need for FFP and the adequacy and outcomes of therapy is essential.94
Cryoprecipitate
A total of 1.1 million units of cryoprecipitate were transfused in 2008 in the United States at a mean average cost of $65.10/unit.1 Cryoprecipitate is prepared by thawing and centrifuging FFP below 6° C and resuspending the precipitated proteins in a small volume of supernatant plasma. Each unit is a concentrated source of factor VIII (≥80 IU), von Willebrand factor (50% of original plasma content), fibrinogen (≥150 mg), factor XIII (30% of original plasma content), and fibronectin. It is considered to be leukoreduced without additional filtration. Cryoprecipitate offers the advantage of transfusing more specific protein and less total volume than an equivalent dose of FFP. Cryoprecipitate does not require crossmatching, but ABO compatibility with the recipient is preferred.








