23.2 Congenital renal disease241
23.3 Acquired renal disease241
23.4 The ureters247
23.5 The bladder247
23.6 The urethra249
Self-assessment: questions250
Self-assessment: answers252
The urinary tract comprises the kidneys, ureters, bladder and urethra. In males, the prostate gland surrounds a small proportion of the mid-to-lower urethra. This chapter will discuss the pathology of the kidney, ureter, bladder and urethra. Chapter 22 deals with the pathology of the prostate, testis, penis, foreskin and scrotum (i.e. the male genital tract). Some of the pathological processes of the urinary tract are very common (e.g. urinary tract infection), but some are rare (e.g. glomerulonephritis). Urine, the modified ultrafiltrate of blood produced by the kidneys, is often used to assess the general health of a person. There are many simple, colour-based ‘stick tests’ that enable patients and doctors to monitor substances that may leak into the urine (e.g. glucose, blood, protein).
23.1. The kidneys
Structure and function
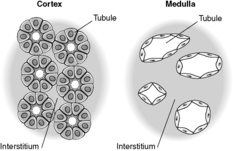
Each kidney lies in the upper retroperitoneum and has a large number of complex functions including salt and water balance and pH homeostasis. The kidneys receive about 25% of the cardiac output. With the naked eye, the kidney is seen to have a tough external capsule covering an outer ‘rind’ of cortex, which itself covers the inner medulla (see Figure 61). Histologically, the kidney is made up of four discrete, but interdependent, compartments:
• glomeruli
• tubules
• interstitium
• vessels.
 |
| Figure 61 |
It is probably easiest to tackle each compartment in turn.
Glomeruli
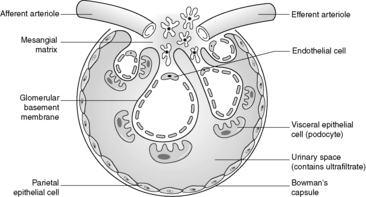
Despite the confusing nomenclature surrounding the pathologies that affect the glomerulus, it is a relatively simple structure. The glomerulus is essentially a highly specialised, incredibly leaky, sieve under high hydrostatic pressure. It is composed of tiny, anastomosing capillaries held together by a specialised matrix and covered by two layers of epithelium (Figure 62). The glomerular capillary wall is the main filtration barrier of the glomerulus and has a unique structure, directly related to its function.
 |
| Figure 62 |
There are some general ‘rules’ that apply to many of the diseases that affect the glomerulus:
• most primary glomerular diseases are mediated by the immune system (and involve the deposition of immunoglobulins and/or complement in some part of the glomerular structure)
• often, glomerular diseases will present with proteinuria (if heavy, the nephrotic syndrome) or haematuria
• some diseases that affect the glomerulus are relatively acute/sudden in onset and are entirely reversible (clinically and histologically). However, chronic glomerular diseases can lead to scarring of the glomeruli, death of nephrons and eventually renal failure.
Tubules
The kidney is made up of about a million nephrons, each of which is composed, simplistically, of a glomerulus and its attached tubule (into which the filtrate from the glomerular sieve percolates). This tubule is hollow and is lined, along its complex, writhing course, by epithelial cells. Essentially, the structure of the tubular epithelial cell varies with its function (usually related to its position along the course of the tubule). The proximal tubular cells reabsorb much of the sodium, water, glucose and protein that filters through the glomerulus. It is not surprising, therefore, that their structure (microvilli projecting into the tubular lumen to increase surface area and plentiful mitochondria providing energy for absorption pumps) is much more complex than many distal tubular cells (flat, nondescript cells that may function only to ‘line’ the tubule and make it watertight). The specialised function and microanatomy of the proximal tubular cells make them more susceptible to certain cellular insults such as ischaemia/poisons.
Interstitium
Previously thought to be an inert, fibrous compartment of the kidney that binds the glomeruli, tubules and vessels together, we now realise that the interstitium is very important in renal function. Indeed, scarring of the interstitium is important in the degree of impairment of renal function and progression of renal disease (see below). The cortical interstitium is inconspicuous in the normal kidney (the tubules are virtually back to back), but contains peritubular capillaries and fibroblast-like cells (these latter cells communicate with each other and with their neighbouring tubular epithelial cells by means of growth factors and other molecules). In the medulla, however, the amount of interstitium increases and the tubules are more separated from each other (Figure 63).
There are some general ‘rules’ that apply to many of the diseases affecting the tubules and interstitium:
• most diseases that affect the tubules and/or interstitium are mediated by ischaemia, toxins or infection
• many diseases of the tubules and/or interstitium present as acute renal failure or abnormalities of urine volume/concentration/electrolytic composition
• diseases of the tubules are potentially entirely reversible. The tubular epithelial cells are stable cells and surviving cells can therefore undergo mitosis, and regenerate and replenish their numbers/make up new tubules. However, diseases targeting the interstitium may lead to scarring and loss of renal function.
Vessels
Combined, the kidneys weigh only a fraction of the total body weight, yet they receive 25% of the cardiac output (the cortex is much more vascular than the medulla). The kidney receives its blood supply from the main renal artery, which subdivides into anterior and posterior branches at the hilum of the kidney. There is then progressive subdivision of the arterial supply until small afferent arterioles enter the glomerulus. Efferent arterioles are formed by merging of the glomerular capillaries. These efferent arterioles form tiny calibre plexuses of vessels, which surround cortical tubules (peritubular capillaries) and are found in the medulla (these arterial ‘vasa recta’ ultimately become the venous vasa recta and drain into the systemic venous system via the intrarenal veins/main renal vein).
Remember that the glomerulus is a leash of capillary-sized vessels, therefore diseases that affect capillaries (e.g. vasculitis) are likely to affect the glomerulus.
The compartments of the kidney, although structurally separate, are intimately interrelated and interdependent. This means that chronic (scarring) pathology in one compartment will almost inevitably lead to scarring/loss of function in the other compartments. For example, chronic glomerular disease, which leads to scarring of the glomerulus, will cause atrophy of the attached tubule, scarring in the interstitium (mediated by the fibroblast-like cells) and loss of the specialised capillary plexuses. This is one of the reasons that nephrologists try to do a biopsy in patients early in the course of their renal disease. If the disease is a chronic (potentially scarring-type) process, eventually, as nephrons are lost and the interstitium fibroses, the kidney will become a shrivelled, scarred structure and the compartment of origin of the disease will not be evident (and the initial compartment target of the disease process may be important in the treatment/prognosis of the disease).
23.2. Congenital renal disease
There are numerous possible congenital abnormalities of the kidneys from non-formation of one kidney (unilateral agenesis), which is compatible with a normal life (and may only be discovered incidentally at autopsy), to congenital absence of both kidneys, which usually leads to death in utero. Sometimes the upper or lower poles of the kidneys are fused (forming a so-called ‘horseshoe kidney’). This type of kidney malformation may be found in fetuses/children who have chromosomal abnormalities such as Turner’s syndrome (45XO). Congenital cystic disease of the kidney is clinically important (Table 49).
| Cystic renal dysplasia | Autosomal dominant polycystic kidney disease | Autosomal recessive polycystic kidney disease | Medullary sponge kidney |
|---|---|---|---|
| • Commonest cystic renal disease in children | • Progressive distension of kidney by enlarging cysts | • Rare, 1 case per 20 000 live births | • Dilated collecting ducts giving ‘spongy’ appearance |
| • Caused by disorganised renal development | • 1–2 cases per 1000 live births | • Gene on chromosome 6 • Liver also alwaysaffected |
• ? 1 case per 5000 population |
| • Can be unilateral or bilateral | • Usually present in adults | • Larger kidneys at birth (may cause death soon after birth due to renal failure) | • May present with renal infections in adult life |
|
• Often associated with poorly formed ureter
• Rarely part of a syndrome
|
• Caused by mutation in two genes PKD1 (85% of cases; chromosome 16) and PKD2 (15% of cases; chromosome 4) (? also PKD3 in rare cases)
• 10% new mutations
• Maybe associated with cysts in liver, pancreas, spleen and cerebral/coronary artery and aneurysms
• About 10% require dialysis/transplantation
|
• No obvious genetic link |
23.3. Acquired renal disease
Glomerular disease
Glomerular diseases (glomerulonephritis/glomerulonephritides; glomerulopathy/glomerulopathies) are often caused by the immune system. Chronic glomerular disease is an important cause of chronic renal failure (CRF) worldwide. Severe chronic renal failure is associated with high morbidity and mortality, and renal function often needs to be maintained by artificial means, either haemodialysis or peritoneal dialysis, or by kidney transplantation from a living or dead person (living-related or cadaveric transplantation). There are numerous glom-erulonephritides and their nomenclature is confusing (Table 50).
| Focal – some glomeruli (often quoted as about ≤20% involved) | |
| Diffuse – majority of glomeruli (≥80% involved) | |
| Segmental – part of a glomerulus | |
| Global – whole glomerulus | |
| Non-proliferative | Proliferative |
|---|---|
| (no increase in glomerular cells) | (increase in one or more cell type in glomerulus) |
| • Minimal change disease | • Mesangial proliferative GN |
| • Focal segmental glomerulosclerosis | • Membrano-proliferative/mesangio-capillary GN |
| • Membranous GN | • Diffuse/post-infectious proliferative GN |
| • Focal segmental necrotising/crescentic GN | • Focal segmental GN • Crescentic GN |
Primary glomerulonephritis
This is essentially a disease process in which the glomerulus is mainly or exclusively affected (i.e. no non-renal organs are involved), e.g. membranous glomerulonephritis.
Secondary glomerulonephritis
This by contrast, involves the glomerulus being affected as part of a widespread, multisystem disease, e.g. diabetes mellitus, amyloidosis and systemic lupus erythematosus (SLE), all of which can affect many organs including the lung, liver, kidney, etc.).
Aetiopathogenesis of primary glomerulonephritis
Using these immunological and ultrastructural techniques (and a wealth of information from experimental models of glomerular disease), it is apparent that there are several possible mechanisms by which immune-mediated glomerular disease occurs.
Pre-formed circulating immune complexes
Pre-formed circulating immune complexes (made up of antibody and antigen) may lodge in any part of the glomerulus and set up a chain of reactions leading to a change in the fine structure/charge of the glomerulus, with altered function. This type of scenario probably occurs in SLE (in which the antigen that sets up this reaction is likely to be normal nuclear-associated protein) and some glomerular diseases associated with infections (e.g. hepatitis B; here the foreign antigen is part of the virus).
No immune complexes
It is now well established that certain glomerular diseases (e.g. minimal change disease) show no evidence of immune complex deposition (using the immunohistological/ultrastructural techniques described above). It is postulated that these diseases may be mediated by T cells or macrophages (or, more specifically, signalling molecules produced by these cells).
Types of glomerulonephritis
As can be seen from Table 50, there are numerous forms of glomerular disease, and it is beyond the scope of this book to detail them all. However, four types of glomerulonephritis will be discussed in an attempt to illustrate important aspects of glomerular pathology:
• post-infectious glomerulonephritis
• minimal change disease
• membranous glomerulonephritis
• IgA disease.
Post-infectious glomerulonephritis
Often considered to be the prototypic glomerular disease, post-infectious glomerulonephritis can occur after a large number of infections (mainly bacterial). Classically, the disease occurs in children/young adults and follows a sore throat caused by group A β-haemolytic streptococci. There is a time lag of about 1–2weeks between the sore throat (often a very bad one) and a feeling of being unwell with the appearance of red- or cola-coloured urine (macroscopic haematuria), poor urine output (oliguria) and a mild-to-moderate elevation of protein excretion (proteinuria). Some patients have high blood pressure (the combination of haematuria, oliguria and hypertension is known as the nephritic syndrome).
Renal biopsy will usually show enlarged, hypercellular glomeruli, which contain numerous neutrophils and increased numbers of swollen endothelial cells with proliferating mesangial cells. Immunohistological stains reveal IgG, IgM and C3 in glomerular capillary walls and mesangial regions, and electron microscopy (EM) will show these immune deposits as electron dense (black) humps on the epithelial side of the glomerular basement membrane (GBM) as well as deposits in the subendothelial and mesangial regions.
Interestingly, the vast majority of patients will get entirely better – the hypercellular glomeruli will return to normal (cell death by apoptosis) and the deposits will be cleared by phagocytosis (mesangial cells are phagocytic). A few (often older) patients may develop very profound renal failure and go on to have chronic renal failure with the need for dialysis/transplantation.
Minimal change disease
Minimal change disease is the commonest cause of the nephrotic syndrome in children (especially in children aged 2–5years). Nephrotic syndrome is defined as heavy protein loss in the urine with low levels of albumin in the blood and peripheral oedema as a consequence of the reduced colloid osmotic pressure. Microscopically, this disease is characterised by normal-looking glomeruli (the tubules, interstitium and vessels are also usually normal). Classically, immunohistological stains are negative (i.e. there are no immune complexes in the glomeruli). The only significant finding is foot process fusion (spreading of the feet of the podocyte, leading to apparent loss of the ‘feet’ on which the podocyte stands on the glomerular basement membrane), seen on electron microscopy (this is actually a non-specific finding as foot process fusion is seen in most glomerular diseases where there is heavy proteinuria). Most patients respond well to oral corticosteroid administration (although the disease may recur when the steroids are reduced/withdrawn).
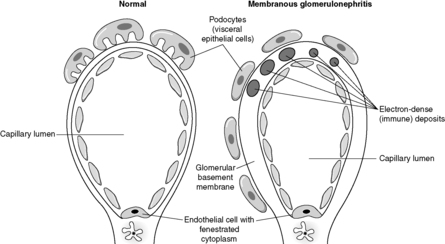
Membranous glomerulonephritis
Membranous glomerulonephritis is the commonest cause of the nephrotic syndrome in adults. In established membranous glomerulonephritis, light microscopy classically shows glomeruli with thick capillary walls. The glomerulus is otherwise normal. A special silver deposition stain will show epithelial-directed ‘spikes’ poking out from the glomerular basement membrane. Immunohistologically, there is immunoglobulin (often IgG) and complement (C3) deposition along/in the capillary walls. Electron microscopy reveals variably sized subepithelial or intramembranous electron-dense deposits (see Figure 64). The ‘spikes’ seen on light microscopy represent ‘fingers’ and ‘tongues’ of glomerular basement membrane insinuating up between the deposits. The disease is idiopathic in about 85% of patients, but can be associated with drugs (e.g. penicillamine), tumours (e.g. lung cancer), infections (e.g. human immunodeficiency virus (HIV) and hepatitis B) and SLE. Prognosis depends, at least in part, on the underlying disease, but at least a third of idiopathic patients will eventually require dialysis or transplantation.
IgA disease
Buy Membership for Pathology Category to continue reading. Learn more here