CHAPTER 44 Transposition of the Great Arteries
Historically, CTGA was first described by Matthew Baillie, a Scottish pathologist, in 1797. The first corrective surgery for CTGA was the atrial switch procedure developed by Senning1 in 1959 and Mustard2 in 1964. Atrial switching effectively converts a CTGA to a CCTGA, in which the RV pumps the systemic circulation. Although this circulation is compatible with life, the systemic RV is prone to early failure. To address the shortcomings of atrial switching, Jatene and colleagues3 perfected the arterial switch procedure in 1975, in which the LV pumps the systemic circulation. It has become the preferred surgical treatment for CTGA. The Rastelli procedure4 is used when the CTGA is associated with pulmonary stenosis. CCTGA was first described by the famous Bohemian pathologist Carl von Rokitansky in 1875. Early treatments focused on correcting secondary lesions, such as ventricular septal defect (VSD). In favor of an anatomically correct circulation, Ilbawi and associates5 and others have advocated the double switch procedure, which combines the atrial switch and arterial switch procedures, or the atrial switch and Rastelli procedures. This procedure restores the LV as the systemic ventricle.
Definition and Classifications
CTGA is defined by ventriculoarterial discordance. It also can be described by the inlet-outlet features of the ventricles: the presence of subaortic infundibulum (Fig. 44-1), the absence of subpulmonary infundibulum, and mitral-pulmonary fibrous continuity (Fig. 44-2). Subaortic infundibulum implies a connection of the aorta to the infundibulum, which is a morphologic feature of the RV. The absence of subpulmonary infundibulum means that the pulmonary trunk is not connected to the RV. Because fibrous continuity of the inlet-outlet valves is a feature of the LV, a mitral-pulmonary fibrous continuity implies that the pulmonary trunk is connected to the LV.
CTGA is known by a number of other names. Because CTGA comprises 90% of all transposition cases, it is sometimes referred simply as transposition of the great arteries. Other designations are simple transposition and dextrotransposition of the great arteries (D-TGA). The letter D refers to D-loop of the ventricles and not the positional relationship of the great arteries. D-loop refers to the rightward folding of the heart tube during early embryologic development. This crucial event leads to the normal positions of the LV and RV at birth (Fig. 44-3). Usually, D-TGA corresponds with the physiology of CTGA, but this is not always the case. In situs inversus, the CTGA physiology corresponds to L-loop ventricles and CCTGA physiology corresponds to D-loop ventricles. To avoid confusion, it is best to use the terms CTGA and CCTGA, which correctly describe the underlying physiology.
CCTGA is defined by the presence of atrioventricular and ventriculoarterial discordance. Alternative names for CCTGA are corrected transposition, double discordance, and levotransposition of the great arteries (L-TGA). In a patient with normal situs, L-TGA implies L-loop ventricles (Fig. 44-4) and atrioventricular discordance, in addition to ventriculoarterial discordance. Like D-TGA, the term L-TGA is best avoided in favor of CCTGA.
Van Praagh proposed a three-letter scheme that labels the sidedness of the atrial, ventricular, and arterial segments,6 and it has been used to categorize various anatomic manifestations of TGA. In this scheme, the first letter denotes the atrial situs, with S meaning solitus and I meaning inversus. The second letter denotes the ventricular loop, with D meaning D-loop, and L meaning L-loop. The third letter denotes the positions of the great arteries, with D meaning that the aortic root is on the right side of the pulmonary root (Fig. 44-5) and L meaning that the aortic root is on the left side of the pulmonary root (Fig. 44-6). In situs solitus and CTGA, the most common segmental anatomy is S, D, D, although S, D, L exists in a minority of cases. In situs inversus and CTGA, the most common pattern is I, L, L, which is the mirror image of S, D, D. In CCTGA, the segmental anatomy is usually S, L, L for situs solitus and I, D, D for situs inversus. Van Praagh and coworkers,7 in a pathology series of CCTGA, reported one case of S, L, D.
Epidemiology and Genetics
CTGA occurs in 5% to 7% of cases of congenital heart disease, with an incidence of 20 to 30/100,000 live births. It is the second most common cyanotic heart disease, behind tetralogy of Fallot, and it is the most common cyanotic heart disease that manifests in the neonatal period. There is no known racial predilection, but there is a 2 : 1 male predominance. CTGA has been reported in trisomies 18 and 21,8 although the vast majority of cases have no identified genetic defect. Chromosomal 22q11 deletion, commonly found in conotruncal anomalies, is not associated with CTGA. Furthermore, the risk of recurrence in the offspring of individuals with CTGA is very low.9 It has a greater incidence in mothers with diabetes. These observations suggest that genetics may play a less important role in the development of CTGA than environmental factors, possibly intrauterine hormonal disturbance or exposure to teratogens. CTGA is rarely associated with other extracardiac anomalies, with the possible exception of coarctation.
Etiology and Pathophysiology
Embryology
The embryology of CTGA is not well understood. The loss of the normal spiral relationship between the ascending aorta and the pulmonary trunk in CTGA suggests a problem with the process of conotruncal septation. In normal development during the fifth week of gestational age, two intertwined, spiraling flow streams in the conotruncus are thought to induce the proper spiraling septation that divides the conotruncus into the aorta and pulmonary trunk.10 The spiraling flow streams may be disturbed, leading to abnormal conotruncal septation and CTGA. This may also explain why the infundibulum septum, formed during the same process, is often abnormal in CTGA.
Associated Cardiac Anomalies
Knowledge of the coronary artery anatomy is crucial for the surgical management of CTGA. In CTGA, as in normal cardiac anatomy, the two main coronary arteries most often arise from the two aortic sinuses of Valsalva closest to, or facing, the pulmonary valve. In the most common coronary pattern for CTGA, which occurs in 67% of cases, the right coronary artery (RCA) originates from the right facing sinus and the left main coronary artery (LMCA) originates from the left facing sinus (Fig. 44-7). The LMCA then bifurcates into the left anterior descending (LAD) artery and left circumflex (LCx) artery, as expected. Less commonly, the LCx arises from the RCA in 22% of cases. Other variations include a single RCA (9.5%), a single LMCA (3%), reversed RCA and LMCA (3%), and others (Fig. 44-8).11
Only a small fraction (1% to 9%) of CCTGA cases are isolated without any associated anomalies.12 The most common associations are VSD and pulmonary stenosis. VSD occurs in 60% to 80% of cases (Fig. 44-9). It can be of any type and the VSD can be multiple. Pulmonary stenosis occurs in 30% to 50% of cases, and it can be valvular or subvalvular. There is a frequent association with tricuspid abnormalities, which include dysplastic leaflets, Ebstein-like displacement of the leaflets, and a tricuspid valve straddling the ventricular septum. Other less common associated anomalies are atrial septal defect (ASD), patent ductus arteriosus (PDA), pulmonary atresia, mitral valve abnormalities, coarctation, and interrupted aortic arch. CCTGA manifests with dextrocardia in 25% of cases and situs inversus in 5% of cases.
The aortic root position is typically anterior and to the left of the pulmonary root. Together with normal situs and L-loop ventricle, S, L, L is the most common segmental description for CCTGA. Variations in coronary anatomy are common, but the branching pattern of the coronary arteries generally follows the ventricles. Because the left ventricle is positioned on the right, the LMCA commonly arises from the right-facing sinus and travels to the right before branching into a LAD and a LCx. The latter lies in the right atrioventricular groove. Similarly, the RCA commonly arises from the left-facing sinus and lies in the left atrioventricular groove. Single coronary origin is also common.13
MANIFESTATIONS
Clinical Presentation
In practice, some mixing of the oxygenated and deoxygenated blood occurs through shunts between the pulmonary and systemic circulations, providing some oxygen to the systemic organs. The degree of cyanosis depends on (1) the size and direction of these shunts and (2) the degree of obstruction to the pulmonary flow. In CTGA-IVS, the most common anatomic arrangement, shunting is limited and the neonate presents with cyanosis within hours of birth. Cyanosis worsens as the PDA closes in the first few days of life. Prognosis in this scenario is poor, with a 30% survival rate at 1 month. In CTGA-VSD, the VSD is usually large and mixing occurs freely at the ventricular level. Patients may present with mild cyanosis, even after the PDA closes. As the pulmonary resistance drops in the first week of life, a large shunting from the systemic RV to pulmonary LV may occur. This can lead to congestive heart failure and later pulmonary vascular obstructive disease. The latter is the cause of pulmonary hypertension and Eisenmenger syndrome. The survival rate at 1 year is 50%, which is better than that for CTGA-IVS. In CTGA-VSD-LVOTO, the LVOT obstruction limits flow to the pulmonary circulation. The physiology in this situation is similar to that of tetralogy of Fallot. Severe obstruction would lead to diminished pulmonary flow and worsening cyanosis. Little obstruction would lead to large shunting and the same outcome as CTGA-VSD. If the obstruction is balanced just right, the pulmonary circulation may be protected from harmful shunt flow at a level of tolerable cyanosis. The survival rate for CTGA-VSD-LVOTO is 50% at 3 years, with some surviving into adulthood. Left untreated, the overall survival rates for CTGA are 70% at 1 week, 50% at 1 month, and 10% at 1 year.14

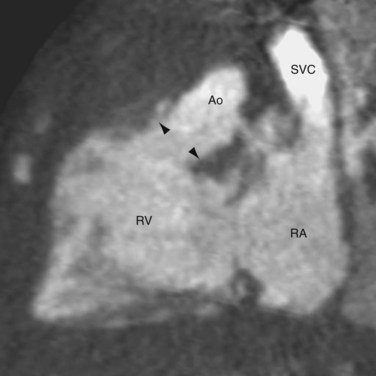
 FIGURE 44-1
FIGURE 44-1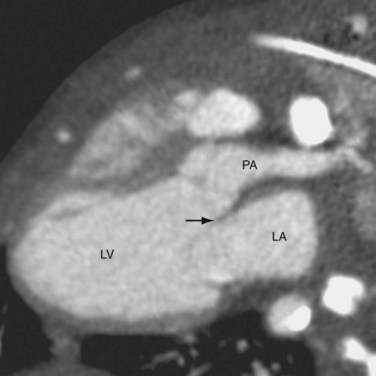
 FIGURE 44-2
FIGURE 44-2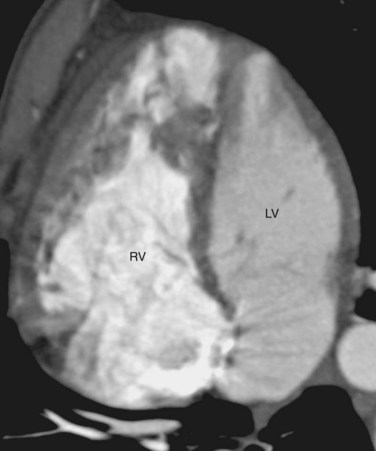
 FIGURE 44-3
FIGURE 44-3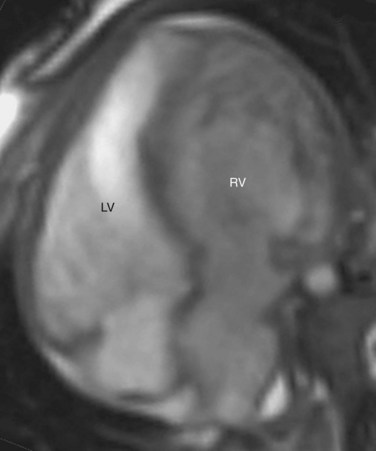
 FIGURE 44-4
FIGURE 44-4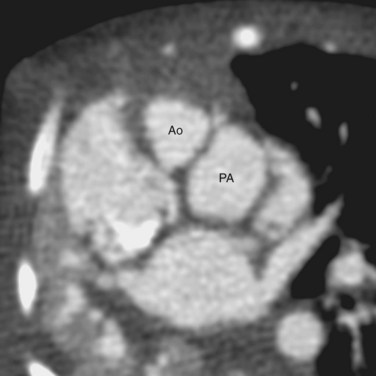
 FIGURE 44-5
FIGURE 44-5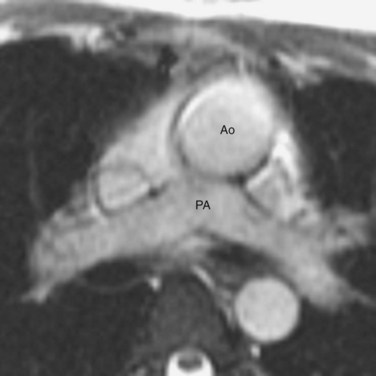
 FIGURE 44-6
FIGURE 44-6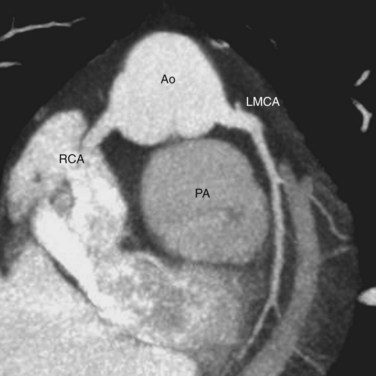
 FIGURE 44-7
FIGURE 44-7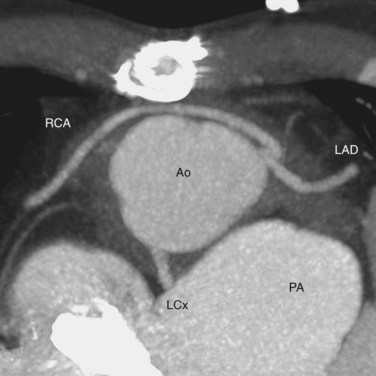
 FIGURE 44-8
FIGURE 44-8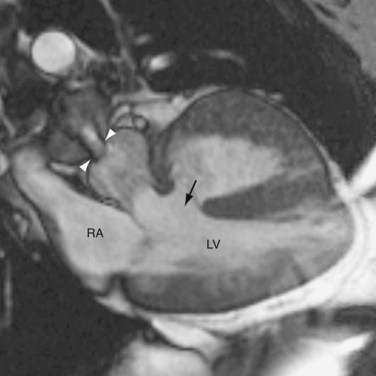
 FIGURE 44-9
FIGURE 44-9