Transfusion Therapy
Blood and Blood Products
Transfusion of blood components (red cells, white cells, platelets, whole plasma, or plasma fractions) is commonplace in the emergency department (ED). Annually in the United States, 15 million blood donations take place and 14 million units of red blood cells (RBCs) are transfused.1 Although acute hemorrhage is the most common emergency indication for blood transfusion, more nonemergency transfusions and blood component therapy now occur in the ED as a result of the general migration of health care away from inpatient settings. Technical advances have made component therapy directed at specific acute and chronic pathologic conditions practical, safe, and affordable.
Background
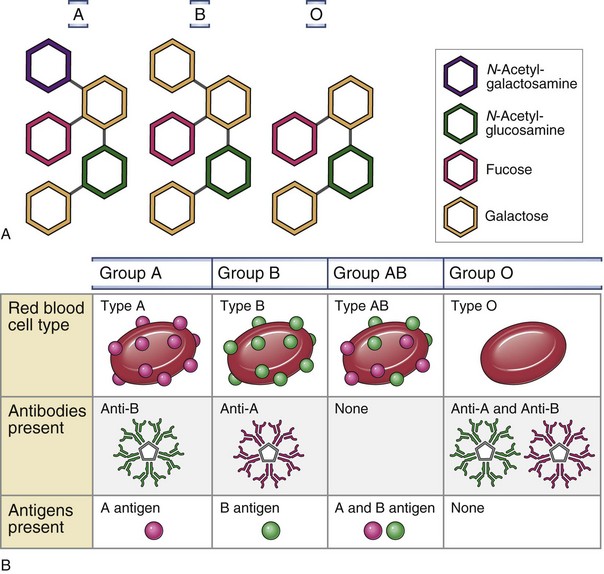
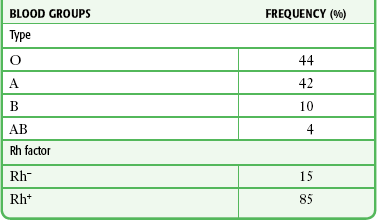
RBC membranes contain a series of glycoprotein moieties, or antigens, that give the cell an individual identity. Two different genetically determined antigens, type A and type B, occur on the cell surface. Any individual may have one, both, or neither of these antigens. Because the type A and type B antigens on the surface of the cell make the RBC susceptible to agglutination, these antigens are termed agglutinogens. The presence or absence of agglutinogens is the basis for the ABO blood group classification, and the blood types are named accordingly as A, B, or AB. Blood type O contains neither the A nor the B agglutinogen. These blood type antigens are represented in Figure 28-1. The relative frequencies of the different blood groups are listed in Table 28-1.
TABLE 28-1
Frequency of Blood Groups in the U.S. General Population
From Guyton AC, ed. Textbook of Medical Physiology. 6th ed. Philadelphia: Saunders; 1981.
Within the first year of life, antibodies begin to form against the standard red cell agglutinogens not present in the individual patient. These agglutinins are γ-globulins of the IgM and IgG types and are probably produced by exposure to agglutinogens in food, bacteria, or exogenous substances other than blood transfusions. In the absence of type A agglutinogens (blood types B and O), anti-A antibodies, or agglutinins, spontaneously develop in the plasma. Similarly, in the absence of type B agglutinogens (blood types A and O), anti-B antibodies develop. When both A and B agglutinogens are present (blood type AB), no agglutinins are formed. Blood groups and their genotypes and constituent agglutinogens and agglutinins are shown in Figure 28-1.
Types of RBC Preparations
The normal blood volume of a healthy adult and healthy child is approximately 70 and 80 mL/kg, respectively. Though intuitively an ideal transfusion agent, whole blood is seldom used except for autologous transfusions (e.g., autotransfusion) and for exchange transfusions. Whole blood is not indicated for the treatment of hypovolemic shock, which can be treated effectively with a combination of crystalloids (e.g., lactated Ringer’s [LR] solution, 0.9% sodium chloride), colloids (e.g., plasma protein, albumin), and packed red blood cells (PRBCs). It is also not indicated for the correction of thrombocytopenia, replacement of coagulation factors, or treatment of anemia.2 The incidence of transfusion reactions following transfusion with whole blood is approximately 2.5 times greater than that with PRBCs.3 Whole blood contains antigenic leukocytes and serum proteins, which carry higher risk (approximately 1%) for an allergic reaction. Nevertheless, warm fresh whole blood has seen increased popularity in military settings and has been proposed as an alternative to component therapy for civilian use in massive transfusion protocols.4
PRBCs
PRBCs are prepared by centrifugation and removal of most of the plasma from citrated whole blood. One unit of PRBCs contains the same red cell mass as 1 unit of whole blood at approximately half the volume and twice the hematocrit (55% to 80%) in 250 mL of volume.5 One unit of PRBCs raises the hematocrit approximately 3% in an adult or increases the hemoglobin level of a 70-kg individual by 1 g/dL. In children, there is an approximate rise in hematocrit of 1% for each 1 mL/kg of packed cells. For example, if 5 mL/kg of PRBCs is transfused, the hematocrit will rise by approximately 5%. Actual changes depend on the state of hydration and the rate of bleeding. Because most of the plasma has been removed, PRBCs cause fewer transfusion and allergic reactions than whole blood does.
PRBCs contain less sodium, potassium, ammonia, citrate, hydrogen ions, and antigenic protein than whole blood does. This may offer advantages in patients with reduced cardiovascular, renal, or hepatic function. The rate of urticaria is still relatively high at 1% to 3% of transfusions, but the incidence of adverse reactions to packed cells is approximately one third of that noted with whole blood. The benefit of increased hemoglobin must be weighed against the potential for volume, electrolyte, and acid-base imbalances following PRBC administration. In cases of massive transfusion (>10 units), there is a significant risk for metabolic and respiratory acidosis, as well as hypocalcemia, which can reach life-threatening levels. Although underlying illness or injury obviously plays a major role in the cause of death, the overall mortality of patients requiring massive PRBC transfusions is approximately 60%.6,7
Transfusion of PRBCs is indicated to provide additional oxygen-carrying capacity and expansion of volume. Packed cells are most commonly used to treat acute hemorrhage and anemia not amenable to nutritional correction. When treating acute hemorrhage, PRBCs are usually given (1) if the hemoglobin level falls below established critical levels for that particular given patient population (see the section “Transfusion Thresholds”), (2) after rapid crystalloid infusion fails to restore normal vital signs, or (3) concurrently with crystalloid infusion in the treatment of obvious life-threatening blood loss.
Specially prepared or screened types of red cells are listed in the following sections. Their indications for use are presented in Box 28-1.
Leukocyte-Reduced RBCs
Leukocyte-reduced blood products contain less than 5 × 106 leukocytes/unit, whereas standard RBC units contain 1 to 3 × 109 leukocytes. Reduction can be performed at the time of collection, in the transfusion laboratory, or at the bedside during transfusion. Leukocyte-reduced products are used to decrease the likelihood of febrile reactions, immunization to leukocytes, and transmission of disease. Currently, about 60% to 75% of the U.S. blood supply is leukoreduced.8 Several groups advocate the use of 100% leukocyte-reduced blood products because of the many adverse transfusion reactions that are associated with leukocytes. Non–leukocyte-reduced products are virtually the exclusive method of transmission of several viruses, including human T-lymphotropic virus 1 and 2, Epstein-Barr virus (EBV), and cytomegalovirus (CMV). Additionally, they help reactivate and disseminate CMV and human immunodeficiency virus (HIV). Moreover, increased rates of bacterial contamination and postoperative and line infections have been associated with the use of non–leukocyte-reduced products. Furthermore, leukocytes lead to HLA alloimmunization, which results in increased graft rejection and platelet refractoriness.9
Infectious Complications of Transfusions
Between 1985 and 1999, 694 deaths associated with transfusion were reported to the Food and Drug Administration (FDA). Seventy-seven (11.1%) of these deaths were caused by bacterial contamination. However, sepsis is an uncommon occurrence because both the citrate preservative and refrigeration kill most bacteria. Concern over sepsis is responsible for the practice of completing transfusions within 4 hours and returning unused blood products to the blood bank refrigerator for future use only if they have been unrefrigerated for less than 30 minutes. Both gram-negative and gram-positive organisms are transmitted, with gram-negative virulence being more commonly associated with mortality. A prospective observational study found that the rate of nosocomial infections was significantly higher in patients receiving blood transfusion. Leukoreduction did not significantly reduce the rate of infection.10 A recent multicenter study by the Centers for Disease Control and Prevention further evaluated the risk for bacterial contamination in the blood pool. The results showed the rate of bacterial sepsis to be much lower than previously thought. Only 0.21 cases and 0.13 deaths per million red cell transfusions occurred. The rate was slightly higher for platelet transfusions, with 10 cases and 2 deaths per million transfusions.11 Mandatory screening of platelets for bacterial contamination began in 2004 and has further reduced the rate of reported death.
Syphilis may theoretically be transmitted by transfusion, but both refrigeration and citrate markedly reduce the survival of Treponema pallidum. Thus, only fresh blood or platelet transfusions are of concern for its transmission. The incubation period for syphilis transmitted by transfusion is 4 weeks to 4 months, and the initial clinical manifestation is commonly a rash. No cases of transfusion-transmitted syphilis have been recognized for many years.12,13
Most blood products have the potential to transmit hepatitis. Routine testing of blood donors for hepatitis C virus (HCV) has occurred since 1991, but the initial screening tests were relatively inaccurate. Since April 1999, the use of nucleic acid amplification testing (NAAT) to detect HCV RNA has been mandatory. This test has essentially eliminated false positives and has a sensitivity of greater than 99%.14,15 The American Association of Blood Banks reported the risk for transmission of HCV to be less than 1 per 1,000,000 transfusions. The incubation period for HCV is 2 to 12 weeks following parenteral infusion. The reported risk for transmission of hepatitis B virus (HBV) is higher at 1 per 137,000 transfusions.
Both CMV and EBV may cause a mononucleosis-like syndrome 2 to 6 weeks after a transfusion. Indications for CMV- and EBV-negative preparations are listed in Box 28-1. Alternatively, leukocyte-reduced products can help protect against CMV and EBV.
Transmission of WNV by blood transfusion was first documented in the United States in 2002.16,17 Since 2003, universal screening for WNV by investigational NAAT occurs on all blood donations. From 2003 to 2005, 1400 potentially infectious donations were removed from the blood pool. Since that time, however, multiple cases of transfusion-associated transmission of WNV have been confirmed. This residual risk for transmission is due to blood units with low levels of viremia. Public health authorities continue to look for ways to eliminate this risk from the blood pool.17,18
Emerging infectious risks to the blood supply are always under investigation. Blood-transmitted infections under current surveillance include parvovirus B19, dengue virus, and the prions that cause Creutzfeldt-Jacob disease. Although a viremic phase of human herpesvirus-8, avian flu (H5N1), H1N1, and Lyme disease has been well documented, no cases of transmission through transfusion have been noted.18,19
A summary of infections risks associated with red cell transfusion is presented in Table 28-2.
TABLE 28-2
Estimated Risks of Transfusion per Unit PRBCs in the United States
| RISK | RATE |
| Major allergic reactions | 1/100 |
| Anaphylaxis | 1/20,000-50,000 |
| Anaphylactic shock | 1/500,000 |
| Hemolytic reaction (minor) | 1/6000 |
| Hemolytic reaction (fatal) | 1/100,000 allergic reactions |
| Death from sepsis (RBC) | 1/5 million |
| Death from sepsis (platelets) | 1/500,000 |
| Parasitic infections (Lyme, malaria, Chagas) | <1/million—data lacking |
| Hepatitis C | <1/million |
| Hepatitis B | 1/140,000 |
| Parvovirus, Creutzfeldt-Jacob disease | Extremely rare—data lacking |
| HTLV 1/2 infection | 1/200,000 |
| HIV infection | 1/2 million |
| West Nile virus | Extremely rare—data lacking |
| CMV/Epstein-Barr | Rare—data lacking |
| Acute lung injury | 1/500,000 |
| Graft-versus-host disease | Extremely rare—data lacking |
| Immunosuppression | Unknown |
| Syphilis‡ | No cases reported currently |
Transfusion Reactions
Acute Reactions
Allergic: The most common manifestation of a minor allergic transfusion reaction is urticaria; however, wheezing and angioedema can also be observed. The allergic response is due to the presence of atopic substances that interact with antibodies in the donor or recipient plasma, but the severity is not dose related.5 Whenever a transfusion reaction is suspected, the first step in management is to stop the transfusion. Treatment is the same as for other allergic reactions and includes antihistamines, steroids, and epinephrine if needed. For mild reactions (e.g., those limited to skin findings), the transfusion can be resumed once treatment has been given.
Anaphylactic: The reported incidence of transfusion-associated anaphylaxis is 1 in 20,000 to 50,000. Anaphylaxis occurs most commonly in IgA-deficient patients who have IgA-specific antibodies of the IgE class.20 Manifestations of an anaphylactic transfusion reaction include shock, hypotension, angioedema, dyspnea, bronchospasm, and laryngospasm. The symptoms are typically rapid in onset and begin within seconds to minutes of starting the transfusion. If this type of reaction occurs, the transfusion must be stopped immediately. Treatment includes airway management as necessary, epinephrine, fluids, and steroids, followed by appropriate supportive care and continued close observation. If a transfusion is still required, the patient needs to be pretreated with steroids and antihistamines 30 to 60 minutes before the transfusion. Alternatively or in addition, washed cellular products can be used.
Febrile (Nonhemolytic): A febrile, nonhemolytic reaction is defined as an increase in temperature of 1°C or higher during or within 6 hours of the transfusion. The mechanism for this type of reaction is most commonly attributed to an interaction between recipient antibodies and donor leukocytes.21,22 This stimulates the release of cytokines such as interleukin-1, which ultimately produces a febrile response. Although this type of reaction is not life-threatening, it is difficult to distinguish from more serious transfusion reactions. Accordingly, all patients with a fever attributable to a transfusion must have the transfusion stopped. Symptoms can be treated with acetaminophen or nonsteroidal antiinflammatory drugs. There is no role for antihistamines in the treatment of this type of reaction. Although controversy exists, premedication with antipyretics and antihistamines may prevent these transfusion reactions.23
Acute Hemolytic: An acute hemolytic reaction is usually the result of donor-recipient major ABO incompatibility. This in turn is most commonly the result of blood product misassignment related to clerical error. Hemolytic transfusion reactions are estimated to occur once per every 6000 blood units transfused, with a fatality rate of 1 per every 100,000 units transfused.
When incompatible blood is given, the result may range widely from no effect to death. If the recipient does not have antibodies (naturally occurring or acquired) directed against the foreign RBC antigen received, there will be no immediate reaction, but antibodies to the infused blood may develop within weeks, thus limiting the safety of subsequent transfusions from the same donor or same antigenic type. If the recipient’s serum has preformed antibodies directed against the donor RBCs (e.g., an incompatibility in the major crossmatch), the recipient will begin to hemolyze the donor cells within seconds or minutes. In most cases of major crossmatch reactions, RBCs of the donor blood are agglutinated and hemolyzed. It is rare for transfused blood to produce agglutination of the recipient’s cells because the plasma portion of the donor blood becomes diluted by the plasma of the recipient. This reduces the titer of the infused agglutinins to a level too low to cause significant agglutination. Because the recipient’s plasma is not diluted to any significant degree, the recipient’s agglutinins can react with donor cells. The end result of antigen-antibody incompatibility is red cell hemolysis. Occasionally this occurs immediately, but more often the cells first agglutinate. They are then trapped in small vessels and become phagocytized over a period of hours to days and release hemoglobin into the circulatory system.24 Clinical manifestations of acute hemolysis include chills, fever, tachycardia, abdominal pain, back pain, hypotension, fainting, and a feeling of “impending doom.” Derived from the liberation of intracellular material associated with hemolysis, vasoactive substances may cause hypotension and shock; other substances may precipitate disseminated intravascular coagulation and high-output cardiac failure. Acute renal failure may also result. The presence of hemoglobinemia and hemoglobinuria is essential in making the diagnosis. A decrease in hematocrit and haptoglobin or an increase in lactate dehydrogenase (LDH) may also be seen.
Treatment of an acute hemolytic reaction begins with immediate cessation of the transfusion. The blood bank should be alerted immediately because a second patient is now at risk for receiving the wrong product. Resuscitation and supportive care along with close monitoring of laboratory values are essential. A sample of blood from the recipient needs to be obtained for a direct antiglobulin test, plasma-free hemoglobin, and repeated type and crossmatch. Urine can also be tested for free hemoglobin. Renal function and electrolytes should be monitored for evidence of renal failure and hyperkalemia. Dialysis is occasionally required.24,25 Fluid resuscitation and diuresis with normal saline are recommended to maintain urine output above 100 to 200 mL/hr. LR solution should be avoided because calcium can precipitate clotting.
Drug-Induced Hemolysis: Drug-induced hemolysis is not a transfusion reaction per se; however, it can be indistinguishable from an acute hemolytic reaction in patients receiving blood transfusions. In this case, both autologous and transfused cells are affected. A patient’s serum can react with red cells in the presence of certain drugs. Two examples of drugs that can cause this type of reaction are cefotetan and ceftriaxone.
TRALI
Transfusion-related acute lung injury (TRALI) refers to noncardiogenic pulmonary edema occurring during or shortly after the transfusion of blood products. A leading cause of transfusion-related mortality and morbidity, TRALI has been reported to occur in as many as 3% of patients receiving transfusions.26,27 TRALI appears to be associated with components from female plasma; preferential distribution of male plasma by the American Red Cross has recently decreased its incidence.28
The potential for TRALI is one reason why some authorities are reluctant to transfuse high ratios of plasma to PRBCs in massive transfusion protocols. TRALI is thought to result from the activation of recipient neutrophils in the lung and the production of vasoactive mediators, which leads to increased pulmonary capillary permeability and leakage. Initial symptoms include respiratory distress, hypoxia, hypotension, fever, and bilateral pulmonary edema; however, the spectrum of TRALI can also include much milder reactions.29,30
Treatment of TRALI is supportive and includes supplemental oxygen, endotracheal intubation, and cardiovascular support as necessary. Diuresis and corticosteroids are not effective.31,32
Delayed
Delayed Hemolytic: Even when major and minor crossmatches indicate compatibility, delayed hemolytic transfusion reactions can occur days to weeks after transfusion. This is due to antibody production by either the donor or recipient B cells in response to exposure to antigens on red cells. Usually seen in multiply transfused patients or in multigravida women, these reactions may be unavoidable without complete RBC antigen typing, a procedure occasionally indicated for recipients of repeated transfusions. An incompatibility in the minor crossmatch does not usually result in a serious reaction, although the recipient’s red cells can be hemolyzed if the titer of the antibody is sufficiently large. Fortunately, 90% of transfusions are now given as PRBCs, which contain a very small volume of plasma, thus minimizing the chance of a transfusion reaction occurring as a result of donor sensitization.
GVHD: GVHD is a transfusion complication most commonly associated with allogeneic hematopoietic cell transfusions. However, it can occur whenever immunologically competent lymphocytes are transfused, especially in immunocompromised hosts. Donor lymphocytes engraft in the recipient and then attack host tissue. Symptoms are typically observed 7 to 14 days after the transfusion and include fever, rash, and diarrhea. Hepatitis and marrow aplasia also occur. GVHD is often fatal; failure of the host’s marrow leads to overwhelming infection or bleeding. The use of gamma-irradiated cellular components prevents this complication by making the donor lymphocytes incapable of proliferating.21,29
Posttransfusion Purpura: In rare cases, profound thrombocytopenia can develop 1 to 3 weeks after a transfusion associated with an antibody response to a platelet antigen. A probable pathophysiologic mechanism for this is the production of low-affinity antibodies that cross-react with autologous platelets. Eventually, as the immune response matures, the low-affinity antibody is eliminated and the thrombocytopenia resolves spontaneously. Only patients at risk for bleeding or hemorrhage need to be treated. Treatment consists of high-dose immune globulin, plasmapheresis, or platelet transfusion.
Miscellaneous Transfusion Issues
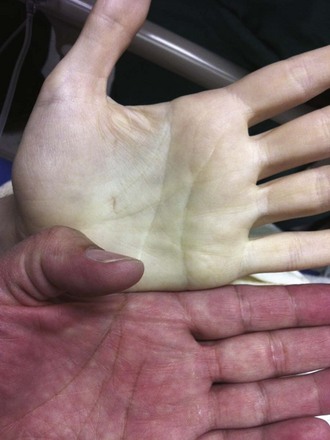
PRBCs are a precious commodity. Guidelines to limit transfusions to those that are absolutely necessary have set transfusion thresholds or “triggers.” A liberal transfusion trigger is approximately 10 g/dL of hemoglobin, whereas restrictive thresholds are set at 7 to 8 g/dL. The limits for restrictive thresholds stem from the finding that aerobic metabolism can still occur at hemoglobin concentrations as low as 5 g/dL.33,34 Clinically, however, almost all patients show signs of physiologic stress at hemoglobin concentrations of less than 6 g/dL.34,35 It is at this level that patients will begin to reliably demonstrate the symptoms and signs of anemia: dyspnea on exertion or even at rest; pallor, particularly of the palms and mucous membranes (Fig. 28-2); and resting tachycardia.
The Transfusion Requirements in Critical Care study compared a strategy of restrictive transfusion triggers with conventional, more liberal triggers.36 The authors concluded that a restrictive strategy appears to be at least as effective as a more liberal strategy, with the possible exception of patients with coronary insufficiency. In trauma patients, more liberal use of blood has also been questioned.37 One of the risks associated with restrictive transfusion thresholds is an increased incidence of infection.37 Nonetheless, mortality, cardiac events, and length of hospital and intensive care unit (ICU) stay appear to be unaffected by more restrictive thresholds.38
No single criterion should be used as an indication for red cell component therapy. Multiple factors related to the patient’s clinical status and oxygen delivery needs should be considered. Current practice focuses on the needs of the individual patient. Particularly close attention should be paid to the subset of patients at risk for coronary ischemia, with more liberal triggers possibly being applied to these patients. Wu and colleagues,39 in a U.S.-based study of Medicare patients with acute myocardial infarction, found RBC transfusions to be beneficial in elderly patients when hematocrit values were lower than 33%. In the setting of severe sepsis, a more conservative threshold of 10 g/dL may also be appropriate.40 In contrast, new data from pediatric ICU settings suggest that adopting a threshold of 7 g/dL imparts no worse clinical outcome and may result in benefits in long-term mortality and morbidity.41 Similar findings were documented in pediatric postsurgical patients.42
In addition to cost and transmitted infections, there is a risk for systemic inflammatory response syndrome with transfusion. This syndrome is closely associated with diminished organ function and mortality in critically ill adult patients and its incidence increases with the administration of more than 4 units of PRBCs.43,44 Red cell administration is also associated with an increased risk for life-threatening acute respiratory distress syndrome45 and multiple-organ failure.46,47 This has been linked to immunologic alterations and their effects on plasma cytokine and cytokine receptor concentrations. In patients receiving more than 15 units of PRBCs, levels of both interleukin and soluble tumor necrosis factor were elevated.31,46 It remains unclear whether the use of leukocyte-reduced PRBCs can mitigate the expected inflammatory response.
The judicious and restricted administration of PRBCs appears to be clinically founded in the majority of patients. By implementing a restrictive transfusion strategy, the probability of a patient requiring blood can be decreased by 42% and the volume of PRBCs transfused can be decreased by 0.93 units per patient.38,48,49
Another area of study focuses on the projected need for administration of PRBCs in any given patient. Knowing which patients will probably need blood based on their initial findings can be helpful in resource allocation and determination of the need for crossmatching. Studies have correlated the base deficit (BD) with the need for transfusion by using a cutoff of −6 mEq/L. Patients with a BD larger than −6 mEq/L have a 72% chance of requiring blood, whereas those with BDs smaller than −6 have only an 18% of requiring PRBCs. More elaborate scales have been proposed based on easily assessable parameters. The emergency transfusion score is a point system based on systolic blood pressure, the presence of free fluid on focused assessment with sonography for trauma (FAST), an unstable pelvic ring fracture, advanced patient age, admission from the scene, motor vehicle collision, or a fall as being predictors of future transfusion requirements.50
Massive Transfusion
Massive transfusion is loosely defined. In the 1970s it was considered to be the transfusion of more than 10 units of blood to an adult, equivalent to 1 blood volume, within 24 hours. Historically, massive transfusion was associated with dismal survival rates (<10%).51,52 As blood banking technology and storage methods have improved, the mortality associated with massive transfusions has decreased significantly. There is no clear physiologic threshold to define a massive transfusion. Mortality in patients receiving fewer than 5 units is currently around 10%, in those receiving 6 to 9 units it is approximately 20%, and in those receiving 10 or more units it is greater than 50%.53 Some sources have recently narrowed the definition of massive transfusion to include only patients who receive more than 50 units of PRBCs within the first 24 hours of resuscitation. Alternative triggers for initiation of a massive transfusion protocol are now being proposed, including elevation of the international normalized ratio (INR) and BD, in addition to hemoglobin. Despite the challenges of treating the expected posttransfusion inflammatory and immunologic complications, patients requiring massive transfusions can have good outcomes.
Transfusion Coagulopathy
Pathologic hemostasis occurs following massive blood transfusions.52,54–57 Coagulopathy and subsequent uncontrolled bleeding are major contributors to trauma-related deaths.58,59 Although such abnormalities rarely develop within the time frame of the initial resuscitation in the ED, an understanding of the problem can lead to a more thoughtful approach to transfusion practices and the anticipation of potential problems. Significant alterations in blood and blood products occur during storage. Moreover, in patients who are given a transfusion equal to 2 blood volumes, only approximately 10% of the original elements remain. The development of transfusion coagulopathy is multifactorial; important factors include tissue injury, acidosis, the duration of shock, and hypothermia, in addition to activation, consumption, and dilution of coagulation factors.60–62
Disseminated intravascular coagulation (from a hemolytic reaction) may play a secondary role in posttransfusion bleeding. Factors V and VIII are labile in stored blood and absent in packed cells. Fibrinogen is relatively stable in stored blood but is absent in packed cells. A deficiency of most clotting factors, especially factors V and VIII and fibrinogen, occurs with massive transfusions. This deficiency probably occurs on a “washout” (i.e., dilutional) basis, although the dynamics are poorly understood. Replacement of these factors may be required. Specific assays for the individual factors are available, but it is more practical to measure the prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen levels. Plasma has been used to correct clotting factor abnormalities secondary to dilution from massive transfusions, but its effectiveness has not been firmly established. Cryoprecipitate has also been used to replace factor VIII and fibrinogen, but it is rarely required because plasma contains some fibrinogen. Fresh frozen plasma (FFP) should be infused to correct the coagulopathy as indicated by clotting studies. Cryoprecipitate may be required if fibrinogen levels fall below 100 mg/dL despite the use of plasma. Although blood component therapy can be based on measured coagulopathy parameters, as a general guide, 1 to 2 units of plasma for each 5 to 6 units of blood may be given empirically.63,64
Numerous massive transfusion protocols exist. Traditionally, transfusion-related coagulopathies have been evaluated and treated as per laboratory indicators, but rapid or massive transfusions do not allow equilibration or timely laboratory analysis. Although this approach is quite acceptable in most patients, the aim of transfusion protocols is to prevent transfusion-related coagulopathy before it occurs. Obviously, one cannot simply continue to transfuse only PRBCs to patients experiencing significant blood loss, and some combination or ratio of plasma to PRBCs to platelets should be adopted. The ideal combination is, however, not known with certainly, and it is largely dependent on the underlying need for transfusion and specific patient characteristics. Most protocols recommend a plasma-to-PRBC ratio of 1 : 1.5 or 1 : 1.8.65 Other protocols also include empirical platelet administration and use ratios of RBCs to platelets to FFP of 1 : 1 : 1.66 At this time, however, no specific transfusion ratio has been proved superior. Strict adherence to any protocol must be balanced against the risk for multisystem organ failure and infection associated with high doses of platelets and plasma. All protocols recommend warming of blood and blood products because hypothermia occurs quickly during massive transfusions and can contribute to further coagulopathy.
Severe Trauma and Coagulopathy: A transfusion coagulopathy often develops in individuals injured during military combat who received transfusions because of widespread tissue trauma. The U.S. military has advocated an approach to transfusion therapy that includes prompt initiation of 1 : 1 : 1 resuscitation ratios with RBCs, pre-thawed universal-donor AB plasma, and apheresis platelets, with conversion to fresh whole blood as soon as it can be obtained. This ratio has not been universally adopted by civilian hospitals.
Emergency Transfusions
In an emergency, three alternatives to fully crossmatched blood exist. The preferred substitute is type-specific blood with an abbreviated crossmatch. The abbreviated crossmatch includes ABO and Rh compatibility. In addition, the recipient’s serum is screened for unexpected antibodies, and an immediate “spin” crossmatch is performed at room temperature. This abbreviated crossmatch requires approximately 30 minutes. Many institutions are now using this procedure as their standard crossmatch for most patients. The safety and utility of the type-specific abbreviated crossmatch have been demonstrated repeatedly, with transfusion reactions occurring only rarely.67,68 Brickman and coworkers demonstrated that bone marrow aspirates obtained with an intraosseous needle can also be used for crossmatching.69
A third alternative to fully crossmatched blood is group O blood, although type-specific blood is generally preferable.5,70 Commonly, this is available at the point of care and has the advantage of being immediately available in cases of severe shock with ongoing bleeding. Thus, despite the theoretical preference for type-specific blood in emergency situations, type O is often a reasonable and practical alternative.
One may transfuse both Rh-positive and Rh-negative group O packed cells into patients who are in critical condition. It is a common misconception that patients who are Rh negative will have an immediate transfusion reaction if given Rh-positive blood. There is no particular advantage in determining the Rh factor because preformed, naturally occurring anti-Rh antibodies do not exist. Theoretically, individuals who are Rh negative may become sensitized either through pregnancy or by previous transfusions, and a delayed hemolytic transfusion reaction will result if Rh-positive blood is transfused. However, this scenario is very rare and is of little significance when compared with life-threatening blood loss. Sensitization to the Rh factor is most problematic for Rh-negative women of reproductive age.71,72 Any sensitized patient may experience a transfusion reaction if exposed again to Rh-incompatible blood. However, significant subsequent transfusion reactions with Rh-incompatible blood in men sensitized to the Rh factor are very rare. Many advise routine use of the more widely available type O Rh-positive packed cells in all patients in whom the Rh factor has not been determined, except in females of childbearing age, for whom future Rh sensitization may be an important consideration. Once resuscitated with Rh-positive packed cells, patients may receive their own type without a problem. Because individuals with type O Rh-negative blood represent only 15% of the population and the blood may be in short supply, it is reasonable to save type O Rh-negative blood for Rh-negative females of childbearing potential and to use type O Rh-positive packed cells routinely as the first choice for emergency transfusions. In a study of emergency blood needs, Schmidt and colleagues reported 601 units of blood into 262 untyped patients, including 8 Rh-negative women, before the blood type was determined.71 No acute hemolytic reactions occurred, and no women were sensitized. A non–emergency-based study found the rate of Rh sensitization in Rh-negative recipients receiving Rh-positive blood to be about 8%, and this figure may be reduced if Rh immune globulin is given after transfusion.73,74 Thus, prophylaxis with Rh immune globulin is recommended only for Rh-negative women of childbearing potential receiving Rh-positive blood.
Rh immune prophylaxis with Rh0(D) human immune globulin (RhoGAM) is also indicated for Rh-negative pregnant women who may be bearing Rh-positive children and may have fetomaternal transplacental hemorrhage, including bleeding in early pregnancy, such as spontaneous or elective abortion, ectopic pregnancy, and other potential causes of antepartum hemorrhage such as trauma. RhoGAM suppresses the immune response of Rh-negative women to Rh-positive RBCs and is effective when given up to 72 hours after exposure to fetal erythrocytes. Standard doses are 50 µg for women up to 12 weeks of pregnancy and 300 µg in the second and third trimester. In the setting of fetal-maternal transfusion greater than 15 mL (usually only in the third trimester when fetal blood volume becomes more substantial), higher doses may be necessary. In such circumstances, the correct dose may be calculated by quantitative testing for fetal erythrocytes in the mother’s blood (Kleihauer-Betke test).75
Metabolic Disturbances
RBCs undergo metabolic, biochemical, and molecular changes during storage that are collectively known as the erythrocyte “storage lesion.”76,77 These changes are generally subtle but can be measured: a decrease in levels of 2,3-diphosphoglycerate (2,3-DPG), pH, and intracellular potassium and a concomitant increase in supernatant potassium.
Directed and Autologous Donations
The system of “directed donations” by which friends or family members may donate blood for a specific individual has been proposed in response to concerns about the transmission of infectious disease. At this time, directed donation systems are in place in some institutions but the practice has not been widely supported. Directed donations probably do not decrease the risk for infectious disease transmission and may disrupt the normal anonymous blood donor system and thus leave fewer units available for other needy patients.78,79
Though of limited clinical applicability in emergencies, autologous donations are commonplace in elective surgery. It has been suggested that up to 10% of the blood supply could be provided through this mechanism. However, current studies show that at its peak, autologous donations represented less than 2% of the total blood collections, and this number is declining.80