Thoracentesis
Anatomy and Physiology of the Pleural Space
Understanding this basic anatomy is important because it underlines the similarities and differences between the two linings, which in turn determines the physiology and pathophysiology of the pleural space. Both the visceral and parietal pleurae are thin layers of connective tissue through which both fluid and protein can leak. Embedded in each membrane are capillary beds that generate both hydrostatic and oncotic pressure. The visceral pleura is supplied by the bronchial arteries and empties into the pulmonary veins, whereas the parietal pleura is supplied by the intercostal arteries and empties into the intercostal veins. In the healthy state there is a small net movement of fluid across the pleural and parietal capillary beds into the low-pressure pleural space. Over the course of a day, the lymphatic system maintains a minimum pleural space outflow of 0.01 mL/kg/hr, with a 30-fold capacity of 3 mL/kg/hr.1,2 Overall, in a healthy state a small amount of fluid, estimated at 0.26 mL/kg of body mass, is present in the pleural space at any given time.3
It is believed that in most instances, the development of a pleural effusion requires both an increase in fluid entry into the pleural space and a decrease in its removal.4
Etiology of Pleural Effusions
The most common causes of pleural effusion in adults are congestive heart failure (CHF), pneumonia, malignancy, pulmonary embolism (PE), and viral disease.4,5 The most common cause of pleural effusions in children is pneumonia, followed by congenital heart disease, malignancy, renal disease and trauma.6 Pleural effusions can be classified as either transudates or exudates. Distinguishing between transudates and exudates narrows the differential diagnosis and directs management and therapy. A comprehensive list of causes can be found in Box 9-1. The most common are discussed in the following sections.
Transudates: Overwhelming the System
The most common cause of a transudate is CHF. The increased hydrostatic pressure in patients with CHF results in a net flow of fluid into the pulmonary interstitium. This fluid readily moves across the leaky visceral pleura into the pleural space,7 and when the volume of fluid exceeds the capacity of the lymphatics for drainage, a pleural effusion develops. In addition, the elevated systemic hypertension associated with CHF also increases fluid flow across the parietal pleura and decreases lymphatic flow out of the thorax. Any process that results in compromised left ventricular outflow can result in a pleural effusion, including myocardial infarction, cardiomyopathy, and valvular disease.
Patients with cirrhosis are frequently hypoalbuminemic, which leads to a chronic state of decreased plasma oncotic pressure. The imbalance between the hydrostatic and oncotic forces across the pleural membrane results in an effusion.8 In addition, experiments have shown that high volumes of ascites can stretch the diaphragm enough to allow fluid to pass through preexisting microdefects.
Exudates: Pathology of Tissues, Destroying the System
The main mechanism of cancer-related pleural effusion is obstruction. Neoplasms can either damage functional lymphatic stomata in the parietal pleura or prevent outflow more distally via involvement of the mediastinal lymph nodes. Other mechanisms involving neoplasm include metastasis to the visceral pleura, increasing capillary permeability, and obstruction of the thoracic duct with consequent chylothorax.9
A pleural effusion develops in at least 30% of those with PE.10 Therefore, when the cause of the effusion is unclear, PE should be strongly considered. The effusions associated with PE are often too small to require thoracentesis, but recent studies have shown them to be uniformly exudative, probably resulting from ischemia- and infarction-induced increases in pulmonary capillary permeability.11
Diagnosis of Pleural Effusion
Radiologic Diagnosis
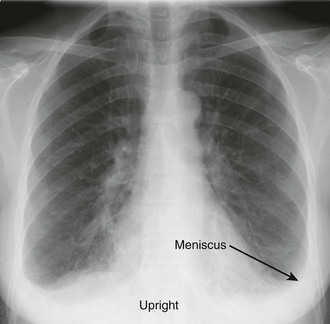
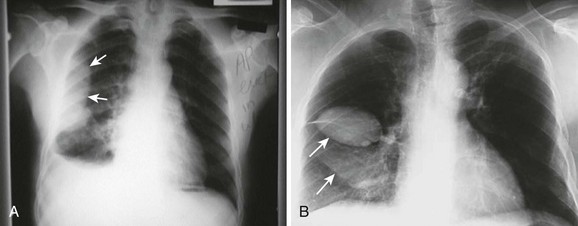
The earliest recognized sign of a pleural effusion on an upright chest radiograph is blunting of the lateral costophrenic angle, which may be seen on either the frontal or the lateral view (Fig. 9-1). With larger free-flowing effusions, the pleural fluid appears as a meniscus that curves downward toward the mediastinum in the frontal view and appears “lowest” midway through the thoracic cavity on the lateral view (Fig. 9-2). The true height of an effusion corresponds to the highest portion of the meniscus. The presence of a pneumothorax or abscess may alter the appearance of the meniscus to more of a straight line (air-fluid level).
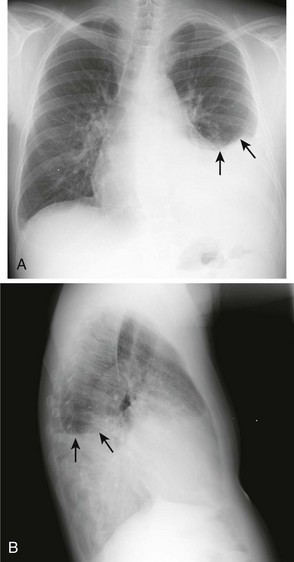
Occasionally, up to 1000 mL of fluid can collect in the subpulmonic space and cause neither blunting of the costophrenic angles nor a meniscus appearance on the upright radiograph. This is called a subpulmonic effusion (Fig. 9-3) and should be suspected if the hemidiaphragm is elevated and the dome peaks more laterally than expected on the anteroposterior radiograph.
Pleural effusions are more challenging to identify on a chest radiograph in a supine patient. If the effusion is large enough, a diffuse haziness may be appreciated (Fig. 9-4). Other findings include apical capping, obliteration of the hemidiaphragm, partial opacification of a hemithorax, and a widened minor fissure.
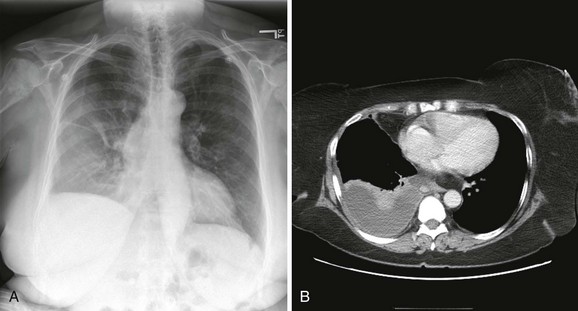
Figure 9-4 A, Supine radiograph of a patient with a large pleural effusion. Note the generalized homogeneous (“ground glass”) increase in radiopacity of the right side of this patient because of posterior layering of a pleural effusion. Also note the difference in the appearance of the pleural effusion when the patient is supine. As opposed to the upright radiograph (see Fig. 9-2), there is minimal blunting of the costophrenic angles and the vascular opacities are preserved in the overlying lung. B, A chest computed tomography (CT) scan of the same patient confirms the presence of a right-sided pleural effusion. Note the typical sickle-shaped appearance of the effusion on CT.
Obtain bilateral decubitus radiographs when a pleural effusion is seen or suspected. With the side of the effusion down, a simple pleural effusion will follow gravity and layer between the floating lung and the chest wall (Fig. 9-5). Unusual shapes reflect the presence of loculations, contained abscesses, or masses. A lateral decubitus view on the opposite side draws the fluid toward the mediastinum and allows visualization of the lung parenchyma to determine whether infiltrates or masses are present.
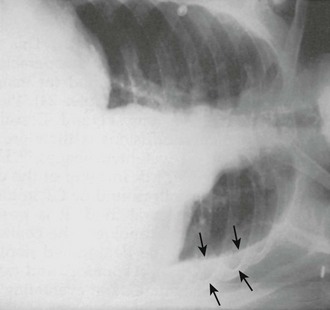
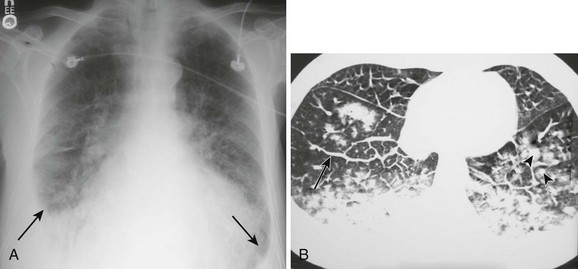
With diseased or scarred lung, tissue adhesions can trap pleural fluid within the parietal, visceral, or interlobar surfaces. Because these adhesions anchor the fluid collection, loculated effusions are often described as “D-shaped” (Fig. 9-6). Fluid loculated in the fissures assumes a lenticular shape.
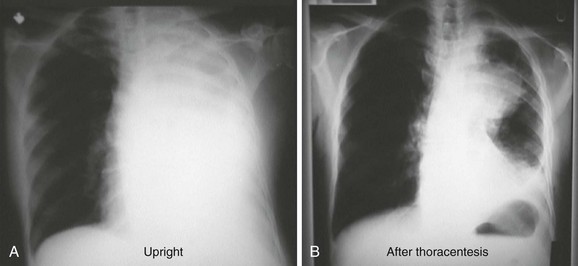
In the case of a massive pleural effusion, the entire hemithorax is opacified (Fig. 9-7). On such films, identification of mediastinal shift is a key to identifying the underlying process. In the absence of a diseased lung or mediastinum, large fluid accumulations push the mediastinum contralaterally. When the mediastinum is shifted toward the effusion, the lungs and main stem bronchi are diseased or obstructed (or both). When the mediastinum is fixed midline, it is likely that it is invaded by a tumor.12,13 As discussed later, differentiation of these disease processes is best done with computed tomography (CT). CHF often produces bilateral pleural effusions, which is generally first evident on the right side (Fig. 9-8).
CT
Although thoracentesis is usually performed on the basis of findings on plain radiography, CT is more sensitive than plain films in detecting very small effusions and can readily assess the extent, number, and location of loculated pleural effusions. Loculated lesions can appear vague on plain films. In the distinct anatomic relationships shown on cross-sectional CT views, free-flowing pleural fluid will form a sickle shape in the most dependent regions (see Fig. 9-4), whereas loculated fluid collections will remain lenticular and relatively fixed in space. In addition, CT can be used to assess pleural thickening, irregularities, and masses that are suggestive of malignancy and other diseases that result in exudative effusions. With intravenous contrast dye, CT differentiates lung parenchymal disease, such as a lung abscess. Pulmonary emboli can also be detected with the use of intravenous contrast enhancement. CT is also useful in identifying mediastinal pathology and in differentiating ascites from loculated subpulmonic pleural fluid.
Ultrasound
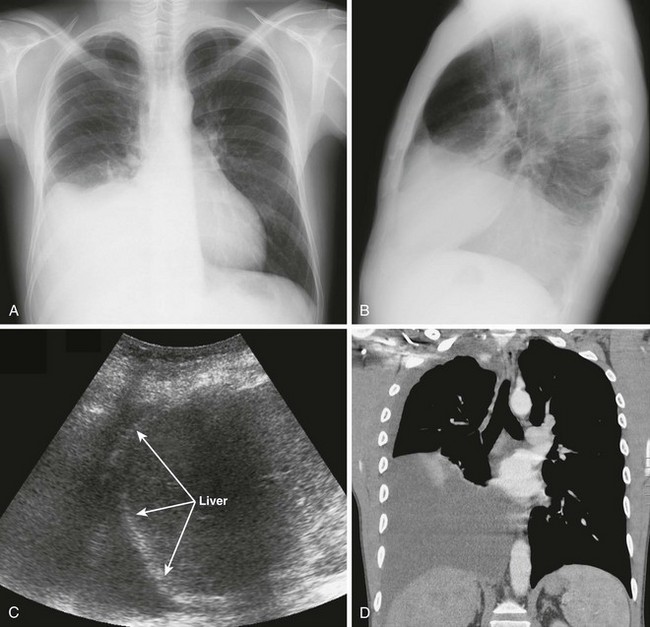
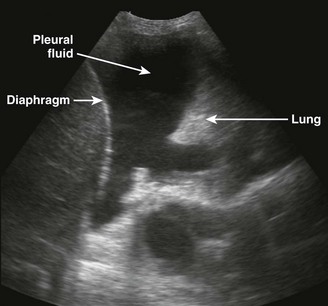
There are definite advantages to using US for assessment of pleural effusions as well. In particular, it is easy and noninvasive and can be performed at the bedside. US is superior to chest radiographs in diagnosing effusions14–16 and can detect effusions as small as 5 mL.17 Although some details can be seen only with CT, US can identify fluid loculations, separate fluid from pleural thickening, and distinguish solid from fluid pleural lesions. US can also be used to identify both pulmonic and abdominal causes of the pleural effusion (Fig. 9-9). Furthermore, US is a useful bedside tool when performing thoracentesis because it allows rapid identification of both the diaphragmatic location and the intercostal level that correlates with the superior margin of the effusion.
Contraindications
There are no absolute contraindications to thoracentesis. Recent studies indicate that if performed under real-time US guidance, thoracentesis is safe despite abnormal coagulation parameters.18 Closely watch all patients with coagulation abnormalities, including those with renal failure, for signs of bleeding after the procedure. Avoid skin puncture through a site of cellulitis or herpes zoster by choosing an alternative insertion site or patient position. Use real-time US guidance when performing thoracentesis on patients who are undergoing mechanical or manual ventilation because the positive pressure associated with mechanical ventilation may place the patient at increased risk for the development of a pneumothorax.