Chapter 73 Support of the failing liver
The Failing Liver
A failing liver is a serious condition that warrants a multimodal approach. The best option for a failing liver from either acute liver failure (ALF) or decompensated liver failure, is liver transplantation (Burroughs et al, 2006). However, the rapidity of progression and variable course of ALF, which includes progression to hepatic coma and the possibility of cerebral herniation, limit the effectiveness of transplantation. Furthermore, the downside of orthotopic liver transplantation is lifelong immunosuppression, and the side effects of these drugs include nephrotoxicity, diabetes mellitus, and infections.
The incidence of ALF in the United States is 2000 to 3000 persons per year (Ostapowicz et al, 2002), but the number is much higher for patients with acute decompensation of chronic liver failure (>200,000). The outcome of ALF varies by etiology: those with favorable prognoses are acetaminophen overdose, hepatitis A, and ischemia, with approximately 60% spontaneous survival (Lee et al, 2008). Etiologies with poor prognoses are drug-induced ALF, hepatitis B, and idiopathic cases, with approximately 25% spontaneous survival.
Medical Therapy
Once a patient is diagnosed with ALF, medical management must be initiated in a coordinated, systematic fashion (see Chapter 72). The goals of medical management are to 1) facilitate recovery, 2) reduce ongoing liver injury with N-acetyl cysteine, 3) prevent systemic manifestations such as cerebral edema, 4) correct hemodynamic instability, and 5) start preparing for liver transplantation if necessary. Treatment measures are initiated according to the severity of hepatic encephalopathy (HE).
For patients in stage I HE, evidenced by behavior changes with no changes in consciousness, baseline laboratory values are obtained for serum electrolytes, Mg2+, Ca2+, lactate, arterial blood gases, liver function tests, lactate dehydrogenase (LDH), ammonia, complete blood count (CBC), albumin, and coagulopathy panel. Twice-daily glucose checks are also recommended. Vitamin K is administered to help prevent or correct coagulopathy (Pereira et al, 2005), and lactulose is given orally for at least two loose stools per day because elevated blood ammonia can be detrimental. Ulcer prophylaxis is also started (Macdougall et al, 1977). Therapeutic measures are initiated when indicated, such as N-acetyl cysteine for acetaminophen overdose (Smilkstein et al, 1988).
If a patient progresses to stage II HE—with disorientation, delayed mentation, and asterixis—the measures for stage I HE are continued, and the patient is transferred to the intensive care unit (ICU) for a multidisciplinary evaluation from a critical care intensivist or hepatologist in consultation with a neurologic intensivist and a liver transplant surgeon. The patient’s level of consciousness is scored using both the Glasgow Coma Scale and the Mayo Clinic FOUR (Full Outline of UnResponsiveness) score (Wijdicks et al, 2005). Laboratory work obtained for stage I HE patients is continued on a routine schedule.
A head computed tomographic (CT) scan or a magnetic resonance image (MRI) scan is obtained to evaluate brain edema and rule out other causes for mental status change, such as hematoma. Depending on the clinical picture, either enteral or parenteral nutrition is begun, with a protein load not to exceed 0.5 g/kg/day and with caloric intake based on Harris-Benedict guidelines: ideal body weight plus 20%. Branched-chain amino acids (HepatAmine) are used at the discretion of dietetics, and antifungal and antiviral prophylaxes are considered because of the high incidence of opportunistic infections in the setting of ALF (Rolando et al, 1996). If these patients require sedation, we advocate the use of the short-acting agent propofol (Wijdicks et al, 2002).
As patients progress to stage III HE, endotracheal intubation is performed to protect the airway and initiate mechanical ventilation. Goals of ventilatory support are to maintain a PaO2 greater than 70 mm Hg, preferably using an FiO2 less than 40%. Mannitol is used to control intracranial pressure, with a goal serum osmolality of 310 to 320 mEq/L (Canalese et al, 1982); it is discontinued if oliguria develops. Simple maneuvers are initiated to lower intracranial pressure (ICP), such as keeping the head of the bed elevated between 30 and 45 degrees, limiting endotracheal suctioning, decreasing stimuli from lighting, and controlling agitation with propofol. IV fluids are adjusted to maintain serum Na+ between 140 and 150 mEq/L. Vasopressors are used to increase the mean arterial pressure (MAP) if the cerebral perfusion pressure (CPP) falls below 60 mm Hg. Vasodilators are used to decrease MAP if the CPP is above 100 mm Hg. More invasive monitoring systems are also instituted, including an arterial line and an ICP monitor (Vaquero et al, 2005). Prior to ICP monitor placement, a coagulation profile is obtained and INR is corrected to 1.5 or less with fresh frozen plasma. If correction requires large volumes of fresh frozen plasma, we consider the addition of recombinant Factor VII (Shami et al, 2003). The cerebral perfusion pressure (CPP = MAP − ICP) is maintained between 60 and 100 mm Hg. If the CPP falls below 60 mm Hg or the ICP rises above 20 mm Hg for more than 5 minutes, the patient is hyperventilated to a PaCO2 of 25 to 30 mm Hg (Strauss et al, 1998). Hyperventilation is only maintained short term.
Intracranial hemorrhage must be excluded if ICP remains high. Ultrasounds to assess hepatic mass and vasculature are obtained every 2 days. Hemodynamic instability often occurs secondary to a systemic inflammatory response (Rolando et al, 2000) or intravascular depletion. Intravascular volume is monitored with a Foley catheter for continuous urine output assessment and either a central venous or pulmonary artery catheter and is maintained for adequate end-organ perfusion. Human 5% albumin is the volume expander. Continuous venovenous hemodialysis is preferred in renal failure and ALF because of the risk of hypothermia and reduced CPP during intermittent hemodialysis (Davenport et al, 1993).
Hypothermia (33° to 35° C) is considered if cerebral edema is refractory to other therapies (Jalan et al, 2004). The duration has not been standardized, although some centers discontinue hypothermia before transplantation because of the risk of bleeding. Other centers continue hypothermia for 24 hours after transplantation because of late risks of cerebral edema.
Nonbiologic Liver Support
Plasma Exchange and Hemodiafiltration
Plasma exchange was a natural outgrowth of the less effective blood-exchange transfusion technique. The goals of plasma exchange in ALF are to reduce the level of circulating toxins and to replace deficient essential factors, such as clotting factors produced by the liver. Plasma exchange is achieved by apheresis, with removal of the patient’s jaundiced plasma and replacement with normal plasma. The results of early clinical trials were discouraging; encephalopathy often improved temporarily, but patient survival was not affected. Therapeutic gains, such as reduction in serum bilirubin and partial recovery from coma, were short lived and seen predominantly in patients with drug-induced ALF (Freeman et al, 1986; Lepore et al, 1972; Sabin et al, 1968). The overall survival rate of ALF patients treated by plasma exchange remained less than 50% (Takahashi et al, 1991). In addition, a significant complication rate was reported with plasma exchange, including chemical toxicity, viral infections, and death from lung and brain complications (Brunner et al, 1987). However, the effects of repeated, high-volume plasma exchange in patients with ALF have been studied under nonrandomized conditions (Kondrup et al, 1992).
In 1999, Clemmesen and colleagues investigated the effect of repeated, high-volume (15% of body weight) plasma exchange in 23 patients: 14 patients with ALF and 9 with decompensated cirrhosis (Table 73.1). The etiologies of ALF were acetaminophen in 8, hepatitis in 3, and nonhepatitis (A, B, C) in 3. Of the patients with acetaminophen intoxication, 25% died, and 21% were bridged to transplantation.
Table 73-1 Overview of Important Biologic and Nonbiologic Extracorporeal Liver Support Device Trials
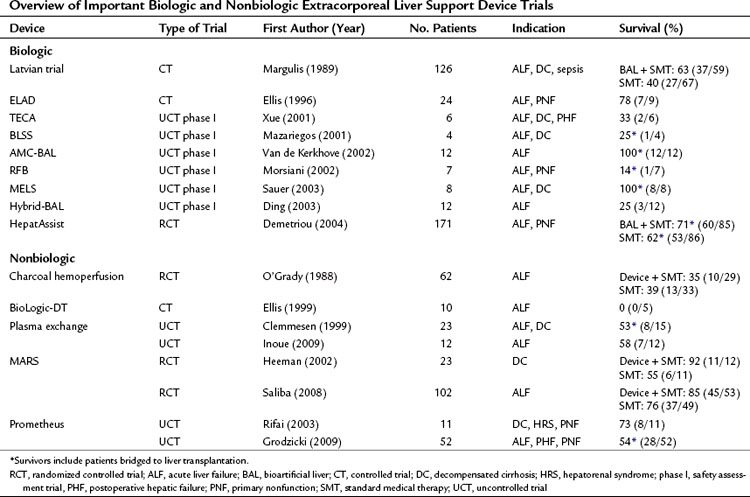
In Japan, where the option of liver transplantation is limited by the low rate of cadaveric organ donation, a dual approach that combines hemofiltration with plasma exchange has become popularized as a treatment of ALF (Inoue et al, 2009). In this Japanese study, all patients regained consciousness, and no patient developed brain edema or hepatorenal syndrome during plasma exchange and hemodiafiltration treatment. The median number of treatment sessions was 21 (range, 4 to 30), and 4 of 7 patients (57%) with indeterminate etiology and 1 of 5 patients (20%) with acute hepatitis B infection survived.
Historic Blood Purification Options
Throughout the 1960s and 1970s, it was believed that small (<5 kD) dialyzable molecules caused coma in ALF (Kiley et al, 1956; Opolon, 1979). As a result, numerous attempts were made to treat ALF patients with hemodialysis (Kiley et al, 1958; Merrill et al, 1950) and charcoal hemofiltration (O’Grady et al, 1988) for removal of these small toxins. Although case reports and controlled studies of both therapies have shown reversal of hepatic encephalopathy and improved survival (Gimson et al, 1982; O’Grady et al, 1988), neither therapy has been proven successful in prospective randomized trials of either ALF or decompensated cirrhosis.
MARS
MARS was developed by Stange and collegues (1993) in the early 1990s. Briefly, MARS is a two-circuit system composed of a blood circuit and a secondary albumin circuit; these are separated by a high-flux dialyzer membrane with a pore size and nominal molecular weight cutoff (MWCO) of approximately 50 to 60 kD. The pore size of this membrane makes it impermeable to albumin but permeable to smaller water and nonpolar waste substances. On the opposing side of this membrane is an albumin circuit that consists of supraphysiologic levels (>10%) of human albumin dialysate. Waste removal occurs by the concentration gradient between the patient’s blood and the albumin dialysate in the secondary circuit. The high concentration of albumin is believed to facilitate removal of nonpolar molecules known to bind to albumin. Detoxification of these nonpolar waste molecules occurs when the albumin passes over adsorbent columns, including an anion exchange resin column and an activated charcoal column (Steiner et al, 2004). The secondary circuit also provides conventional low-flux dialysis for detoxification of water-soluble molecules.
One of the earlier MARS trials was an uncontrolled trial of 13 patients who had not responded to standard medical treatment of decompensated liver failure (Stange et al, 1999). The etiologies of liver cirrhosis were hepatitis C in one patient and alcohol abuse in the rest; the precipitating events were unknown in 10 cases. This early uncontrolled study showed an overall survival of 69% (9 of 13). A prospective, controlled trial of the MARS system (n = 12) versus standard medical therapy (n = 11) was performed by Heemann and colleagues (2002) in patients with decompensated cirrhosis. The authors concluded that albumin dialysis was associated with a significant improvement in 30-day survival (11 of 12 survived with MARS, 6 of 11 survived in the control group). An editorial to this study raised concerns regarding stratification, standard medical care before randomization, and the inclusion criteria (Kamath, 2002). A larger randomized controlled trial of the MARS system plus standard medical therapy (n = 39) versus standard medical therapy alone (n = 31) in the treatment of hepatic encephalopathy in advanced cirrhosis was reported by Hassanein and colleagues (2007). In contrast to previous reports on MARS, mostly from European centers, this trial was conducted mostly at centers in the United States. This trial demonstrated that the use of MARS therapy was associated with an earlier and more frequent improvement of grades 3 and 4 hepatic encephalopathy compared with standard medical therapy alone. This 5-day trial did not attempt to assess the role of MARS therapy on survival or cirrhotic patients. A trial that did evaluate survival was reported by Saliba and colleagues (2008). This randomized prospective trial of MARS in the treatment of ALF included 102 patients randomized to MARS therapy (n = 53) or standard medical treatment alone (n = 49). Survival at 6 months was greater after MARS treatment (84.9% vs. 75.5%); however, this difference did not reach a significant level by log rank test.
Along with hepatic encephalopathy, MARS has been shown to improve other secondary end points associated with a failing liver, including renal dysfunction, jaundice, and hemodynamic parameters such as systemic vascular resistance and mean arterial pressure (Schmidt et al, 2003). One prospective randomized controlled trial showed significant improvement in survival in patients with hepatorenal syndrome (Mitzner et al, 2000). MARS therapy has been associated with prolonged relief of intractable pruritus in patients with cholestatic liver disease (Pares et al, 2004). In addition, in a study by Novelli and colleagues (2007), MARS therapy was associated with an improvement in Model of End-Stage Liver Disease (MELD) scores at 1 and 3 months after treatment.
A meta-analysis of MARS use evaluated patients with ALF or decompensated cirrhosis in four randomized clinical trials involving 67 patients and two nonrandomized trials involving 61 patients. This meta-analysis failed to show a significant survival benefit with MARS treatment (Khuroo et al, 2004). As a result, many clinicians are still hesitant to use MARS therapy on their patients with liver failure because a reproducible survival benefit has not been shown.
Prometheus
Prometheus is another form of albumin dialysis for supporting a failing liver. Prometheus functions by fractionating the plasma component of blood, in which the fractionated plasma is detoxified as it passes through two adsorption columns. Prometheus and MARS differ in the MWCO of their blood-separation membranes. MARS uses a 50- to 60-kD MWCO membrane that does not allow passage of albumin from the blood, whereas Prometheus uses a membrane with a 250-kD MWCO that allows the passage of albumin. Therefore greater potential removal of albumin-bound toxins from the patient’s blood is possible with the Prometheus system (Rifai et al, 2003). There have been concerns of dropping the patient’s albumin levels using Prometheus (Krisper et al, 2007) and concerns of losing clotting factors, presumably because of reduction in protein C and protein S concentrations after the fractionation and adsorption process (Meijers et al, 2007). As with MARS, a survival advantage of Prometheus over standard medical therapy has not yet been shown in a prospective randomized trial.
Biologic Liver Support
Ex Vivo Liver Perfusion
In 1965, Eiseman and colleagues reported the use of xenoliver (porcine) cross-perfusion to treat eight comatose patients. None of these patients survived, but transient clinical improvement, such as awakening from the comatose state, was reported. Later, in 1967, Burnell and colleagues reported the use of human-human cross-circulation in the treatment of three patients with fulminant hepatic failure. Moreover, livers from a variety of species—porcine, dog, and bovine—have been used (Abouna et al, 1970; Chari et al, 1994; Eiseman et al, 1965). Evident after the treatments were symptoms of hyperacute rejection—gastrointestinal bleeding, hemolysis, and thrombocytopenia—and less specific symptoms such as fever and nausea that would subside after each session. Human organs found not to be suitable for transplantation were used in a cross-perfusion setting reported in 1993; in this study, two of three patients were successfully bridged to transplantation, but the last patient did not improve clinically (Fox et al, 1993).
Hepatocyte Transplantation
Transplantation of liver cells, specifically hepatocytes, is promising for patients with inherited liver disorders such as tyrosinemia (Grompe et al, 1994) and hyperbilirubinemia Crigler-Najjar syndrome (Fox et al, 1998), which eventually lead to liver failure.







