Subacute and Riedel’s Thyroiditis
Subacute Thyroiditis
The term subacute thyroiditis (SAT) describes a self-limited inflammatory disorder and the most common cause of thyroid pain, probably of viral origin.1–5 It was first reported by Mygind6 in 1895, who described 18 cases of “thyroiditis akuta simplex.” The name De Quervain traditionally has been associated with this condition, however, probably because he described the pathology of this disorder thoroughly in 19047 and again in 1936.8 SAT occurs in 5% of patients with clinical thyroid disease9 and frequently follows an upper respiratory tract infection. Its incidence correlates with the peak incidence of enterovirus.10 Other viruses, such as Epstein-Barr virus and cytomegalovirus, also have been reported, but so far clear evidence for a viral cause is still lacking.11 There is a strong preponderance of women over men with this condition.1
SAT has a multiplicity of synonyms, some reflecting misconceptions regarding the etiology or pathology of the condition. These include De Quervain’s thyroiditis, viral thyroiditis, granulomatous thyroiditis, acute or subacute diffuse thyroiditis, acute simple thyroiditis, noninfectious thyroiditis, struma granulomatosa, pseudogranulomatous thyroiditis, giant cell thyroiditis, pseudo–giant cell thyroiditis, migratory “creeping” thyroiditis, and pseudotuberculous thyroiditis. The term subacute thyroiditis connotes a temporal quality that might apply to any inflammatory process of intermediate severity and duration. As the term is generally employed, however, it specifically includes only patients showing a pseudogranulomatous pathologic appearance in the thyroid gland (which is virtually specific for the disease) and a characteristic clinical syndrome in which the painful tender goiter also is associated with considerable malaise, fever, and evidence of thyroid dysfunction (described more fully later).1–5 It generally is distinguishable from a similar disorder, painless or silent thyroiditis, which disturbs thyroid function in a manner similar to SAT but without pain or tenderness and with a different pathologic appearance.
Incidence
Few epidemiologic studies of SAT have been reported.10,14–22 Compared with other thyroid diseases, SAT is uncommon, occurring at the rate of about 1 case per 5 cases of Graves’ disease and 1 case per 15 or 20 cases of Hashimoto’s thyroiditis.19 Although the cause is most likely viral, SAT, similar to all other thyroid conditions, occurs most commonly in women who are 40 to 50 years old. The reported female-to-male ratio is 3 to 6 : 1.19 it has been noted as a rare cause of hyperthyroidism in pregnancy.20 It is rare in children and seems to occur in any season of the year,10,22 with a trend toward more cases in fall and spring.22 Familial or geographic aggregation of cases is seldom noted. SAT has been reported most commonly from the temperate zone, having been observed in North America, Europe, and Japan. Recently, a few cases were reported in Western Saudi Arabia,21 although it is rarely reported from many other parts of the world. Associated autoimmune conditions do not seem more common than autoimmune conditions observed in the general population.22
Although complete recovery is the rule, recurrence after several years has been reported.24–27 In one study, four recurrent episodes of SAT occurred in 3 of 222 patients (1.4%). The recurrent episodes were similar to the first episodes of SAT.27 In a larger study that evaluated data for 3344 patients with SAT between 1970 and 1993, SAT recurred in 48 of 3344 patients (1.4%) (mean 14.5 ± 4.5 years after the first episode). Five patients experienced a third episode (mean 7.6 ± 2.4 years after the second episode).27 Another cohort study showed a 4% recurrence rate after many years.22 Theoretically, late recurrence possibly occurs after the disappearance of immunity to the previous viral infection. During an evaluation of subtypes of hypothyroidism over a 4-year period in Denmark, an incidence of subacute thyroiditis of 1.8% was found in a cohort of 685 patients with hypothyroidism.28
Etiology
In 1952, Fraser and Harrison29 were the first to propose that SAT represents a viral infection of the thyroid gland. Since then, considerable indirect evidence suggests that SAT is most likely the result of a viral infection30–32 that rarely recurs after a complete recovery, possibly because of immunity to the offending virus.
Clinically, the disease has several characteristics typical of viral infections, including a typical prodrome with myalgias, malaise, and fatigue; absence of leukocytosis; and usually a self-limited course.1–5 Additionally, clusters of the disease have been reported during outbreaks of viral infection.1–5,10 It has been described in association with mumps, measles,1 influenza,1 the common cold,1 adenovirus,1 infectious mononucleosis,1,12 coxsackievirus,1 myocarditis,1 cat-scratch fever,1 St. Louis encephalitis,1 hepatitis A,18 parvovirus B19 infection,19 and cytomegalovirus infection.11,13
In an extensive study reported by Volpé and colleagues,33 32 of 71 patients with SAT, who had no evidence of specific viral disease, showed at least fourfold increases in viral antibodies during the thyroid illness. These viral antibodies included antibodies to coxsackievirus, adenovirus, influenza virus, and mumps virus. Coxsackievirus antibodies were found most commonly, and the changes in their titers most closely approximated the course of the disease. In a later study of 10 patients in Singapore, no such antibodies were observed, however.34 It is possible that the presence of these antibodies may not reflect pathogenic significance, but instead may result from an anamnestic response to the inflammatory thyroid lesion. The thyroid responds with the clinical picture of thyroiditis after invasion by a variety of viruses, and a variety of agents may be causative in the syndrome of SAT.
Certain nonviral infections, such as malaria and Q fever, have been associated with a clinical syndrome that at least simulates SAT.1 The significance of these observations remains to be determined. In addition, a case of SAT occurring simultaneously with giant cell arteritis has been reported.35 Several cases of SAT that developed during interferon-α treatment for hepatitis C have been described,36–38 and more recently, a case of SAT that developed after long-term immunosuppression and lithium therapy following an allogeneic bone marrow transplant was reported.39
Several autoimmune phenomena have been described in SAT. Thyroid autoantibodies (antithyroglobulin and antithyroid peroxidase antibodies) have been found in 42% to 64% of patients with SAT.33 In most of these patients, the antibody titer gradually decreased and remained low or disappeared as the disease faded. Thyroid-stimulating hormone (TSH) receptor antibodies also have been reported in patients with SAT,40–42 although changes in antibody titer did not correlate with disease activity.40 Autoantibodies to several novel, uncharacterized thyroid antigenic determinants were found in eight of nine patients with SAT tested.43 These autoantibodies persisted, and their level did not decrease over 39 months after onset of SAT. These antibodies likely arise secondary to the damage caused by viral infection of the thyroid gland because they are typically polyclonal in nature.43
There is evidence that T-cell-mediated immunity against thyroid antigens may play a role in the pathogenesis of SAT. During the initial phase of the disease, the gland is infiltrated by T cells, and sensitization of T cells against thyroid antigens has been shown in such patients.44–46 This sensitization was transitory, however, and likely represented a secondary immune response to the inflammatory release of antigen induced by the viral infection of the gland.1
It has been suggested that thyroid-destructive events in the course of SAT may trigger, under a genetic background, thyroid autoimmune disease of various kinds.47,48 Patients with a previous history of SAT, in about 1% of cases,2 may develop hypothyroidism as a consequence of previous thyroid damage. The occurrence of Graves’ disease after SAT also has been described, although such evidence seems to be extremely rare, with fewer than 20 cases reported in the literature.49–53
Although SAT is shown to be associated with thyroid autoimmune phenomena, after recovery all immunologic phenomena should disappear. This is in contrast to the continuing presence of these abnormalities in autoimmune thyroid disease.54 The transitory immunologic markers observed during the course of SAT seem to be secondary to the release of antigenic material from the thyroid and seem to be a normal, physiologic response to the inflammatory destruction of the gland.55
In light of the previous observations, the lack of any direct evidence, and because it is rare for SAT to progress to either Graves’ disease or Hashimoto’s disease, the corollary is still consistent with the view that antigen-driven events can produce a transient immunologic disturbance, but does not, or is most unlikely to, culminate in chronic autoimmune thyroid disease. It is possible that the illness of SAT might act as a nonspecific stress acting on the immune system to precipitate Graves’ disease in a favorable genetic background.54
An association between SAT and HLABw35 has been noted in all ethnic groups tested.56–59 This haplotype seems to confer an unusual susceptibility to SAT, perhaps because it allows one or more viruses to trigger an immune response directed against thyroid tissue.60 Histocompatibility studies show that 72% of patients with subacute thyroiditis manifest HLA-Bw35.57–60 Familial occurrence of subacute thyroiditis associated with HLA-B35 has been reported.61,62 Another HLABw67 was found in 87% of a Japanese population and correlated with a seasonal appearance and a mild course of disease. Thus the susceptibility to subacute thyroiditis is genetically influenced, and it has also been suggested that subacute thyroiditis might occur by transmission of viral infection in genetically predisposed individuals.13
Clinical Features
Half of patients have a history of an antecedent of upper respiratory infection, followed in days or weeks by the clinical manifestations of SAT itself.1–5,22,63 SAT begins with a prodrome of generalized myalgias, pharyngitis, low-grade fever, and fatigue. The patient notes pain of varying degrees in the region of the thyroid gland. This pain may involve one lobe, part of a lobe, or the whole thyroid, and it typically radiates from the thyroid gland to the angle of the jaw and to the ear of the affected side. If not bilateral initially, the pain and tenderness often spread to the uninvolved side of the thyroid within days or weeks. It also may radiate to the anterior chest or may be centered only over the thyroid. Moving the head, swallowing, or coughing may aggravate it. Transient vocal cord paresis may occur.23
Symptoms of mild to moderate hyperthyroidism occur in the early phase in most patients.22 Fifty percent of patients have symptoms of thyrotoxicosis, and the usual symptoms of nervousness, tremulousness, weight loss, heat intolerance, and tachycardia predominate.1–5,63–66 On physical examination, most patients appear uncomfortable and flushed, with variable fever. The thyroid gland may be only slightly to moderately enlarged, with one lobe larger than the other. The consistency of the involved area is usually firm or hard. With time or treatment, the thyroid tenderness subsides, and the goiter generally disappears within several weeks or months. Signs of mild to moderate hyperthyroidism are present in 50% of cases. About 8% to 16% of patients with this condition are noted to have a preexisting goiter. Cervical lymphadenopathy is rare.
In most patients, SAT lasts 2 to 4 months, although it may last 1 year. When the course is prolonged, the major manifestation is persistent, painful, tender thyroid enlargement, the thyrotoxicosis almost always having subsided earlier. Recurrences after recovery have been reported but are unusual, on the order of 2.3% per year26 or 4% over 21 years after the first episode.22
Sometimes hyperthyroidism may not be apparent clinically but can be detected by biochemical means.64 This situation is due to a disruptive process within the thyroid gland, with continuous leakage of the colloid into the interstitial spaces, where it is broken down into its component parts, liberating thyroid hormones, thyroglobulin, and other iodoamino acids into the circulation.26,64–74 Because the thyroid cells during this phase are virtually incapable of producing new thyroid hormone, the colloid that has been stored within the follicles is depleted within 2 to 3 months, resulting in a phase of transient hypothyroidism in patients in whom the process has persisted over the interval.75 Because disruption of the thyroid parenchyma can continue for months, hypothyroidism may persist for several weeks. As recovery continues, the follicles regenerate, the colloid is repleted, and normal thyroid function is restored. With recovery, the thyroid is reconstituted, repleted with colloid; thyroid function is restored; and a variable amount of interstitial fibrosis persists.1–5,64–75 This transient hypothyroidism may be subclinical or overt and occurs in about two thirds of patients.
SAT rarely progresses to permanent hypothyroidism.1–5,75,76 In these cases, progression may be due to total destruction of the thyroid, with consequent fibrosis. As mentioned before, in rare instances, the disorder may seem to culminate in autoimmune thyroiditis after recovery from SAT.48,51,53
Diagnosis
The typical painful SAT usually is obvious when the patient is first seen and should present no difficulties in diagnosis for the endocrinologist.67,68 In patients who have only a sore throat or ear pain, however, the diagnosis is less obvious, and many patients are initially misdiagnosed with pharyngitis.64 It is important that the thyroid gland be palpated carefully in patients presenting with upper respiratory infections or complaints of sore neck or throat or earache.
Eventually, patients with Hashimoto’s thyroiditis and a few with silent thyroiditis may present with a painful, tender thyroid enlargement that is indistinguishable from SAT.54 The radioactive iodine uptake is rarely as completely suppressed in Hashimoto’s thyroiditis as it is in SAT, and the titers of thyroid autoantibodies are usually high enough to suggest lymphocytic thyroiditis. Acute suppurative thyroiditis initially may mimic SAT, but with time, the findings of fever, more localized tenderness and swelling, and erythema over the involved area of the thyroid should become obvious. A rapidly growing anaplastic carcinoma77 of the thyroid or hemorrhage into a thyroid nodule can cause thyroid pain and tenderness. In anaplastic carcinomas, the lesion is usually obvious by virtue of its large size, adherence to adjacent structures, lymphadenopathy, and characteristic progressive course. Hemorrhage into a thyroid nodule presents with a localized nature, and the obvious nodule usually leads to the correct diagnosis.
The hallmark of SAT is a markedly elevated erythrocyte sedimentation rate. The serum thyroglobulin and C-reactive protein concentration are similarly elevated.78 The leukocyte count is normal or slightly elevated. Peripheral blood thyroid hormone concentrations are elevated, with ratios of thyroxine (T4) to triiodothyronine (T3) of less than 20, reflecting the proportions of stored hormone within the thyroid,79 and serum concentrations of thyrotropin are low or undetectable. Serum thyroid peroxidase antibody concentrations are usually normal. The 24-hour radioactive iodine uptake is low (<5%) in the toxic phase of SAT, distinguishing this disease from Graves’ disease. Color-flow Doppler ultrasonography also may help to make this distinction; in patients with Graves’ disease, the thyroid gland is hypervascular, whereas in patients with painful SAT, the gland is hypoechogenic and has low to normal vascularity.80
Laboratory and Imaging Findings
Dynamic changes in thyroid function studies occur with the onset of thyroid inflammation (Fig. 13-1). The destruction of the thyroid follicles results in release and breakdown of the colloid into the interstitial tissue and into the circulation of iodinated materials—protein, proteases, peptides, and amino acids. An increase in serum T4, T3, and thyroglobulin and in urinary iodine results.1–5,64,74
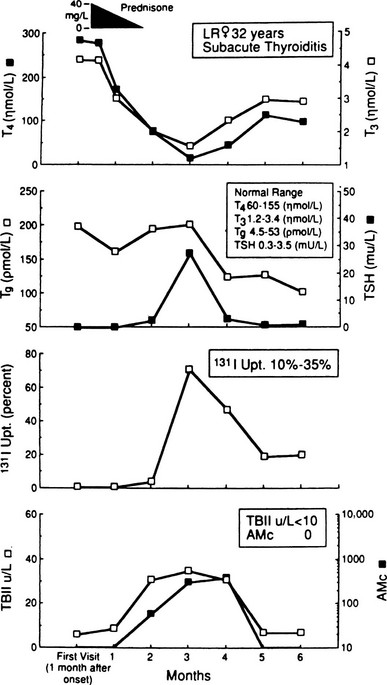
FIGURE 13-1 Salient laboratory features during the course of subacute thyroiditis. AMc, Antimicrosomal (antithyroperoxidase) antibody; T4, thyroxine; T3, triiodothyronine; TBII, thyrotropin-binding inhibitory immunoglobulin; Tg, thyroglobulin.
In addition, iodoproteins, such as thyroglobulin and iodoalbumin, are discharged from the gland into the circulation.69 Plasma thyroglobulin may remain elevated long after all other evidence of the inflammatory process has subsided.71 The decline in plasma T4 is exponential during the first week, and this phase of hyperthyroidism can continue only until the gland is depleted of its preformed colloid.73 TSH is usually undetectable in the hyperthyroid phase,72,73 and the TSH response to thyrotropin-releasing hormone, as expected, is diminished at this time.81,82
At the same time, the damage to the thyroid follicular cells results in impaired iodine transport; the 24-hour radioactive iodine uptake is characteristically suppressed to 0% to 1%, revealing a patchy and irregular distribution of the tracer.1–5,64,69,83,84 Even if only part of the gland is involved, the uptake may be similarly depressed as a result of suppression of pituitary TSH owing to the elevated levels of thyroid hormone.72,73 Increased perfusion is shown in studies with technetium-99m sestamibi during the acute stage of SAT. This increased uptake in the thyroid region suggests the inflammatory phase of this disease.85 SAT is one of the hyperthyroid conditions associated with high levels of thyroid hormones but a low radioactive iodine uptake, and such observations are characteristic in the early phase of this disorder. Under these circumstances, only minimal thyroid hormone biosynthesis is sustained, and what is produced leaks out.69
Evidently, thyroid cell damage reduces the ability of the gland to respond to TSH so that large doses of TSH generally do not cause a rise in the radioactive iodine uptake except when some parts of the gland are uninvolved.82 This lack of response to exogenous TSH administration persists during the first weeks of the disease, reflecting continuing thyroid cell impairment and failure of the iodide-concentrating mechanism. Also, the administration of perchlorate or thiocyanate generally does not cause release of excessive amounts of iodine from the gland.74
The erythrocyte sedimentation rate is characteristically elevated (often >100 mm/h) in SAT.1–5,86 If the test is normal or only slightly elevated, the diagnosis of SAT should be suspected. The leukocyte count is normal in about half of patients and elevated in the remainder1–5,8,86 and has been reported as high as 18 × 109/L. The leukocyte counts correlate with serum concentrations of granulocyte colony-stimulating factor.87 There may be a mild normochromic anemia, and an increase in α2-globulin frequently is seen as a nonspecific inflammatory response.88 Alkaline phosphatase and other hepatic enzymes may be elevated in the early phase.89 It has been suggested that SAT actually represents a multisystem disease also affecting the thyroid gland.90 There also are increases in serum ferritin,94 soluble intercellular adhesion molecule-1,95 selectin,96 and interleukin-697 levels during the inflammatory phase.
Ultrasound examinations show hypoechoic focal areas and can be used for guided fine-needle cytology.84–103 Magnetic resonance imaging of the thyroid also can help distinguish SAT from Graves’ disease during the hyperthyroid phase. The ADC values obtained from the diffusion-weighted images of the patients with Graves’ disease are significantly higher than the values of patients with SAT.104
Recovery Phase
As the process subsides, the serum T4, T3, and thyroglobulin levels decline, but the serum TSH level remains suppressed. The normal concentrations of sex hormone–binding globulin in the hyperthyroid phase probably reflect the short duration of exposure to increased thyroid hormone.105
Later, during the recovery phase, the radioiodine uptake becomes elevated with the resumption of the ability of the thyroid gland to concentrate iodide. The serum T4 concentration may fall below normal; the TSH level may become elevated. Usually, after several weeks or months, all the parameters of thyroid function return to normal. Restoration of iodine stores seems to be much slower and may take more than 1 year after the complete clinical remission.106,107 Ultimate recovery is the general rule. An occasional patient remains permanently hypothyroid.
Tests of thyroid antibodies are positive in a few cases; these develop several weeks after the onset and tend to decline and disappear thereafter.33,108 An antibody against an unpurified thyroid antigen persists for years, however, after clinical features have subsided.43 Also, as mentioned before, antibodies to the TSH receptor, either of the stimulating or of the blocking variety, may appear transiently without relationship to the thyroid functional state.40–42 In about 2% of patients, SAT may trigger autoreactive B cells to produce TSH receptor antibodies, resulting in TSH antibody–associated dysfunction.109
Pathology
From histologic examination, the process may be diffuse or irregular in its involvement, with various stages of the disease sometimes found within the same specimen.110 Initially, there is extensive follicular cell destruction, extravasation of colloid, and infiltration of lymphocytes and histiocytes. The lymphocytes and histiocytes tend to congregate around masses of colloid and coalesce into giant cells. With time, there is a variable degree of fibrosis, and areas of follicular regeneration are seen. After recovery, the thyroid appears normal except for minimal residual fibrosis.
The follicular cells sometimes virtually disappear, leaving a fine follicular lining. The initial phase is characterized by the appearance of neutrophils, followed by large mononuclear cells and lymphocytes (Fig. 13-2). The follicles appear much larger than normal, with disruption of the epithelial lining and hyperplasia of the surviving follicular cells. Histiocytes congregate around masses of colloid within the follicles and in the interstitial tissues, producing “giant cells.” Because often these giant cells actually consist of masses of colloid surrounded by large numbers of individual histiocytes, they should in such cases be termed pseudo–giant cells. True giant cells and granulomas also appear in this disease, however.111 Marked interstitial edema also is present with lymphocytic infiltration.
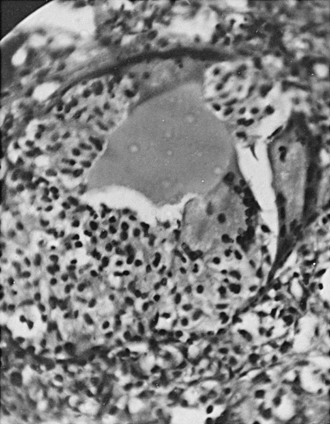
FIGURE 13-2 Pathologic findings in subacute thyroiditis. Note the severe destruction of the thyroid follicle, with the remaining colloid being surrounded by large numbers of histiocytes, giving the appearance of a giant cell (pseudo–giant cell). Marked interstitial edema is noted, with cellular infiltration and considerable destruction of the thyroid parenchyma.
The process often is irregularly distributed in either or both lobes.114 With recovery, the inflammatory reaction recedes, and a variable amount of fibrosis may appear. Areas of follicular regeneration are seen, but there is no caseation, hemorrhage, or calcification. The degree of recovery is generally virtually complete, aside from the residual fibrosis already mentioned. Only in rare instances is there complete destruction of the thyroid parenchyma leading to permanent hypothyroidism.
Thyroid tissue obtained by fine-needle aspiration biopsy (Fig. 13-3






