Pulmonary Embolism and Deep Vein Thrombosis
Perspective
Pathophysiology of Thrombosis
VTE forms as a result of excessive fibrin production from the action of thrombin on fibrinogen. Factors that enhance fibrinogen synthesis and promote its catalysis to fibrin include systemic inflammation, traumatic or immune-related vascular trauma, inherited thrombophilias and hemoglobinopathies, cancer, pregnancy, and sluggish blood flow. Venous injury, slow blood flow, and hypercoagulability are the cardinal inciting mechanisms for VTE, and most clinical decision rules for VTE incorporate these factors. In addition, each year of life independently increases the likelihood of imbalanced clot formation.2
Deep Vein Thrombosis
Clinical Presentation
The initial symptoms of DVT can be as subtle and nonspecific as a mild cramping sensation or sense of fullness in the calf, without objective swelling, and may be difficult to differentiate clinically from myriad other, unrelated disorders (Box 88-1). Many patients use the term charley horse to describe the sensation of an early DVT. It is precisely at this early stage, however, that DVT can be treated most effectively to minimize the potential morbidity and mortality associated with VTE. Because the left iliac vein is vulnerable to compression by the left iliac artery, leg DVT occurs with a slightly higher frequency in the left leg compared with the right; bilateral leg DVT is found in fewer than 10% of ED patients diagnosed with DVT. Likewise, the clinical signs of DVT vary and may include unilateral swelling, edema, erythema, and warmth of the affected extremity; tenderness to palpation along the distribution of the deep venous system; dilation of superficial collateral veins; and a palpable venous “cord.” The classic Homan’s sign (pain felt in the calf or posterior aspect of the knee on passive dorsiflexion of the foot while the knee is extended) is insensitive and nonspecific for DVT and has no role in clinical assessment of the patient. Upper extremity DVT refers to a thrombosis in the axillary vein and causes arm swelling on the same side as an indwelling catheter or recent intravenous infusion site. In the absence of a catheter, the most frequent location of arm DVT is on the dominant hand side. Patients frequently note that their rings become tight as an early sign of DVT.
Diagnosis
Diagnosis of DVT and PE starts with an estimation of the pretest probability (PTP). This estimation may be accomplished either by the clinical gestalt of an experienced practitioner or in conjunction with a clinical decision tool, such as that derived and validated by Wells and colleagues (Table 88-1). Patients with a low PTP can have DVT excluded with a normal quantitative D-dimer.
Table 88-1
Clinical Model for Estimating the Pretest Probability of Deep Vein Thrombosis
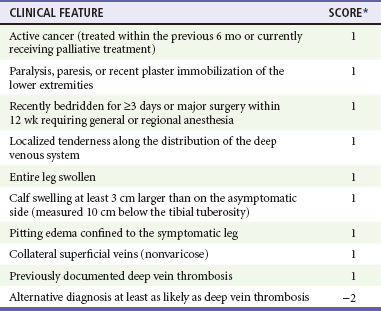
*A score of <2 indicates that the probability of deep vein thrombosis is low.
Adapted from Wells PS, Anderson D, Bormanis J: Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 350:1795, 1997.
Radiographic Evaluation
DVT evaluation is by a combination of D-dimer testing and duplex venous ultrasonography.
Venous duplex ultrasonography, performed by a certified sonographer and interpreted by a board-certified radiologist or similarly credentialed expert, has a sensitivity and specificity of approximately 95% for proximal DVT and is the diagnostic test of choice in most centers. It is important for emergency physicians to know the technique of the examination. Most radiology departments use the three point sequence (common and superficial femoral veins and popliteal vein, excluding the calf and saphenous veins). Although management of calf vein and saphenous clots remains controversial, the diagnostic sensitivity of a single venous ultrasound for the exclusion of a clot at risk of progressing to a proximal DVT is increased significantly by including the calf and saphenous veins. A patient at low risk may have the diagnosis of DVT effectively excluded by a negative three-point venous duplex ultrasound. However, for patients at higher than low risk, a single negative three-point ultrasound is inadequate as a sole method to exclude DVT, whereas a single normal whole-leg ultrasound (including normal calf and saphenous veins) is sufficient to exclude DVT with any PTP.1 A negative three-point ultrasound together with a negative quantitative D-dimer is sufficient to exclude DVT with any PTP. In a patient with a high PTP in whom the D-dimer is elevated (or not performed), a negative three-point ultrasound at the index visit should be followed by a repeat ultrasound in 2 to 7 days, which if negative is sufficient to exclude PE. An expertly performed and interpreted positive ultrasound is sufficient to confirm the diagnosis of DVT. Ultrasound cannot be used to rule out iliac or pelvic vein thrombosis.
Many emergency physicians use bedside ED ultrasound in their daily practice, but at present the data are conflicting as to whether emergency physician–performed ultrasound (EPPU) for lower-extremity DVT has adequate diagnostic accuracy. Follow-up with formal diagnostic duplex ultrasound seems reasonable.2
Indirect computed tomography (CT) venography (CTV) is not a primary imaging modality for DVT but may be performed in conjunction with CT pulmonary angiography (CTPA) of the chest during the evaluation of suggested PE. Adding CTV to CTPA provides an incremental increase in the sensitivity for VTE, identifying DVT in approximately 2% of patients in whom the CTPA is read as negative for PE but at the expense of significant additional radiation exposure to the pelvis and lower extremities.3 Interobserver agreement among radiologists interpreting CTV appears to be less than that for the CTPA portion of the study, possibly because of poor venous opacification in many cases.4 At this time, routine use of CTV is not necessary when CTPA is performed.11
Treatment
Superficial Leg Thrombophlebitis
Based on the results of a large randomized controlled trial, patients with a clot in the greater saphenous vein that extends above the knee are at risk for progression to DVT via the saphenous-femoral vein junction and may require an abbreviated course of anticoagulation.5 The published evidence suggests that saphenous vein thrombophlebitis can adequately be treated with nonsteroidal anti-inflammatory drugs, heat, and graded compression stockings (fitted to exert 30-40 mm Hg of pressure at the ankle) followed by a mandatory repeat ultrasound in 2 to 5 days. If the clot is extending, then anticoagulation is indicated. The duration of anticoagulation treatment remains uncertain, but full-dose low molecular weight heparin or fondaparinux for 10 days followed by repeat ultrasound seems reasonable.
Isolated Calf Vein Thrombosis
The optimal management strategy for thromboses of the tibial or peroneal veins remains controversial.7 Approximately 25% of isolated calf vein thromboses propagate proximally, prompting recommendations for treatment with anticoagulation as for proximal leg DVT.8 However, most of these data were from hospitalized or postoperative patients with a higher risk of propagation than ambulatory patients.9 For tibial or peroneal vein thrombosis in an otherwise healthy, ambulatory patient with no other indication for anticoagulation, I recommend antiplatelet therapy with aspirin (325 mg of enteric-coated acetylsalicylic acid per day) and close follow-up with repeat duplex ultrasound scan at 2 to 5 days to evaluate for clot propagation.
Upper Extremity Venous Thromboses
DVTs of the upper extremity have become more common in association with increased use of indwelling venous catheters and wires for electronic cardiac devices. Upper extremity DVT can cause PE, and all patients with DVT above the elbow require definitive treatment.10–12
Complications
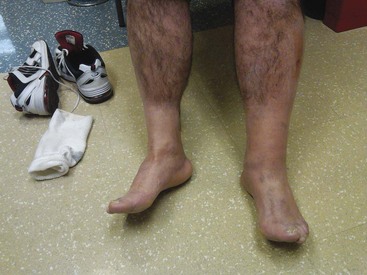
Although the most feared complication of DVT is fatal PE, DVT damages venous valves, causing venous insufficiency. Venous insufficiency, in turn, manifests as a spectrum ranging from painless varicosities to severe postphlebitic syndrome, which can cause unremitting pain and swelling, varicose veins, skin changes, and nonhealing ulcers. Figure 88-1 shows the leg of a construction worker with a femoral DVT that produces swelling on the job, impairing his ability to work.
Pulmonary Embolism
PE results from a clot that formed hours, days, or weeks earlier in the deep veins and dislodged, traveled through the venous system, and traversed the right ventricle into the pulmonary vasculature. What the patient experiences during this process varies widely, ranging from no symptom to cardiovascular collapse. No one knows exactly how many patients pass through the ED with PE because there is no reliable way of identifying missed cases. Assuming that ED populations have a risk for PE somewhere between that of hospitalized patients (who are at high risk for PE) and outpatients (who are at lower risk), approximately 1 in every 500 to 1000 ED patients has PE. About 8% of ED patients with PE die within 30 days, even when PE is promptly diagnosed and treated.10
Pathophysiology of Pulmonary Vascular Occlusion
In contrast, if asked in a detailed and structured way, about 90% of patients with noninfarcting emboli admit to the sensation of dyspnea. The dyspnea may be constant and oppressive or may be intermittent and perceived only with exertion, possibly because of an exercise-induced increase in pulmonary vascular resistance. Rest dyspnea seems to be the clinical manifestation of distorted and irregular blood flow within the lung, referred to as ventilation-perfusion inequality. With each breath a patient with PE wastes ventilation because of increased alveolar dead space (alveoli that are ventilated but not perfused). A lodged clot can redistribute blood flow to areas of the lung with already high perfusion relative to ventilation and therefore cause more blue blood to pass through the lung without being fully oxygenated. This venous admixture is probably the primary cause of hypoxemia with PE and the increased alveolar-arterial oxygen difference (the A-a gradient). About 15% of patients with PE have a normal A-a gradient of oxygen (with normal defined as age in years/4 + 4), however, and the A-a gradient is abnormally high in most patients who are evaluated for PE but ultimately found to not have PE. In a multicenter registry of 348 patients with PE, 37 (10.6%) had a pulse oximetry reading of 100% at the time of arrival to the ED, while breathing room air. Despite its shortcomings as a single diagnostic step, the presence of hypoxemia (pulse oximetry <95%, breathing room air) that cannot be explained by a known disease process increases the probability of PE. Conversely, a normal oxygen saturation can be used only when considered together with multiple other clinical features and should not alone or independently be used to forego testing for PE (Box 88-2).18,19 In addition, when PE is diagnosed, the severity of hypoxemia represents a powerful independent predictor of patient outcome.
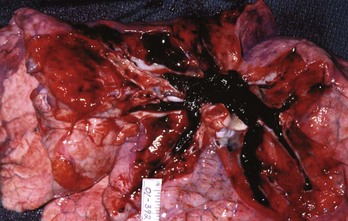
PE also causes highly variable effects on vital signs. In the ED, about half of all patients with PE have a heart rate greater than 100 beats/min.11 Tachycardia from PE probably results from impaired left ventricular filling, leading to a pathophysiologic process that parallels that of hemorrhagic shock. In one study, the probability of PE was not reduced in patients who normalized any vital sign while in the ED.12 When PE obstructs more than 50% of the vasculature, it usually causes an acute increase in right ventricular pressure. In contrast to the left ventricle, the right ventricle does not show an elastic response to acutely increased afterload; it quickly dilates, showing echocardiographic hypokinesis early in the course. In about 40% of cases, the right ventricular damage persists for at least 6 months and probably longer. Arterial hypotension represents an ominous hemodynamic consequence of PE; it occurs in only about 10% of patients but signifies a fourfold increase in risk of death compared with normotensive patients.20 In its most extreme form, PE can obstruct the right ventricular outflow entirely, either by casting the entire pulmonary vascular tree (Fig. 88-2) or by acutely occluding the main pulmonary artery. Pulseless electrical activity (PEA) is the most common electrocardiogram (ECG) result from obstructive PE. The survival rate for cardiac arrest from PE is abysmally low, even if the arrest is witnessed and heroic treatment is initiated.