Chapter 31
Preoperative Management
Matthew J. Eagleton, Jeanwan Kang
Based on a chapter in the seventh edition by Tamara N. Fitzgerald and Alan Dardik
Preoperative assessment and management of vascular patients are of utmost importance because patients with vascular disease have multisystem involvement, including cardiac, cerebrovascular, renal, and peripheral arterial disease, as well as multiple other comorbid conditions. Consequently, they are at high risk for complications after surgery. A focused preoperative evaluation should identify potential treatable risk factors so that complications can be minimized and optimal choices can be made for the timing of elective surgery.
General Preoperative Risk Assessment
Every patient undergoing elective vascular surgery should have a preoperative assessment that includes a thorough history and physical examination, blood analysis, and electrocardiogram (ECG). In addition, if there are patient-specific areas of concern, these should receive a more detailed, directed evaluation. A complete blood count should be obtained to screen for the presence of infection, to ensure an adequate red blood cell volume, and to rule out a serious hematologic abnormality. Serum electrolyte concentrations should be evaluated and corrected when abnormalities exist. Of special importance are serum potassium, calcium, and magnesium levels because if they are abnormal and not corrected, they can lead to deleterious cardiac effects. Furthermore, because renal disease is so prevalent in vascular patients and some vascular interventions may compromise renal function, a baseline creatinine level should be obtained. All patients should also have serum glucose concentration measured, and in diabetic patients, glucose levels should be controlled before, during, and after intervention. Measures of coagulation, such as the prothrombin time and international normalized ratio, should be determined to identify coagulation abnormalities, and in patients taking warfarin or other anticoagulants, an appropriate anticoagulation scheme should be decided on before surgery (Box 31-1).
Because cardiac disease is so prevalent among patients with peripheral vascular disease, ECG should be performed in all patients. ECG not only provides critical information about the presence of a rhythm abnormality or previous infarction but also provides a baseline assessment should an adverse cardiac event occur in the postoperative period. Chest radiography may be helpful in some patients if undiagnosed underlying disease is suspected on the basis of the history and physical examination. In select patients, more advanced testing may be required, such as dobutamine stress echocardiography or pulmonary function tests when cardiac or pulmonary disease is suspected. Finally, overall assessment of health can be quantified by the American Society of Anesthesiology classification (Table 31-1).
Table 31-1
American Society of Anesthesiology (ASA) Classification
| ASA I | Normal, healthy patient with good exercise tolerance |
| ASA II | Controlled medical conditions without significant systemic effects |
| ASA III | Medical conditions with systemic effects; functional compromise |
| ASA IV | Medical condition with significant dysfunction; potential threat to life |
| ASA V | Critical medical condition; little chance of survival with or without surgery |
| ASA VI | Brain death; anesthesia performed for organ donation |
Specific Risk Factor and Organ System Evaluation
Cardiac Evaluation
Heart disease is present in more than 50% of patients with peripheral vascular disease, so it is imperative that all vascular patients be appropriately screened for cardiac disease. Cardiac disease is discussed in more detail in Chapter 39, but a brief overview of the preoperative evaluation is provided here.
Factors that identify patients with heart disease include coronary artery disease, heart failure, previous myocardial infarction (MI), aortic stenosis, and advanced age. Other predictors are diabetes, hypertension, and poor functional capacity. During the preoperative evaluation, the surgeon should acquire a detailed cardiac history, including information on current exercise tolerance, angina, dyspnea, fatigue, syncope, arrhythmias, previous MI, and prior cardiac interventions. A thorough cardiovascular examination should also be performed and an ECG obtained in all patients both to serve as a baseline assessment and to identify dysrhythmias or signs of current or previous myocardial ischemia. On the basis of this information and the clinical presentation of the patient, the decision is then made as to whether a more detailed preoperative cardiac assessment is necessary.
The American College of Cardiology Foundation and the American Heart Association have provided recommendations to guide physicians with regard to pursuing further cardiac workup (Table 31-2).1
Table 31-2
ACCF/AHA Recommendation for Perioperative Cardiac Assessment
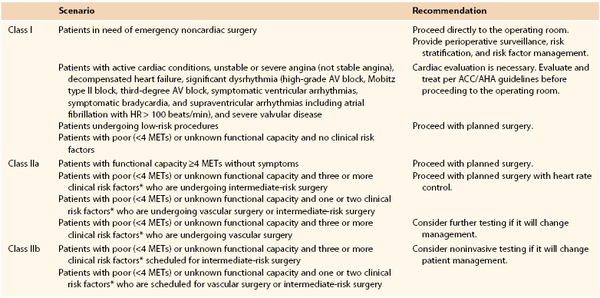
* Clinical risk factors include ischemic heart disease, compensated or prior heart failure, diabetes mellitus, renal insufficiency, and cerebrovascular disease.
Class I recommendations suggest that procedures/treatments should be performed; Class IIa recommendations suggest that it is reasonable to perform the procedure/treatment; Class IIb recommendations imply that the procedure/treatment should be considered; and in Class III the intervention should not be performed because it may not be helpful and may potentially be harmful to the patient.
ACCF/AHA, American College of Cardiology Foundation/American Heart Association; AV, atrioventricular; HR, heart rate; METs, metabolic equivalents.
Major vascular procedures represent the highest risk procedures. Endovascular aortic aneurysm repair and carotid endarterectomy, however, are considered within the intermediate-risk category, distinct from the open vascular surgery procedures. Dipyridamole-thallium imaging or dipyridamole stress echocardiography may be indicated for patients at intermediate or high cardiac risk who are undergoing vascular surgery (as outlined before), but it is probably unnecessary in low-risk individuals. In addition, those who have undergone coronary revascularization within 5 years or have normal findings on coronary angiography or cardiac stress testing within 2 years may also proceed to surgery without further cardiac evaluation provided there have been no significant functional changes since that time.
Preoperative Medical versus Interventional Therapy for Cardiac Disease
Perioperative use of beta blockers has long been the standard of care for most patients with cardiac disease undergoing vascular surgery. Their use, however, has engendered significant controversy during the past several years. The POISE (PeriOperative Ischemic Evaluation) trial confirmed a reduction in primary cardiac events with perioperative beta-blockade therapy.2 Nearly 8000 patients undergoing noncardiac surgery were randomized to a fixed higher dose, extended-release metoprolol started the day of surgery, or no treatment. Whereas patients receiving beta-blockade therapy experienced a reduction in cardiovascular death, MI, and cardiac arrest, this benefit was offset by an increased risk of stroke and total mortality. The results suggested that the administration of high-dose beta blockers in the absence of dose titration may be harmful in beta-blocker naive patients. In the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography trial (DECREASE-IV), patients were randomized to receive beta blockade only, statin only, beta blockade and statin, or neither medication before elective noncardiac surgery.3 Beta-blocker use, initiated well before surgery and titrated for heart rate (50-70 beats/min), proved to be cardioprotective to intermediate-risk patients, without an increase in perioperative stroke or death. The results of this trial, however, have been called into question.3a On the basis of these studies as well as several others, the American College of Cardiology Foundation/American Heart Association adjusted their recommendations for perioperative beta-blocker use in 2009 (Table 31-3).
Table 31-3
ACCF/AHA Guidelines for Perioperative Beta-Blocker Use
| Scenario | Recommendation | |
| Class I | Patients undergoing surgery who are receiving beta blockers for treatment of conditions with ACCF/AHA Class I guidelines for the drugs | Continue perioperative beta blockers. |
| Class IIa | Patients undergoing vascular surgery who are at high cardiac risk owing to CAD Preoperative assessment for vascular surgery identifies high cardiac risk as defined by more than one clinical risk factor* Preoperative assessment identifies CAD or high cardiac risk (identified by the presence of >1 clinical risk factor*) in patients who are undergoing intermediate-risk surgery |
Beta blockers titrated to heart rate and blood pressure are reasonable. |
| Class IIb | Patients undergoing either intermediate-risk procedures or vascular surgery in whom preoperative assessment identifies a single clinical risk factor* in the absence of CAD Patients undergoing vascular surgery with no clinical risk factors who are not currently taking beta blockers |
The usefulness of beta blockers is uncertain. |
| Class III | Patients undergoing surgery who have absolute contraindication to beta blockade Routine administration of high-dose beta blockade in the absence of dose titration in patients not currently taking beta blockers who are undergoing noncardiac surgery |
Use is contraindicated. |
* History of ischemic heart disease, heart failure, cerebrovascular disease, diabetes mellitus, and renal insufficiency.
ACCF/AHA, American College of Cardiology Foundation/American Heart Association; CAD, coronary artery disease.
In addition to beta blockade, the patient’s current cardiovascular regimen, including other antihypertensives and statins, should be maintained. Historically, some recommended that aspirin be discontinued before surgery to avoid hemorrhagic complications; current guidelines, however, recommend continued aspirin perioperatively, given its value in cardiovascular health and low risk of associated bleeding.4 Few data are available to recommend the cessation or continuation of clopidogrel before major vascular surgery, although most consider there to be increased risk of bleeding associated with its continued use. Before considering stopping of antiplatelet agents, however, one must take into consideration whether the patient has had a recent percutaneous coronary intervention. If aspirin and clopidogrel need to be discontinued perioperatively, elective surgery should be postponed 4 to 6 weeks after placement of a bare metal stent and 12 months after a drug-eluting stent.4 If surgery cannot be postponed, aspirin therapy should be continued throughout the perioperative period. In general, statin agents should not be discontinued before surgery but should be continued throughout the hospitalization, in particular in patients with vascular disease. There is evidence demonstrating the effectiveness of statins in preventing cardiovascular events among high-risk patients through their effects on endothelial function, reduction in vascular inflammation, and stabilization of atherosclerotic plaque.1
It is often debated whether coronary artery revascularization should be performed before elective peripheral vascular surgery. In support of a conservative management plan, procedure-related complications in patients undergoing coronary artery revascularization are frequent and often lead to delays in the intended vascular surgery. Furthermore, patients who have recently (<5 years) undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) before vascular surgery do not have an obvious survival advantage over patients at high cardiac risk without previous coronary interventions. In addition, the Coronary Artery Revascularization Prophylaxis (CARP) trial demonstrated that in patients with stable coronary artery disease, coronary artery revascularization (with either CABG or PCI) before elective major vascular surgery does not improve long-term survival or reduce short-term postoperative outcomes such as death, MI, or length of hospital stay.5 The trial did exclude patients with unstable coronary syndromes, more than 50% stenosis of the left main coronary artery, and left ventricular ejection fraction below 20%. In further support of these results, the DECREASE-V pilot study screened 1880 patients scheduled to undergo major vascular surgery to identify a high-risk cohort.6 The final cohort of 101 patients were randomized to best medical therapy alone or best medical therapy and revascularization before vascular surgery. Thirty-day and 1-year all-cause death and nonfatal MI were similar between the two groups. Thus, current recommendations are that CABG or PCI be reserved for patients with unstable cardiac symptoms or advanced coronary disease, for whom a survival benefit with CABG or PCI is clear.1
Preoperative Management of Hypertension
Hypertension must be carefully controlled in the perioperative period because excessive swings in blood pressure and heart rate place a patient with coronary artery disease at risk for cardiac ischemia. In addition, because chronic hypertension resets cerebral circulatory autoregulation, reductions in blood pressure may induce cerebral ischemia. Therefore, an appropriate history should be obtained for each patient, including onset of hypertension, degree and strategy of control, and current medications. Although most hypertension is primary, the surgeon should take care to assess the possibility of hypertension from a secondary source, such as renal artery stenosis, coarctation of the aorta, pheochromocytoma, Conn’s syndrome, Cushing’s syndrome, or other causes that may prove dangerous during a surgical procedure.
Blood pressure should always be measured in a relaxed setting with an appropriately sized cuff to prevent erroneous values. The diagnosis of hypertension is made when two or more readings of diastolic blood pressure are higher than 90 mm Hg or systolic blood pressure is higher than 140 mm Hg. Accelerated hypertension is defined as markedly elevated blood pressure plus grade 3 retinopathy (hemorrhage and exudates), whereas malignant hypertension is defined as markedly elevated blood pressure plus papilledema. Complicated hypertension includes cardiovascular end-organ damage, such as stroke, renal failure, MI, or aortic aneurysm.
Several measures can be taken to reduce the operative risks associated with hypertension. Beta blockade is a good choice for blood pressure control in patients with vascular disease because it is also valuable for cardioprotection. In addition, beta blockade has been shown to limit the magnitude of the rise in blood pressure during intubation and the amount of ischemic changes on the ECG during surgical procedures. The use of α2 agonists, such as clonidine, can lead to reduced rates of perioperative MI and mortality. Patients should be maintained with their current antihypertensives as withholding of these medications before surgery may result in rebound hypertension. The exceptions are angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and diuretics, which should be withheld the day of surgery, particularly in patients with renal insufficiency.
Elective surgery should be delayed if systolic blood pressure is higher than 200 mm Hg or if diastolic blood pressure is higher than 120 mm Hg, and acute control within hours of surgery is inadvisable. Pain can elevate blood pressure; therefore, appropriate analgesia with nonsteroidal anti-inflammatory drugs or opioids should be administered to alleviate pain. Agents with minimal hemodynamic effects should be considered, such as fentanyl rather than morphine. Before induction of anesthesia, patients should also be well hydrated because volume loading can minimize the drastic swings in blood pressure that are characteristic of hypertensive patients. Refer to Chapter 30 for a more detailed discussion of hypertension in vascular patients.
Pulmonary Evaluation
A thorough discussion of pulmonary care is provided in Chapter 40, but a brief review of preoperative pulmonary assessment is covered here. Postoperative pulmonary complications play a significant role in the risk for surgery and anesthesia. As such, preoperative pulmonary evaluation should be completed in all patients and should begin with a history and physical examination, which are the most sensitive evaluation to identify “at-risk” patients. Risk factors for postoperative pulmonary complications include patient-related risk factors (i.e., chronic obstructive pulmonary disease, age older than 60 years, American Society of Anesthesiology class II or higher, functionally dependent, and congestive heart failure). Obesity and mild to moderate asthma do not increase the risk of pulmonary complications.7 The surgeon should pay particular attention to any history of smoking, exercise intolerance, unexplained dyspnea, or coughing. On physical examination, decreased breath sounds, wheezes, crackles, or prolonged expiratory phase should be noted. Procedure-related risks should likewise be assessed. The duration of surgery, the need for emergent surgery, and the surgical site all influence the risk of perioperative pulmonary complications. Aortic aneurysm repair, thoracic surgery, abdominal surgery, upper abdominal surgery, emergency surgery, and vascular surgery all increase the risk of pulmonary complications.7
In some situations, the at-risk patient will require further pulmonary evaluation and risk stratification. Whereas clinicians frequently obtain chest radiographs as part of a routine preoperative evaluation, few show unexpected abnormalities or influence the management of the patient. Despite this, there is some evidence that chest radiographs may be helpful for patients with known cardiopulmonary disease and those older than 50 years who are undergoing upper abdominal, thoracic, and open abdominal aortic aneurysm surgery.7 The value of pulmonary function testing before lung resection surgery and in determining the candidacy for coronary artery revascularization is well documented. Its value outside of these cases, however, remains controversial. In addition, available data do not suggest a prohibitive threshold on these studies below which the risks of surgery are not acceptable. Currently the American College of Physicians recommends preoperative pulmonary function testing only in patients who are thought to have undiagnosed chronic obstructive pulmonary disease. Other factors, such as low serum albumin concentration (<35 g/L), are powerful, independent markers of increased risk for postoperative pulmonary complications.8 Several indices incorporating multiple factors can be used to stratify pulmonary risk.
Pulmonary risk factors have been added to the Goldman Cardiac Risk Index and include the following: obesity (body mass index >27 kg/m2), cigarette smoking within 8 weeks of surgery, productive cough within 5 days of surgery, wheezing within 5 days of surgery, ratio of forced expiratory volume in 1 second to forced vital capacity of less than 70%, and PaCO2 within 45 mm Hg. Patients with a combined score of more than 4 points (of 10) are 17 times more likely to have complications.
Once a patient is identified as at risk for pulmonary complications, risk reduction strategies should be used. These include smoking cessation, inspiratory muscle training, optimization of nutritional status, intraoperative strategies, and postoperative lung expansion techniques when appropriate. Patients who currently smoke have a twofold increased risk for postoperative complications, with the highest risk in patients who have smoked within the last 2 months. It has been questioned, therefore, whether preoperative smoking cessation could reduce the perioperative risk. In a prospective randomized trial in men undergoing hip and knee replacement surgery, patients were randomly assigned to usual care versus a smoking cessation program including weekly meetings and nicotine replacement.9 Overall complications were lower in the intervention group (18% vs 52%), but this was primarily due to fewer wound complications and urinary tract infections. No differences in pulmonary measures were identified, but there was a trend toward shorter hospital stays and fewer cardiac complications in the intervention group. Smoking cessation may paradoxically increase postoperative complications for smokers who stop or reduce smoking shortly (<2 months) before surgery.10







