Chapter 4 Physiology of Male Gametogenesis
INTRODUCTION
The contribution of the male to the biology of reproduction is to produce a genetically intact spermatozoa that will fertilize an oocyte. The end product of male gametogenesis, the mature spermatozoa, is designed for one purpose: to deliver the male contribution of the genetic makeup to the embryo. The biology of gamete production is different in males compared to females. Gamete production in females is intimately part of the endocrine responsibility of the ovary. If there are no gametes, then hormone production is drastically curtailed. Depletion of oocytes implies depletion of the major hormones of the ovary. In the male this is not the case. Androgen production will proceed normally without a single spermatozoa in the testes.
ORGANIZATION OF THE TESTES
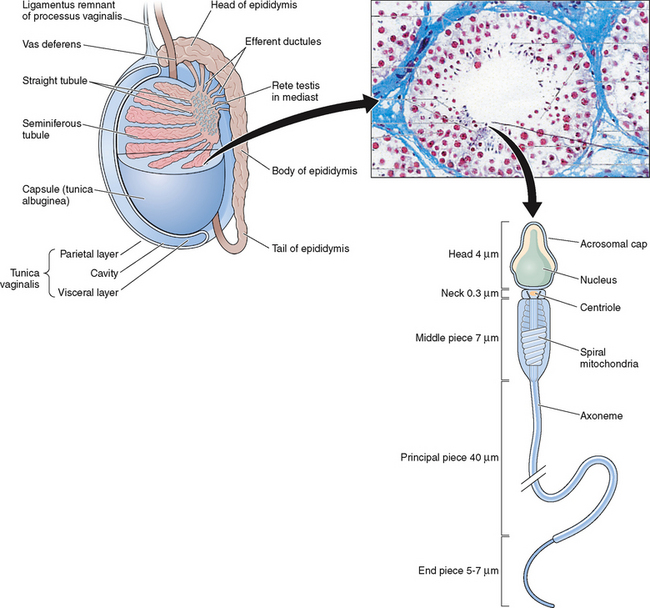
The testes are ellipsoid in shape, measuring 2.5 × 4 cm in diameter and engulfed by a capsule (tunica albuginea) of strong connective tissue.1 Along its posterior border, the testis is loosely connected to the epididymis, which gives rise to the vas deferens at its lower pole.2 The testis has two main functions: it produces hormones, in particular testosterone, and it produces the male gamete—the spermatozoa. The testis is incompletely divided into a series of lobules. Most of the volume of the testis is made up of seminiferous tubules, which are looped or blind-ended and packed in connective tissue within the confines of the fibrous septa (Fig. 4-1). The fibrous septae divide the parenchyma into about 370 conical lobules consisting of the seminiferous tubules and the intertubular tissue. The seminiferous tubules are separated by groups of Leydig cells, blood vessels, lymphatics, and nerves. The seminiferous tubules are the site of sperm production (see Fig. 4-1). The wall of each tubule is made up of myoid cells of limited contractility and also of fibrous tissue. Each seminiferous tubule is about 180 μm in diameter; the height of the germinal epithelium measures 80 μm, and the thickness of the peritubular tissue is about 8 μm.3 The germinal epithelium consists of cells at different stages of development located within the invaginations of Sertoli cells, namely spermatogonia, primary and secondary spermatocytes, and spermatids. Both ends of the seminiferous tubules open into the spaces of the rete testis4 (see Fig. 4-1). The fluid secreted by the seminiferous tubules is collected in the rete testis and delivered in the ductal system of the epididymis.
SUPPORTING CELLS
Leydig Cells
The Leydig cells are irregularly shaped cells with granular cytoplasm present individually or more often in groups within the connective tissue.5,6 Leydig cells are the prime source of the male sex hormone testosterone.7–9 The pituitary hormone luteinizing hormone (LH) acts on Leydig cells to stimulate the production of testosterone. This acts as a negative feedback on the pituitary to suppress or modulate further LH secretion.8 Compared with the testosterone levels in the blood, the intratesticular concentration of testosterone is many times higher, especially near the basement membrane of the seminiferous tubule.
The Sertoli Cell
The seminiferous tubules are lined with highly specialized Sertoli cells that rest on the tubular basement membrane and extend into the lumen with a complex ramification of cytoplasm (Fig. 4-2). Spermatozoa are produced at puberty but are not recognized by the immune system that develops during the first year of life. The seminiferous tubule space is divided into basal (basement membrane) and luminal (lumen) compartments by strong intercellular junctional complexes called tight junctions. These anatomic arrangements, complemented by closely aligned myoid cells that surround the seminiferous tubule, form the basis for the blood–testis barrier. The blood–testis barrier provides a microenvironment for spermatogenesis to occur in an immunologically privileged site. Sertoli cells serve as “nurse” cells for spermatogenesis, nourishing germ cells as they develop. These also participate in germ cell phagocytosis. Multiple sites of communication exist between Sertoli cells and developing germ cells for the maintenance of spermatogenesis within an appropriate hormonal milieu. Follicle-stimulating hormone (FSH) binds to the high-affinity FSH receptors found on Sertoli cells, signaling the secretion of androgen-binding protein. High levels of androgens are also present within the seminiferous tubule.
The two most important hormones secreted by the Sertoli cells are antimüllerian hormone and inhibin. Antimüllerian hormone is a critical component of embryonic development and is involved in the regression of the müllerian ducts. Inhibin is a key macromolecule in pituitary FSH regulation. Some of the functions of the Sertoli cell are (1) maintenance of integrity of seminiferous epithelium, (2) compartmentalization of seminiferous epithelium, (3) secretion of fluid to form tubular lumen to transport sperm within the duct, (4) participation in spermiation, (5) phagocytosis and elimination of cytoplasm, (6) delivery of nutrients to germ cells, (7) steroidogenesis and steroid metabolism, (8) movement of cells within the epithelium, (9) secretion of inhibin and androgen-binding protein, (10) regulation of spermatogenic cycle, and (11) provision of a target for hormones LH, FSH, and testosterone receptors present on Sertoli cells.
SPERMATOGENESIS
The process of differentiation of a spermatogonium into a spermatid is known as spermatogenesis.4 It is a complex, temporal event whereby primitive, totipotent stem cells divide to either renew themselves or produce daughter cells that become specialized testicular spermatozoa over a span of weeks. Spermatogenesis involves both mitotic and meiotic proliferation as well as extensive cell remodeling. Spermatogenesis can be divided into three major phases: (1) proliferation and differentiation of spermatogonia, (2) meiosis, and (3) spermiogenesis, a complex metamorphosis that transforms round spermatids arising from the final division of meiosis into a complex structure called the spermatozoon. In humans, the process of spermatogenesis starts at puberty and continues throughout the entire lifespan of the individual. It takes place in the lumen of the seminiferous tubules. In fact, 90% of the testis volume is determined by the seminiferous tubules and their constituent germ cells at various stages of development. Once the gonocytes have differentiated into fetal spermatogonia, an active process of mitotic replication is initiated very early in embryonic development. This appears to be under FSH control and develops the baseline number of precursor cells of the testicle.
Proliferation and Differentiation of Spermatogonia
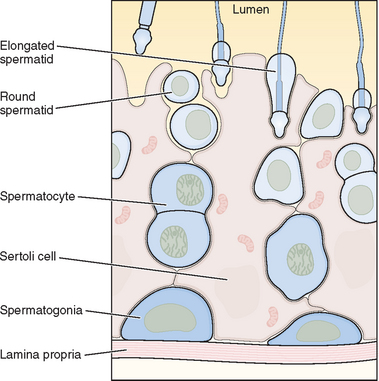
Within the seminiferous tubule, germ cells are arranged in a highly ordered sequence from the basement membrane to the lumen (see Fig. 4-2). Spermatogonia lie directly on the basement membrane, followed by primary spermatocytes, secondary spermatocytes, and spermatids as they progress toward the tubule lumen. The tight junction barrier supports spermatogonia and early spermatocytes within the basal compartment and all subsequent germ cells within the luminal compartment.
Types of Spermatogonia
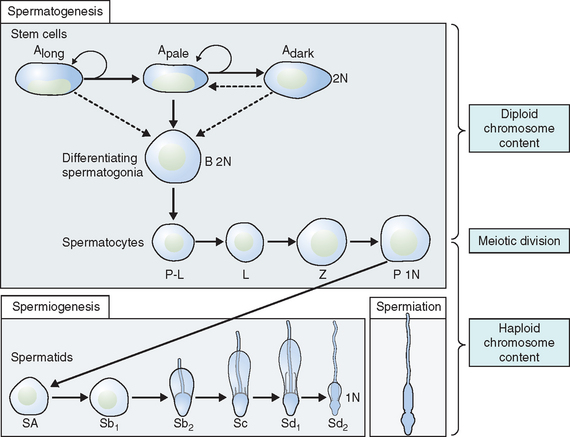
Spermatogonia represent a population of cells that divide by mitosis, providing both a renewing stem cell population as well as spermatogonia that are committed to enter the meiotic process. Germ cells are staged by their morphologic appearance; there are dark type A (Adark) and pale type A (Apale) and type B spermatogonia, primary spermatocytes (preloptotene, leptotene, zygotene, and pachytene), secondary spermatocytes, and spermatids (Sa, Sb, Sc, Sd1, and Sd2) (Fig. 4-3). Other proliferative spermatogonia include Apaired (Apr), resulting from dividing Aisolated (Ais), and subsequently dividing to form Aaligned (Aal). Differentiated spermatogonia include type A1, A2, A3, A4, intermediate, and type B, each a result of the cellular division of the previous type. In humans, four spermatogonial cell types have been identified; these are Along, Adark, Apale, and B.10–12 In the rat, type Ais is believed to be the stem cell13,14; however, it is not clear which human type A spermatogonia is the stem cell. Some investigators have proposed that the type Adark spermatogonia represent the reserve or nonproliferative spermatogonial population that gives rise to Apale.11,15,16 Spermatogonia do not separate completely after meiosis but remain joined by intercellular bridges, which persist throughout all stages of spermatogenesis and are thought to facilitate biochemical interactions, allowing synchrony of germ cell maturation.17
Type B spermatogonia possess considerably more chromatin within the inner nuclear envelope than intermediate or type A spermatogonia. Type B spermatogonia represent the cells that differentiate and enter into the process of meiosis during which they are called primary spermatocytes.11 They are the differential precursors to preleptotene spermatocytes. This last mitotic division helps maintain a pool of stem cells so the process can continue indefinitely.
Mitosis
Mitosis involves proliferation and maintenance of spermatogonia. It is a precise, well-orchestrated sequence of events involving duplication of the genetic material (chromosomes), breakdown of the nuclear envelope, and equal division of the chromosomes and cytoplasm into two daughter cells.18,19 DNA is also spatially organized into loop domains on which specific regulatory proteins interact during cellular replication.19–24 The mitotic phase involves spermatogonia (types A and B) and primary spermatocytes (spermatocytes I). Developing germ cells interconnected by intracellular bridges produce the primary spermatocyte through a series of mitotic divisions. Once the baseline number of spermatogonia is established after puberty, the mitotic component will proceed in order to continue to provide precursor cells and to start the process of differentiation and maturation.
Meiosis
Meiosis is a complex process with specific regulatory mechanisms of its own.25 The process commences when type B spermatogonia lose their contact with the basement membrane to form preleptotene primary spermatocytes. Thus, each primary spermatocyte can theoretically yield four spermatids, although fewer actually result, because some germ cells are lost due to the complexity of meiosis. The primary spermatocytes are the largest germ cells of the germinal epithelium. Meiosis is characterized by prophase, metaphase, anaphase, and telophase. In this, two successive cell divisions yield four haploid spermatids from one diploid primary spermatocyte. As a consequence, the daughter cells contain only half of the chromosome content of the parent cell. After the first meiotic division (reduction division), each daughter cell contains one partner of the homologous chromosome pair, and they are called secondary spermatocytes. These cells rapidly enter the second meiotic division (equational division), in which the chromatids then separate at the centromere to yield haploid early round spermatids. Meiosis assures genetic diversity and involves primary and secondary spermatocytes, which give rise to spermatids.
Spermiogenesis
Spermiogenesis is a process during which the morphologic changes occur during the differentiation of the spermatid into the spermatozoon. It begins once the process of meiosis is completed. Six different stages have been described in the process of spermatid maturation in humans: Sa-1 and Sa-2, Sb-1 and Sb-2, and Sc-1 and Sc-2 (see Fig. 4-3). Each of these stages can be identified by morphologic characteristics. During the Sa-1 stage, both the Golgi complex and mitochondria are well developed and differentiated, the acrosomal vesicle appears, the chromatid body develops in one pole of the cell opposite from the acrosomal vesicle, and the proximal centriole and the axial filament appears. During Sb-1 and Sb-2 stages, acrosome formation is completed, the intermediate piece is formed, and the tail develops. This process is completed during the Sc stages. During the postmeiotic phase, progressive condensation of the nucleus occurs with inactivation of the genome, the histones are converted into transitional proteins, and finally protamines are converted into well-developed disulfide bonds.
Spermiation
The process whereby a mature spermatid frees itself from the Sertoli cell and enters the lumen of the tubule as a spermatozoon is known as spermiation. The spermatids originating from the same spermatogonia remain connected by bridges to facilitate the transport of cytoplasmic products. Spermiation involves the active participation of the Sertoli cell. This may also involve actual cell movement as the spermatids advance toward the lumen of the seminiferous tubules.26 The mature spermatids close their intracellular bridges, disconnect their contact to the germinal epithelium, and become free cells called spermatozoa. Portions of the cytoplasm of the Sertoli cell known as the cytoplasmic droplet may remain as part of the spermatozoon during the process of spermiation. This is a morphologic feature present on immature sperm in semen.27
The Cycle or Wave of Seminiferous Epithelium
A cycle of spermatogenesis involves the division of primitive spermatogonial stem cells into subsequent germ cell types through the process of meiosis. Type A spermatogonial divisions occur at a shorter time interval than the entire process of spermatogenesis. Therefore, at any given time, several cycles of spermatogenesis coexist within the germinal epithelium. In humans, spermatocyte maturation takes 25.3 days, spermiogenesis 21.6 days, and the total estimated time for spermatogenesis is 74 days. Spermatogenesis is not random throughout the seminiferous epithelium. Germ cells are localized in spatial units referred to as stages and represent consistent associations of germ cell steps.28–31 In rodent spermatogenesis, one stage can be found in a cross-section of seminiferous tubule.
The cycle of spermatogenesis can be identified for each species, but the duration of the cycle varies.11 The stages of spermatogenesis are sequentially arranged along the length of the tubule. This arrangement of the stages of spermatogenesis is such that it results in a “wave of spermatogenesis” along the tubule. This wave is in space but the cycle is in time.31 Along the length of the seminiferous tubule there are only certain cross-sections where spermatozoa are released. In the rat, all stages are involved in spermatogenesis, but spermatozoa are released only in stage VIII.
Although it appears that the spatial organization is lacking or poor in the human seminiferous tubule, mathematic modeling indicates that these stages are tightly organized in an intricate spiral pattern.32 In addition to the steps being organized spatially within the seminiferous tubule, the stages are organized in time.31